Graphical abstract

Keywords: Phytase, Liquid-liquid extraction, Reversed micelles, Triticale
Highlights
-
•
The application of the reverse micelles resulted in purification of A. niger phytase.
-
•
It was possible purify phytase from A. niger by reversed micelles in short period time.
-
•
Reversed micelles proved to be a viable alternative for phytase purification.
-
•
Phytase remained active after extraction using AOT/isooctane reversed micelles.
-
•
The phytase purity and activity were confirmed by SDS-PAGE and zymogram analyzes.
Abstract
This work describes the successful extraction of Aspergillus niger phytase from a crude extract (CE) obtained from solid-state fermentation by reversed micelle system using anionic surfactant sodium bis (2-ethylhexyl) sulfosuccinate (AOT) in isooctane achieved in two simple steps: forward and backward extractions. The effects of potassium chloride (KCl) concentration, pH of the aqueous solution, and AOT concentration that affect the system were examined. The best result for the forward extraction was obtained with the CE solution at pH 4.0, 50 mM KCl, and 100 mM AOT, while for the backward extraction the best result was achieved with a stripping aqueous solution at pH 5.5 containing 200 mM KCl, achieving a purification factor of 4.03, 1.15 times higher than that reported for the conventional purification process. Phytase purity was demonstrated by SDS-PAGE (89 kDa) and its activity by zymogram, confirming the efficiency of the process with low time consumption (∼40 min).
1. Introduction
Phytases are enzymes that catalyze the phospho-monoester bonds of phytic acid or its salt, the phytate [myo-inositol (1,2,3,4,5,6) hexakiphosphate], which accounts for more than 80 % of the total phosphorus in cereals (wheat, barley, triticale, etc), in derivatives of myo-inositol and inorganic phosphate. However, phytate is not a source of phosphorus in humans or non-ruminant animals, due to its antinutritional behavior, since these animals do not produce phytase to digest it. Thus, the high index of chelating activity of phytate leads to reduced biodigestability of minerals present in the digestive tract, reduces the energy absorbed by monogastric animals, and generates to several environmental problems, such as water pollution by the discharge of its excrement increasing the risk of eutrophication [1,2]. In view of this, the supplementation of feed with phytases obtained from many source avoids these problems in non-ruminant animals.
Phytases of fungal origin has been widely obtained in commercial-scale due to easy cultivation of fungi by both solid-state fermentation (SSF) and submerged fermentation (SmF) systems. The difference between the two fermentation systems is that the SmF occurs in a liquid medium containing nutrients, while the SSF occurs in solid particles in absence or very small volume of water, that is, environment favorable to microbial growth mainly of fungi. Thus, the SSF has economic advantages compared to the SmF, such as, utilization of inexpensive substrates (agro-industrial waste), not require the addition of complex nutrients, less water requirement, high volumetric productivity, besides generating fewer effluents, what makes SSF to be widely used for enzymes production.
Among the known fungal phytases, the phytases produced by A. niger are the most common used, especially due to its high activity and for its generally recognized as safe” (GRAS) status. Downstream processing of the enzyme involves important unitary operations in the bioprocess industry. Among the separation techniques for the development of the enzymatic purification strategy, we can highlight ultrafiltration, ultracentrifugation, different types of chromatography techniques and liquid-liquid extraction (two aqueous phases, inverse micelles), isolated or conjugated.
Recently our research group purified an A. niger 7A-1 phytase produced by SSF using as substrate triticale residue, cereal resulting from the hybridization of two distinct species, wheat (Triticum) and rye (Secale). Extraction was obtained by microfiltration, ultrafiltration (300, 100 and 30 kDa) and DEAE-Sepharose column chromatography. The enzyme showed up thermotolerant and with high activity in the pH 5–6 range, presenting a potential use in animal feed [3].
The application of liquid-liquid extraction technique by single step using aqueous two-phase system (ATPS) for the downstream processing of phytase from Aspergillus niger NCIM 563, produced under SSF, was studied and compared with the traditional multi-step procedure involving salt precipitation and column chromatography. The results showed that ATPS allowed a higher recovery of phytase (98.5 %) in a short period of time (3 h) as well as an improved thermostability in comparison to the 20 % recovery in 96 h by a regular chromatographic process, which reveals that ATPS is an interesting alternative for simultaneous partitioning and purification of phytases [4].
