Abstract
Over the past few decades, complementary medicine therapy using medicinal plants have been developed in healthcare. Phytochemical studies about medicinal plants have been conducted to verify their potency as medicinal remedies in modern therapeutics. Dipterocarpus littoralis commonly known as Meranti Jawa in Indonesia is traditionally used to treat diseases such as diarrhea, diabetic and malaria. This study aimed to isolate bioactive compounds from D. littoralis using bioguided fractionation method. The bioactivity measured were antioxidant, antidiabetic, and antiplasmodial activity. Alpha-glucosidase and alpha-amylase assays were applied to estimate the in vitro antidiabetic activity of D. littoralis. The antioxidant activities were determined by using the free radical scavenging assays 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2-2″-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS). Analysis of total flavonoid and phenolic contents were expressed as Quercetin Equivalent (QE) and Gallic Acid Equivalent (GAE), respectively. The in vitro antiplasmodial activity test of methanol extract of D. littoralis was also conducted against Plasmodium falciparum strain 3D7. Purification of the ethyl acetate fraction of the methanol extract of D. littoralis resulted in an oligostilbenes namely α-viniferin (1). The structure of the α-viniferin was characterized by comprehensive spectral analysis including IR, 1D and 2D NMR, and in comparison with the literature data. Compound 1 showed an alpha-glucosidase and alpha-amylase inhibitory activity with IC50 values of 256.17 and 212.79 μg/mL, respectively. The in vitro antiplasmodial activity test against Plasmodium falciparum strain 3D7 at a concentration of 100 μg/mL revealed a strong antiplasmodial inhibitory activity with IC50 value of 2.76 μg/mL. Our findings indicated that α-viniferin (1) which is isolated from D. littoralis extract could be regarded as potential antidiabetic and antiplasmodial resources in the future.
Keywords: Natural product chemistry, Pharmaceutical chemistry, Alpha-viniferin, D. littoralis, Alpha-glucosidase, Alpha-amylase, DPPH, ABTS, Antiplasmodial
Natural product chemistry, Pharmaceutical chemistry, Alpha-viniferin, D. littoralis, Alpha-glucosidase, Alpha-amylase, DPPH, ABTS, Antiplasmodial.
1. Introduction
Diabetes Mellitus (DM) defines by The World Health Organization (WHO) as a degenerative disease that occurs when insulin is not produced enough by the pancreas to support the metabolism process in the human body [1]. This hyperglycemia condition is a disorder of body metabolism which is characterized by a high fasting blood sugar. Indonesia seems to have the fastest-growing burden of diabetes and the numbers will continue to grow in years forward. In epidemiology, it is estimated that in 2030 the prevalence of Diabetes Mellitus (DM) in Indonesia reaches 21.3 million people. Lousy dietary habits, lack of sleep and stress increase the risk of diabetes. Obesity is also becoming the leading cause of type 2 diabetes [2, 3].
Another common disease remains one of the world's leading killers in most tropical countries, including Indonesia and as a major public health problem in Indonesia is malaria. The WHO estimated that there were 2.5 million cases of malaria in Indonesia in 2006. In 2018, the malaria situation in Indonesia shows that there were still 10.7 million people living in middle and high malaria-endemic areas. These areas mainly include Papua, West Papua and East Nusa Tenggara province recording a high number of cases of this deadly disease [4]. Many factors have contributed to the outbreaks of malaria in Indonesia. Some of these outbreaks occur under unique conditions of human malaria distribution, prevalence, and drug resistance. The Asia Pacific Leaders Malaria Alliance (APLMA) has recorded that of the 80 percent malaria cases in Indonesia in 2017, Papua province has reported the highest number of cases of the deadly disease, followed by East Nusa Tenggara, Maluku, and West Papua [5, 6].
Studies about medicinal plants have been conducted to verify their potency as medicinal remedies in modern therapeutics. A recent experimental and clinical studies have revealed several biological effects of the medicinal plant include hypolipidemic, antibacterial, anti-inflammatory, antidiabetic, antiplasmodial and antioxidant activities [7, 8, 9, 10, 11, 12]. Many efforts have been done to prevent and treat diabetes and malaria including traditional treatment using the medicinal plants. Ethnobotanically, the use of traditional medicine is based on the knowledge of the ancestors towards healing diseases.
The Dipterocarpaceae is a large tropical plant family including 16 genera with at least 600 species inside approximately. Dipterocarpus is the biggest genus of this family. This genus consist of about 75 species is widely distributed in the Southeast Asia regions especially in Malaysia and Indonesia [13, 14, 15]. Dipterocarpus littoralis is the endemic species of this genus distributed in Nusakambangan, Central Java with local name Meranti Jawa. The D.littoralis wood is used as a building material and furniture industry. The stem bark of this plant is used as a traditional remedy to treat diseases like diarrhea, diabetic and malaria [16]. Oligostilbenes such as resveratrol dimers, trimers, tetramers, hexamers, heptamers octamers are commonly found in Dipterocarpaceae plants abundantly [17]. These compounds presented a variety of bioactivities including anti-cancer, larvacidal, anti-hyperuricemic, anti-inflammatory, and anti-acetylcholinesterase [17, 18, 19, 20, 21]. There is no study has been carried out in terms of D. littoralis chemical constituents. To the best of our knowledge, this is the first report on the antioxidant, antidiabetic and antiplasmodial potency of a chemical constituent from D. littoralis. Thus, the aim of this research was to isolate and characterize chemical constituent from the methanol extract of the stem bark of D. littoralis and evaluate their bioactivities including antioxidant, antidiabetic and antiplasmodial.
2. Materials and methods
2.1. General
All solvents and chemicals used in the study were analytical grade. Ultraviolet (UV) absorbance for determining antioxidant was observed using Genesys UV-Vis Spectrophotometer and microplate reader. TLC analysis on precoated Si-gel plates (Merck Kieselgel 60 F254, 0.25 mm) and detected by UV light (254 nm) and by CeSO4 spraying reagent. Vacuum liquid chromatography (VLC) was carried out using Merck Si-gel 60. Methanol, sucrose, saline water, and dimethyl sulfoxide (DMSO) from Merck. Rat intestinal acetone powder, glucose kit, NaOH, acarbose, HCl, phosphate buffer (pH 6.9), and porcine pancreas α-amylase enzyme from Sigma Company.
2.2. Material
D. littoralis was collected from Nusakambangan island, Central Java. Voucher specimen was identified by staff of Purwodadi Botanical Garden in Purwodadi, East Java. The plant specimen was deposit in Natural Product and Synthesis Chemistry Laboratory, Chemistry Department, Institut Teknologi Sepuluh Nopember.
2.3. The extracts preparation
The stem bark of D. littoralis was separated and air-dried to constant weight at room temperature. Then grinded into a powder. The sample was macerated in 24 h using methanol as a solvent at room temperature to get the extract. The extract then concentrated using a vacuum rotary evaporator to yield a brownish methanol extract.
2.4. Fractionation
The crude extract was fractionated by vacuum liquid chromatography (VLC), eluting with a gradient solvent system. Dichloromethane fraction was collected and concentrated in vacuo. Then, this fraction was prepared by fractionated a portion (40 g) of the total methanol using vacuum liquid chromatography. Further fractionation was done by repeated VLC, eluting with gradient solvent system and purified by Sephadex in order to yield a yellowish-brown powder. Then the purity of the isolated compound was tested by monitoring three eluents and 2-dimensional TLC. The structure of the isolated compound was characterized by comprehensive spectral analysis including IR, 1D and 2D NMR.
2.5. Total flavonoid content and total phenolic content
2.5.1. Total flavonoid content (TFC)
Aluminium chloride colorimetric method (AlCl3) by Zhishen et al. (1999) was applied to get total flavonoid content with quercetin as standard [22]. Extract (1 mL) was put into a volumetric flask containing 4 mL of water, followed by NaNO2 5% (0.3 mL). Approximately 0.3 mL of AlCl3 10% was added after 5 min. The blend was let to endure for 6 min. Then, NaOH 1M (2 mL) was put in and distilled water was poured to get up to 10 mL of the total volume. The absorbance was monitored and recorded at 510 nm. The quercetin standard solution as a standard curve. Total flavonoid was established as quercetin equivalents (QE) (mg/g of dry mass). Samples were presented in triplicate.
2.5.2. Total phenolic content (TPC)
The Folin-Ciocalteau (FC) method was performed to establish a total phenolic compound. About 0.5 mL extracts (10 times diluted) and FC reagents were blended for 5 min. About 4 mL Na2CO3 1 M was then added and mixed for 15 min. The colorimetric method at 765 nm is used to determine phenols. Gallic acid standard solution as a standard curve. Total phenolic content was determined as Gallic acid equivalent (GAE) (mg/g of dry mass). Samples were presented in triplicate.
2.6. Antioxidant test
2.6.1. DPPH assay
The Brand Williams method with modified by Dudonn'e et al was applied to estimate the activities of antioxidants from the extract [23, 24]. About 3 mL 1,1, diphenyl-2-picrylhydrazyl DPPH solution 6 × 10−5 M and samples 100 μL were prepared. The methanol extracted solution and methanol DPPH solution were mixed in various concentrations. The same quantity of methanol was put into the regulator. Then, it took 20 min to incubate the mixture in dark room temperature. The absorbance was observed at 515 nm (As). The blanko DPPH solution was monitored its absorbance at 515 nm (Ab). The DPPH inhibition rate was examined with the formula: Inhibition (%) = [(Ab-As)/Ab] x100.