In addition to liquid-liquid extraction with aqueous two-phase systems (ATPS), reversed micelle system (RMS) extraction has been used in recent decades, especially in the extraction and purification of proteins and enzymes. Liquid-liquid extraction with reversed micelles of surfactants in organic solvents involves two steps: a forward extraction and a backward extraction. The first step is based on the ability of reversed micelles to solubilized, by contact, the proteins from an aqueous phase into the water pool of the surfactant aggregates; the process occurs under agitation between the aqueous phase containing the target protein and an organic or micellar phase (surfactant/nonpolar solvent). The second step is related to the solubilized proteins in the interior of the reverse micelles, whose recover directs the proteins to a new aqueous phase or a stripping aqueous phase (with given pH and ionic strength) by changing the interactions between the proteins and the reversed micelles.
Reverse micelles in sodium bis (2-ethylhexyl) sulfosuccinate (AOT) reverse microemulsions are known to be spherical over a wide range of compositions. The choice of the solvent is normally determined by its compatibility with the surfactant and biocatalyst. Several correlations have been established between solvents and their properties affecting the enzyme behavior and stability. Water/ AOT / isooctane is the most studied system because it does not require the addition of a co-surfactant to form microemulsions and the properties of its aggregate have been examined in detail [[5], [6], [7], [8], [9]]. Studies have proven that AOT reverse micelles can increase the extraction efficiency of proteins. According to ZHAO et al. [10], AOT reverse micelles were still able to improve the functional properties, such as flavor and aroma, and the native conformation (α-helix and β-sheet structure) of peanut proteins comparing to obtained through aqueous buffer extraction.
The present work aimed to recover the A. niger 7A-1 phytase enzyme from crude solid-state fermentation extract (SSF) using AOT / isooctane reverse micelles in order to reduce the steps and time of purification process.
2. Material and methods
2.1. Chemicals
Bis(2-ethylhexyl) sulfosuccinate sodium salt (AOT) with ≥ 99 % of purity was used as anionic surfactant and isooctane (≥ 99 %) was used as solvent organic for the reverse micelles formation were purchased from Merck (Germany). All other chemicals were of analytical grade. The mixture of AOT and isooctane was stirred for 30 min to establish the aggregation of surfactant molecules until they have dissolved and showed a clear (translucent and homogeneous) phase solution.
2.2. Microorganism, cell growth and enzyme production
The Aspergillus niger 7A-1 strain, isolated from the soil of the semi-desert region of Coahuila was provided by Nanobioscience Research Group, Universidad Autónoma of Coahuila, Saltillo city, México. Spores with seven days of growth on potato dextrose agar (PDA) slants at 28 ± 1 °C were collected with a 0.1 % (v/v) Tween 80 solution and adjusted to 1 × 106 spores/mL concentration, to be used as inoculum. The substrate used for solid-state fermentation (SSF), consisted of a mixture of agro-industrial wastes of triticale obtained from Universidad Autónoma Agraria Antonio Narro, México, which was previously washed with distilled water to remove soil and impurities particles, dried at 60 °C, milled to obtain an approximately 0.3 mm particle size and posteriorly sterilized in Petri dishes containing 5 g in each dish, in autoclave at 121 °C for 20 min.
Phytase production was obtained by SSF according to Neira-Vielma et al. [3]. Briefly, the substrate (5 g) contained in each Petri dishes was moistened with 3 mL of sterile solution [dextrose (168 g/L), KCl (2 g/L), lactose (4.8 g/L), NH4NO3 (40 g/L), and Tween 80 (10 mL/L)], to reach 60 % of moisture. Posteriorly, the substrate was inoculated with 0.5 mL of spore suspension (1 × 106 spores/mL) and the content was mixed and incubated at 28 ± 1 °C for 5 days under static condition. After this period, the fermented medium was transferred to Erlenmeyer flasks containing 5 mL of distilled water/g of the fermented substrate, and the mixture was stirred (200 rpm) for 1 h at 25 ± 1 °C and finally centrifuged at 10,000 x g for 10 min. The supernatant with clear brown color and rich in phytase was termed crude extract (CE), which was lyophilized and stored at - 4 °C for further use.