2.6.2. ABTS assay
The method of Re et al (1999) is also applied to determine the activities of antioxidants from the extract [25, 26]. Previously, potassium peroxydisulfate solution 140 mM was prepared and 2, 2 -azino-bis (3-ethylbenzthiazoline)-6-sulfonic (ABTS) solution 7 mM was ready. Then both stock solutions were blended and left to sit for about 12 h at room temperature. ABTS solution (1 mL) and 88 μL extract were blended. Then, it took 4 min to incubate the mixture. After that, the absorbance was monitored at 734 nm. The ABTS inhibition rate was examined with formula: Inhibition (%) = [(Ab-As)/Ab] x100. The concentration of the extracts made linear graphs towards percentage inhibitions formation to estimate IC50 values. The experiment was presented in triplicate.
2.7. Antidiabetic test
2.7.1. Alpha-glucosidase inhibitory
In-vitro α-glucosidase inhibition was determined by an inhibitory method by Khan et al. (2002) with minor modifications [27]. Rat intestinal enzyme was prepared in sodium phosphate buffer 0.1 M. Spectrophotometrically, α-glucosidase inhibition of plant extract was observed using rat intestinal enzyme and substrate sucrose 100 mM at 37 °C and pH 6.9.
Sodium phosphate buffer 150 μL including 20 μL α-glucosidase solution and sample extracts 10 μL were blended. Then, it took 10 min to incubate the mixture at 37 °C in 96-well plates. The absorbance was recorded UV spectrophotometrically at 490 nm for sample absorbance (As) and blanko absorbance (Ab). The absorbance then was estimated as the percentage inhibition of the sample at various concentrations. The α-glucosidase inhibition was examined with the formula: α-glucosidase inhibitory activity (%) = [(Ab-As)/Ab] x100.
2.7.2. Alpha-amylase inhibitory test
The α-amylase activity test was applied according to the standard method with minor modifications [28, 29, 30, 31]. The measurements were performed at 37 °C using potato starch as a substrate. The sample (2 μL dissolved in DMSO) and substrate (50μL) were mixed in 30 μL phosphate buffer 0.1M (pH 6.9). After pre-incubation for 5 min, 20 μL of α-amylase solution were added and the solution was incubated for 15 min at 37 °C. After that, 50 μL HCl 1M and 50 μL iodin were added to the solution. The absorbance was observed at 650 nm by a microplate reader (As). Acarbose was taken as a control. The blank solution was monitored its absorbance at 650 nm (Ab). The α-amylase inhibition was examined with the formula: α-amylase inhibitory activity (%) = [(Ab-As)/Ab] x100.
2.8. Antiplasmodial
The antiplasmodial activity was performed by the procedure described by Budimulja et al (1997) [32,33]. The sample was dissolved in DMSO, then kept the sample at –20 °C until used. The malarial parasite P. falciparum 3D7 clone was propagated in the presence of various concentrations of each sample. The growth of the parasite was observed by making a blood smear fixed with methanol and stained with Geimsa stain. The antiparasitic effect of the sample was measured by growth inhibition percentage as described by Widyawaruyanti et al (2007) [34]. The antiplasmodial activity of each compound was expressed as an IC50 value.
2.9. Statistical analysis
The experiments were replicated three times. SPSS was used to determine mean values (mean ± SD). The concentrations of the extracts made regression equations towards percentage inhibitions of α-glucosidase and α-amylase formation from which IC50 values were estimated. Probit analysis is used to determine percentage inhibition of P. falciparum growth.
3. Result and discussion
3.1. Identification of compound
Methanol (MeOH) extract from the stem bark of Dipterocarpus littoralis was fractionated successively by vacuum liquid chromatography (VLC) into three major fractions. The ethyl acetate (EtOAc) fraction was selected for subsequent studies, on further fractionation and purification of the major component either by VLC, silica gel column chromatography (CC), sephadex LH 20 CC and preparative TLC, which resulted in the isolation of a known compound (1). Moreover, compound 1 was reported for the first time from this species.
Compound 1 was obtained as a brown white powder. A detailed assessment of the NMR data indicated that 1 is an alpha-viniferin, a compound group known as components of plant species. The IR spectrum showed absorption for aromatic (1599 cm−1), aliphatic (2928 cm−1), and hydroxyl (3362 cm−1). The structure of compound 1 was elucidated with 1H- and 13C-NMR spectral data, as well as 2D-NMR techniques (HMBC and HMQC). The 1H-NMR spectrum (in MeOH-d4) showed signals for three sets of two aromatic protons [δ5.79 (1H, d¸ J=1.6 Hz, H-14a), 6.13 (1H, d¸ J=2 Hz, H-12a); δ6.62 (1H, overlap, H-14b), 6.18 (1H, d¸ J=2 Hz, H-12b); δ6.42 (1H, d, J= 2 Hz, H-14c), 6.19 (1H, d¸ J=2 Hz, H-12c)], three sets of four aromatic protons [δ6.91 (2H, d¸ J=8 Hz, H-2a, H-6a), 6.62 (2H, d¸ J=8.8 Hz, H-3a, H-5a); δ7.07(2H, d¸ J=8.8 Hz, H-2b, H-6b), 6.67 (2H, d¸ J=8.4 Hz, H-3b, H-5b); δ6.69 (2H, d¸ J=8 Hz, H-2c, H-6c), 6.64 (2H, d¸ J=8.8 Hz, H-3c, H-5c)] and three series of two aliphatic protons signals [δ6.04 (1H, s, H-7a), 3.88 (1H, s¸ H-8a); δ5.83 (1H, d, J=9.2 Hz, H-7b), 4.56 (1H, d, J=9.2 Hz, H-8b); δ4.63 (1H, d, J=7.2 Hz, H-7c), 4.42 (1H, d, J=7.2 Hz, H-8c)]. Further evidence for the structure of compound 1 was provided by HMBC and HSQC experiments shown in Table 1. Based on these data, the structure of alpha-viniferin was assigned for compound 1 as shown in Figure 1. NMR data were in accordance with literature data [35].
Table 1.
NMR spectroscopic data of compound 1.
| No | δH (Mult., J in Hz) | δC | HMBC |
|---|---|---|---|
| 1a | - | 131.03 | |
| 2a/6a | 6.91 (d, 8) | 127.39 | C-4a, C-6a/2a, C-7a |
| 3a/5a | 6.62 (d, 8.8) | 114.83 | C-1a, C-4a, C-5a/3a |
| 4a | - | 157.17 | |
| 7a | 6.04 (s) | 85.57 | C-2a/6a, C-9a, C-11b |
| 8a | 3.88 (s) | 45.41 | C-1a, C-7a, C-9a, C-10a/14a, C-9b, c-10b, c-11b |
| 9a | - | 140.47 | |
| 10a | - | 117.97 | C-8a, C-14a/10a, C-11a/13a, c-12a |
| 14a | 5.79 (d, 1.6) | 107.70 | |
| 11a | - | 160.69 | |
| 13a | - | 158.60 | |
| 12a | 6.13 (d, 2) | 97.14 | C-10a/14a, C-11a/13a |
| 1b | - | 131.28 | |
| 2b/6b | 7.07 (d, 8.8) | 127.82 | C-4b, C-6b/2b, C-7b |
| 3b/5b | 6.67 (d, 8.4) | 115.24 | C-1b, C-4b, C-5b/3b |
| 4b | - | 157.55 | |
| 7b | 5.83 (d, 9.2) | 89.22 | C-2b/6b, C-9b |
| 8b | 4.56 (d, 9.2) | 52.18 | C-1b,C-7b, C-9b, C-10b, C-14b, C-8b, C-10b, C-12b |
| 9b | - | 138.94 | |
| 10b | - | 119.92 | |
| 11b | - | 159.84 | |
| 12b | 6.18 (d, 2) | 95.70 | |
| 13b | - | 158.67 | |
| 14b | 6.62 (overlap) | 105.35 | C-8b, C-10b, C-12b |
| 1c | - | 131.64 | |
| 2c/6c | 6.69 | 128.81 | |
| 3c/5c | 6.64 | 115.24 | |
| 4c | - | 157.70 | |
| 7c | 4.63 (d, 7.2) | 94.83 | |
| 8c | 4.42 (d, 7.2) | 54.83 | |
| 9c | - | 137.84 | |
| 10c | - | 118.77 | |
| 11c | - | 160.96 | |
| 12c | 6.19 (d, 2) | 96.03 | |
| 13c | - | 160.20 | |
| 14c | 6.42 (d, 2) | 104.90 |
Figure 1.
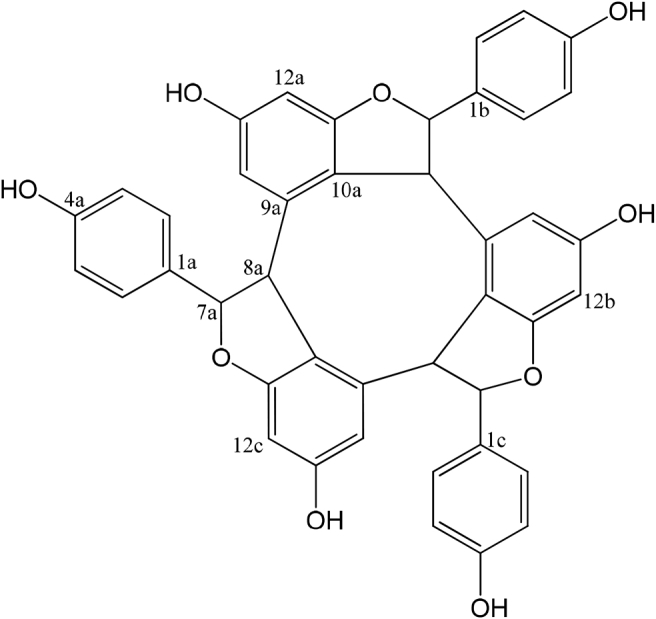
Structure of compound 1.