2.3. Forward extraction and backward extraction of phytase with reversed micelles
The reversed micellar system was constituted by the anionic surfactant, AOT in isooctane. The initial aqueous phase was constituted by CE suspended in 100 mM different buffers according to their pKa value at the pH range 4.0–9.0 (Sodium citrate/citric acid at pH 4.0, Sodium acetate (tri-hydrate)/acetic acid pH at 4.5–5.5, Sodium citrate/citric acid at pH 6.0–6.5, Tris (hydroxymethyl)aminomethane/hydrochloric acid at pH 7.0–9.0) to a final protein concentration of approximately 1.0 ± 0.15 mg/mL. Forward extraction and backward extraction steps of phytase were carried out as follows: In the first step (Forward extraction), to each 10 mL of buffered aqueous phase (with KCl at the range from 0 to 150 mM) was added an equal volume of organic phase also known by micellar phase (AOT in isooctane at the range from 10 to 200 mM) and both phases were mixed for 10 min at 200 rpm at 25 °C for solubilize the protein into reverse micelles aqueous cores. Afterward, the mixture was centrifuged for 10 min at 2400×g, for phase separation into a residual aqueous phase and micellar phase. In the second step (backward extraction), 7.0 mL of micellar phase, with solubilized protein, was added to an equivalent volume of new buffered aqueous solution (fresh stripping solution) at different pH (4.0–8.0) containing KCl 0–300 mM. The mixture was stirred for 10 min at 200 rpm at 25 °C and centrifuged for 10 min at 2400×g to recover the enzyme in the aqueous phase (stripping solution). The results were expressed as the average of three experiments ± standard deviation.
2.4. Protein determination
The protein content in the aqueous phases was determined according to Bradford [11] and in the micellar phases according to Pires et al. [12] using bovine serum albumin as the standard.
2.5. Phytase activity
Phytase activity in the aqueous phase was measured spectrophotometrically according to Harland and Harland [13], modified. Briefly, the reaction mixture consisted in the addition of 250 μL 0.1 M MgSO4*7H2O in 0.2 M sodium acetate buffer (pH 5.15), 600 μL of 0.00682 M phytic acid in 0.2 M sodium acetate buffer (pH 5.15) and 150 μL of enzyme solution which was incubated for 60 min at 55 °C and the reaction was stopped by the addition of 500 μL of 10 % trichloroacetic acid. The color was developed by adding 600 μL of Taussky-Schorr reagent [1 g (NH4)Mo7O24*4H2O, 10 mL 10 N H2SO4 and 5 g FeSO4*7H2O in 100 mL distilled water] to generate a blue chromophore. Then, the contents were mixed and incubated for 30 min, and the liberated inorganic phosphate was measured at 660 nm. One unit of phytase activity (U) was expressed as the amount of enzyme that liberates 1 μmol phosphorus per minute under standard assay condition. The specific activity was calculated as the ratio between the enzymatic activity and the total protein content of the sample and expressed in U/mg. The enzyme activity in the micellar phase was not estimated due to the incompatibility of the method. The purification factor (PF) was defined as the ratio between the specific activity of stripping aqueous phase and the specific activity of the initial aqueous phase.
2.6. SDS–PAGE electrophoresis/ Zymogram
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS–PAGE) was carried out according to Laemmli [14], using 7.5 % (w/v) polyacrylamide. All the samples were previously lyophilized before mixed with sample loading buffer. Polypeptide bands of the initial aqueous phase, the residual aqueous phase of forward extraction, stripping aqueous phase and molecular mass standards (Full-range Rainbow Markers of GE Healthcare, USA), were stained with Coomassie Brilliant Blue R-250. Zymograms were carried out in agreement to Bae et al. [15] to detect the presence of active phytase in the sample purified by the reversed micellar system. Briefly, gels previously obtained (SDS–PAGE) were immersed initially in 1% Triton X-100 for 1 h at 25 °C and then transferred to a 0.1 M sodium acetate buffer (pH 5.0) and maintained for 1 h at 4 °C. Phytase activity was detected by incubating the gels in a 0.1 M sodium acetate buffer (pH 5.0) containing 0.4 % (w/v) sodium phytate, for 16 h. Activity bands were visualized through the immersion of the gels in a 2 % (w/v) aqueous cobalt chloride solution for 5 min at 25 °C. Posteriorly, this solution was replaced with a solution containing equal volumes of 6.25 % (w/v) aqueous ammonium molybdate solution and 0.42 % (w/v) ammonium vanadate solution, freshly prepared. Phytase activity was evident as a clear zone against the opaque background.