3.2. Free radical scavenging assay
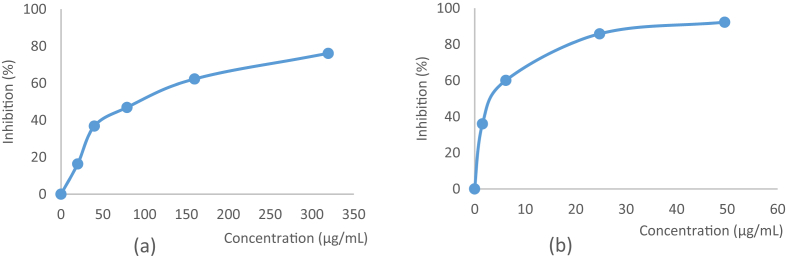
DPPH is a stable free radical in aqueous or methanol solution. In order to evaluate the free radical scavenging activity using the test samples, the change of the optical density of DPPH radicals was monitored [24]. The ability of methanol extract of D.littoralis to scavenge free radicals was assessed by reducing the stable DPPH radicals to the yellow colored 1,1-diphenyl-2-picrylhydrazine. DPPH is nitrogen centered free radical, which color changes from violet to yellow while extract of D.littoralis donates a proton to reduced the radicals. Compound 1 showed a potent antioxidant compound with 76.11% of DPPH radical scavenging capacity and IC50 value of 88.92 μg/mL. Extract of D.littoralis also showed free radical scavenging activities as a potent antioxidants with 77.35 % of DPPH inhibition capacity and IC50 value of 57.59 μg/mL as shown in Table 2. It was compared to Trolox as standard with 97.89% of inhibition rate and IC50 value of 2.46 μg/mL.
Table 2.
Free radical scavenging of D.littoralis using DPPH and ABTS assay.
| Inhibition∗ (%) |
IC50 (μg/mL) |
|||
|---|---|---|---|---|
| DPPH | ABTS | DPPH | ABTS | |
| Methanol extract | 77.35 | 82.61 | 57.59 | 18.86 |
| Compound 1 | 76.11 | 97.64 | 88.92 | 3.34 |
| Trolox | 97.89 | 95.97 | 2.64 | 5.15 |
Values are means, N = 3, P < 0.05 (ANOVA Analysis by SPSS program).
The antioxidant in D.littoralis has also observed using the ABTS scavenging test. The highest antioxidant capacity was observed in compound 1 with 97.64 % of inhibition rate and IC50 value of 3.34 μg/mL. The extract of D.littoralis showed the antioxidant capacity value of 82.61% with IC50 value of 18.86 μg/mL. Trolox is used as a standard with 95.97% of inhibition rate and IC50 value of 5.15 μg/mL. Therefore, the extract of D.littoralis stem bark shown a mild antioxidant activity and may be a very interesting candidate for the research and development of natural, environmental and healthy antioxidant for the pharmaceutical and food industries. High phenolic content in the extract of D.littoralis was shown by the TPC test with 97.54 mg of gallic acid equivalents (GAE) per 100 g of extract. Flavonoid content was also observed in the extract of D.littoralis with 298.89 mg of quercetin equivalents (QE) per 100 g of extract. Gallic acid and quercetin were used as a standard for total phenolic and flavonoid content in the extract of D.littoralis. DPPH and ABTS radical scavenging of α-viniferin which is isolated from D. littoralis extract are shown in Figure 2.
Figure 2.
Radical scavenging activity of compound 1 which is isolated from D. littoralis extract (a) DPPH assay at a concentration of 319.45 μg/mL, (b) ABTS assay at a concentration of 99 μg/mL.
3.3. Alpha-glucosidase and alpha-amylase inhibitory activity
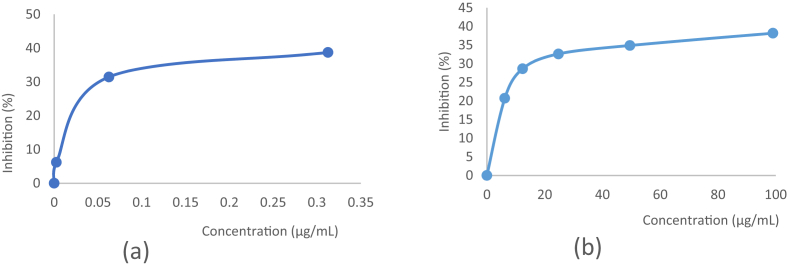
Glucosidase is the key catalyzing enzyme involved in the process of carbohydrate digestion and glucose release. Inhibition of α-glucosidase is one very effective way of delaying glucose absorption and lowering the postprandial blood glucose level, which can potentially suppress the progression of DM [36]. In order to verify the antidiabetic activities, the in vitro α-glucosidase inhibitory studies were demonstrated that compound 1, MeOH extract and EtOAc fraction of D. littoralis had α-glucosidase inhibitory activity. The EtOAc fraction of D. littoralis has an inhibition activity of α-glucosidase (p > 0.05) with 53.99% of inhibitory rate with IC50 value of 206.9 μg/mL. Compound 1 has a higher mean α-glucosidase inhibition activity than methanol extract of D.littoralis (p > 0.05) with 38.7% of inhibitory rate and IC50 value of 256.17 μg/mL as shown in Table 3. The in vitro α-glucosidase inhibitory activity was determined at 312.5 μg/mL by absorbance decrease at 490 nm. Acarbose was taken as standard showed 91.9% of inhibition rate of α-glucosidase activity.
Table 3.
The α-glucosidase and α-amylase of D.littoralis.
| α-glucosidase |
α-amylase |
|||
|---|---|---|---|---|
| Inhibition∗ (%) | IC50 (μg/mL) | Inhibition∗ (%) | IC50 (μg/mL) | |
| Methanol extract | 39.22 | 426.75 | 69.15 | 66.22 |
| Fraction EtOAc | 53.99 | 206.90 | 41.49 | 181.88 |
| Compound 1 | 38.70 | 256.17 | 38.18 | 212.79 |
| Acarbose | 91.9 | 7.42 | 94.11 | 2.61 |
Values are means, N = 3, P < 0.05 (ANOVA Analysis by SPSS Program).
The antidiabetic activity of D.littoralis has also determined using α-amylase inhibitory assay. The experiments result showed inhibition of compound 1 towards α-amylase activity for 38.18% of α-amylase inhibition rate with IC50 value of 212.79 μg/mL as shown in Table 3. The α-amylase inhibitory activity were determined at 99.01 μg/mL by absorbance decrease at 650 nm. The MeOH extract and EtOAc fraction of D. littoralis have an inhibition activity of α-amylase (p > 0.05) with 69.15 and 41.49% of inhibitory rate with IC50 values of 66.22 and 181.88 μg/mL respectively. Acarbose was also taken as standard showed 94.11% of inhibition rate of α-amylase activity. The antidiabetic ability of D.littoralis is linear with its capacity to reduce the α-glucosidase and α-amylase activity. The evaluation of antidiabetic potential using α-glucosidase and α-amylase inhibition is expectedly able to encounter novel therapeutic agents which are applicable harmless for treating DM. The observed activities and the therapeutic properties of the active plants may be still comprised of a synergistic effect of the phytochemicals. The α-glucosidase and α-amylase inhibitory activity of α-viniferin which is isolated from D. littoralis extract are shown in Figure 3.
Figure 3.
The α-glucosidase and α-amylase inhibitory activity of compound 1 which is isolated from D. littoralis extract (a) α-glucosidase assay at a concentration of 312.5 μg/mL, (b) α-amylase assay at a concentration of 99.01 μg/mL.
3.4. Antiplasmodial inhibitory activity
The results of the in vitro antiplasmodial activity with D.littoralis MeOH extract, EtOAc fraction and compound 1 against P. falciparum 3D7 strain are presented in Table 4. Compound 1 presented strong parasite inhibition with 60.55 % of parasite inhibition rate and 2.76 μg/mL IC50 value, followed by EtOAc fraction and MeOH extract of D.littoralis with 36.29 and 51.25 % of parasite inhibition rate with IC50 values of 3.42 and 98.88 μg/mL, respectively. Chloroquine is taken as a standard with IC50 values of 0.002 μg/mL. The result of the test proved that compound 1 which isolated from methanol extract of D.littoralis is available as a new antiplasmodial agent against P. falciparum 3D7 strain. The evaluation of antiplasmodial potential against P. falciparum 3D7 strain is expectedly able to encounter novel therapeutic agents which are applicable harmless against P. falciparum.
Table 4.
The in vitro antiplasmodial of D.littoralis.
| Inhibition∗ (%) | IC50 (μg/mL) | |
|---|---|---|
| Methanol extract | 51.25 | 98.88 |
| Fraction EtOAc | 36.29 | 3.42 |
| Compound 1 | 60.55 | 2.76 |
| Chloroquine | 92.79 | 0.002 |
Values are means, N = 3, P < 0.05 (Probit Analysis by SPSS Program).
4. Conclusion
The inhibitory effects of the isolated compound against rat intestinal α-glucosidase and porcine pancreas α-amylase were evaluated in comparison with the antidiabetic acarbose. The isolated α-viniferin showed an active α-glucosidase and α-amylase inhibitory activities with IC50 values of 256.17 and 212.79 μg/mL, respectively. The in vitro antiplasmodial activity test against Plasmodium falciparum at a concentration of 100 μg/mL revealed a strong antiplasmodial inhibitory activity with IC50 value of 2.76 μg/mL.
The present work reported for the first time the alpha-amylase and alpha-glucosidase inhibitory effects of D. littoralis, in support of their ethnomedicinal use for diabetic and malaria. This report partly defines the reason why this medicinal plant possesses antidiabetic and antiplasmodial properties as it has been used in the local community.
Declarations
Author contribution statement
Theodore Y. K. Lulan: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Sri Fatmawati: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Mardi Santoso, Taslim Ersam: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
Theodore Y. K. Lulan was supported by the Indonesian Ministry of Research and Technology of Higher Education.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to acknowledge Purwodadi Botanical Garden staffs for plant identification and deposition.
References
- 1.WHO . WHO; 2006. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia; p. 50. [Google Scholar]
- 2.WHO Addressing Asia’s fast growing diabetes epidemic. Bull. World Health Organ. 2017;95:550–551. doi: 10.2471/BLT.17.020817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . 2016. Global Report on Diabetes, Switzerland. [Google Scholar]
- 4.WHO . 2018. World Malaria Report 2018, Luxembourg. [Google Scholar]
- 5.Elyazar I.R.F., Hay S.I., Baird J.K. Malaria Distribution, Prevalence, Drug Resistance and Control in Indonesia, in: Adv. Parasitol. first ed. Elsevier Ltd.; 2011. pp. 41–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan W., Xu X., Shi N., Tsang S.W., Zhang H. Antimalarial activity of plant metabolites. Int. J. Mol. Sci. 2018;19:1–40. doi: 10.3390/ijms19051382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fatmawati S., Ersam T., Shimizu K. The inhibitory activity of aldose reductase in vitro by constituents of Garcinia mangostana Linn. Phytomedicine. 2015;22:49–51. doi: 10.1016/j.phymed.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Egharevba G.O., Dosumu O.O., Oguntoye S.O., Njinga N.S., Dahunsi S.O., Hamid A.A., Anand A., Amtul Z., Ujjukuri P. Antidiabetic , antioxidant and antimicrobial activities of extracts of Tephrosia bracteolata leaves. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zahoor M., Zafar R., Rahman N.U. Isolation and identification of phenolic antioxidants from Pistacia integerrima gall and their anticholine esterase activities. Heliyon. 2018;4 doi: 10.1016/j.heliyon.2018.e01007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sukandar E.R., Ersam T., Fatmawati S., Siripong P., Aree T., Tip-Pyang S., Cylindroxanthones A.-C. Three new xanthones and their cytotoxicity from the stem bark of Garcinia cylindrocarpa. Fitoterapia. 2016;108:62–65. doi: 10.1016/j.fitote.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Tian C., Chang Y., Zhang Z., Wang H., Xiao S., Cui C., Mingchun L. Extraction technology , component analysis , antioxidant , antibacterial , analgesic and anti-inflammatory activities of flavonoids fraction from Tribulus terrestris L . leaves. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo S., Gill H., Dias D.A., Li M., Hung A., Toan L., Lenon G.B. The inhibitory effects of an eight-herb formula (RCM-107) on pancreatic lipase : enzymatic , HPTLC profiling and in silico approaches. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muhtadi E.H., Hakim L.D., Juliawaty Y.M., Syah S.A., Achmad J., Latip E.L., Ghisalberti Cytotoxic resveratrol oligomers from the tree bark of Dipterocarpus hasseltii. Fitoterapia. 2006;77:550–555. doi: 10.1016/j.fitote.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Cronquist A. Columbia University Press; New York: 1981. An Integrated System of Classification of Flowering Plants. [Google Scholar]
- 15.Atun S., Achmad S.A., Ghisalberti E.L., Hakim E.H., Makmur L., Syah Y.M. Oligostilbenoids from vatica umbonata (Dipterocarpaceae) Biochem. Systemat. Ecol. 2004;32:1051–1053. [Google Scholar]
- 16.Maury-Lechon G., Curtet L. Biogeography and evolutionary systematics of Dipterocarpaceae. In: Appanah S., Turnbull J.M., editors. A Rev. Dipterocarps Taxon. Ecol. Silvic.; Jakarta: 1998. pp. 5–44. [Google Scholar]
- 17.Rohaiza S., Yaacob W.A., Din L.B., Nazlina I. Cytotoxic oligostilbenes from Shorea hopeifolia. African J. Pharm. Pharmacol. 2011;5:1272–1277. [Google Scholar]
- 18.Aminah N.S., Achmad S.A., Aimi N., Ghisalberti E.L., Hakim E.H., Kitajima M., Syah Y.M., Takayama H., Diptoindonesin A. A new C-glucoside of ε -viniferin from Shorea seminis (Dipterocarpaceae) Fitoterapia. 2002;73:501–507. doi: 10.1016/s0367-326x(02)00179-x. [DOI] [PubMed] [Google Scholar]
- 19.Yu-sheng C., Chao-jun C., Wei Y.A.N., Hui-ming G.E., Ling-dong K. Anti-hyperuricemic and anti-inflammatory actions of vaticaffinol isolated from Dipterocarpus alatus in hyperuricemic mice. Chin. J. Nat. Med. 2017;15:330–340. doi: 10.1016/S1875-5364(17)30053-5. [DOI] [PubMed] [Google Scholar]
- 20.Yang W.S., Lee B., Kim S.H., Kim H.G., Yi Y., Htwe K.M., Kim Y., Yoon K.D., Hong S., Lee W., Cho J.Y. Dipterocarpus tuberculatus ethanol extract strongly suppresses in vitro macrophage-mediated inflammatory responses and in vivo acute gastritis. J. Ethnopharmacol. 2013;146:873–880. doi: 10.1016/j.jep.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 21.Chen C.J., Jiang R., Wang G., Jiao R.H., Tancharoen C., Sudto K., Vajarothai S., Hannongbua S., Ge H.M., Tan R.X. Oligostilbenoids with acetylcholinesterase inhibitory activity from Dipterocarpus alatus. Planta Med. 2014;80:1641–1646. doi: 10.1055/s-0034-1383194. [DOI] [PubMed] [Google Scholar]
- 22.Zhishen J., Mengcheng T., Jianming W. Analytical, Nutritional and Clinical Methods Section : the determination of flavonoid contents in murberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. [Google Scholar]
- 23.Dudonne S., Vitrac X., Coutiere P., Woillez M., Merillon J.-M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH , ABTS , FRAP , SOD , and ORAC assays. J. Agric. Food Chem. 2009;57:1768–1774. doi: 10.1021/jf803011r. [DOI] [PubMed] [Google Scholar]
- 24.Lulan T.Y.K., Fatmawati S., Santoso M., Ersam T. Free radical scavenging activity of artocarpus champeden extracts. In: K. F., Yuly Kusumawati H.J., Fatmawati Sri, Purnomo Adi Setyo, editors. AIP Conf. Proc. AIP publishing; 2018. [Google Scholar]
- 25.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 26.Lulan T.Y.K., Fatmawati S., Santoso M., Ersam T. Antioxidant capacity of some selected medicinal plants in East Nusa Tenggara , Indonesia : the potential of sterculia quadrifida R . Br. Free Radicals Antioxidants. 2018;8:96–101. [Google Scholar]
- 27.Khan T., Zahid M., Asim M., Shahzad-ul-Hussan, Iqbal Z., Choudhary M.I., Ahmad V.U. Pharmacological activities of crude acetone extract and purified constituents of Salvia moorcraftiana Wall. Phytomedicine. 2002;9:749–752. doi: 10.1078/094471102321621386. [DOI] [PubMed] [Google Scholar]
- 28.Thao N.P., Binh P.T., Luyen N.T., Hung T.M., Dang N.H., Dat N.T. α -amylase and α -glucosidase inhibitory activities of chemical constituents from Wedelia chinensis (Osbeck .) merr . Leaves. J. Anal. Methods Chem. 2018;2018:1–8. doi: 10.1155/2018/2794904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kadouh H.C., Sun S., Zhu W., Zhou K. α -Glucosidase inhibiting activity and bioactive compounds of six red wine grape pomace extracts. J. Funct. Foods. 2016;26:577–584. doi: 10.1016/j.jff.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subramanian R., Asmawi M.Z., Sadikun A. In vitro α-glucosidase and α-amylase enzyme inhibitory effects of Andrographis paniculata extract and andrographolide. Acta Biochim. Pol. 2008;55:391–398. [PubMed] [Google Scholar]
- 31.Unuofin J.O., Otunola G.A., Afolayan A.J. In vitro α-amylase , α-glucosidase , lipase inhibitory and cytotoxic activities of tuber extracts of Kedrostis africana ( L .) Cogn. Heliyon. 2018;4 doi: 10.1016/j.heliyon.2018.e00810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Budimulja A.S., Syafruddin, Tapchaisri P., Wilairat P., Marzuki S. The Sensitivity of Plasmodium protein synthesis to prokaryotic ribosomal inhibitors. Mol. Biochem. Parasitol. 1997;84:140–157. doi: 10.1016/s0166-6851(96)02781-8. [DOI] [PubMed] [Google Scholar]
- 33.Boonlaksiri C., Oonanant W., Kongsaeree P., Kiittakoop P., Tanticharoen M., Thebtaranonth Y. An antimalarial stilbene from Artocarpus integer. Phytochemistry. 2000;54:415–417. doi: 10.1016/s0031-9422(00)00074-1. [DOI] [PubMed] [Google Scholar]
- 34.Widyawaruyanti A., Subehan, Kalauni S.K., Awale S., Nindatu M., Zaini N.C., Syafruddin D., Asih P.B.S., Tezuka Y., Kadota S. New prenylated flavones from Artocarpus champeden , and their antimalarial activity in vitro. J. Nat. Med. 2007;61:410–413. [Google Scholar]
- 35.Hadi S. Noviany, the isolation of hopeaphenol, a tetramer stilbene, from shorea ovalis blume. Adv. Nat. Appl. Sci. 2009;3:107–112. [Google Scholar]
- 36.Arise R.O., Idi J.J., Mic-Braimoh I.M., Korode E., Ahmed R.N., Osemwegie O. In vitro Angiotesin-1-converting enzyme , α -amylase and α -glucosidase inhibitory and antioxidant activities of Luffa cylindrical (L) M . Roem seed protein hydrolysate. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e01634. [DOI] [PMC free article] [PubMed] [Google Scholar]