3. Results and discussion
3.1. Effect of pH on the forward extraction of phytase
The pH of aqueous phase has strong influence on the mass transfer in MRS. Taking into account that, in micellar systems, a specific biomolecule can be extracted either by electrostatic interactions or by hydrophobic interactions, it is important to find a suitable pH value in order to obtain a good degree of purification. Although there are several reports about enzyme recovery in the literature such as cutinase [8], alkaline protease [16,17], lipase [18], lectin [19,20], cellulase [21], bromelain [22], there was no reports about phytase recovery with reversed micelles. In this way, this present study demonstrated that the largest amount of protein extracted to the micellar phase was found at pH 4.0, equivalent to 40.94 ± 1.16 %, and progressively decreased at higher pH values, reaching the lowest value at pH 8.0, equivalent to 5.94 ± 0.24 % of protein solubilized into reversed micelles (Fig. 1).
Fig. 1.
Effect of pH on phytase forward extraction from an initial aqueous phase (CE), added with 100 mM KCl, with reversed micelles of 100 mM AOT in isooctane, at 25 °C. (▲) % of protein transferred to the micellar phase; (◼) % of Phytase activity in the residual aqueous phase. Each data point is the average of three determinations and the error bars show the standard deviation.
This higher percentage of protein in the micellar phase can be attributed to the phytase solubilization, since at pH values below the isoelectric point (pI) of 5.0 [23], its charge is positive, promoting its migration into the micelle constituted by an anionic surfactant (AOT), through electrostatic interactions. Regarding the phytase activity in the residual aqueous phase, it was observed an increase up to pH 5.0–6,0 and a decrease at higher pH values until pH 8.0. Similar behavior was found by Neira-Vielma et al. [3] for this extracellular phytase from A. niger 7A-1 strain purified by ultrafiltration followed by ion-exchange chromatography, which showed maximum activity at pH 5.3, corroborating the acid character of the phytases from microbial sources, once have an optimal pH value around 4.5–6.0 [24]. At pH 8.5 and 9.0, it was not possible to form the system and consequently was impossible to obtain an appropriate separation of phases.
Based on these results, pH 4.0 of aqueous phase was selected as the best value of pH for the subsequent experiments.
3.2. Effect of the AOT concentration on the forward extraction of phytase
The concentration of AOT in MRS is an important factor, since it is known that varying of the concentration of any surfactant by varying the dimensions of the inverted micelles. The study of the effect of AOT concentration on the phytase forward extraction with 100 mM KCl solution, pH 4.0, was carried out at an AOT concentration range from 10 to 200 mM (Fig. 2). The results demonstrated that the protein transferred to micellar phase increased from 12.41 ± 1.10–48.57 ± 2.06 % with the increase of the AOT concentration from 10 to 200 mM, whereas the enzymatic activity in the residual aqueous phase decreased from 87.84 ± 1.13–64.23 ± 1.62 %, suggesting that this loss of activity was probably ascribable to the phytase incorporation into micellar phase. Notwithstanding, it may be possible that at low AOT concentration, the micelles have a lower protein retention capacity, on the contrary at high surfactant concentrations, the micelles tend to retain more protein not ensuring that it is only the protein of interest.
Fig. 2.
Effect of AOT concentration on phytase forward extraction from an initial aqueous phase (CE). (▲) % of the protein in the micellar phase, (◼) % of phytase activity in the residual aqueous phase. Extraction condition: 100 mM KCl solution, pH 4.0 stirring at 200 rpm, 25 °C for 10 min. Each data point is the average of three determinations and the error bars show the standard deviation.
However, the specific activity in the residual aqueous phase when it was used AOT at a concentration of 200 mM increased by about 12.6 % in comparison with the 100 mM AOT condition. This indicates that a higher amount of protein contaminants of CE was extracted when a higher concentration of surfactant was used. This behavior may be explained by the effect of the surfactant concentration since the increase in the surfactant concentration leads to an increase in the water content solubilized in the micellar phase (W0) and consequently increase the size of the micelle [8,25] and/or to the increase of the number of micelles [5,26], allowing a more selective and greater transfer of proteins to the organic phase. According to Eskici and Axelsen [25] based on AOT energy, the size of a reversed micelle is determined primarily by a balance between the electrostatic interactions attractive (between sodium cations and anionic AOT head groups) and repulsive (between anionic AOT head groups and between sodium cations), and the availability of water to screen them. On the other hand, higher surfactant concentrations may difficult the backward extraction of proteins into a stripping aqueous phase [8]. Therefore, the optimum surfactant concentration corresponds to the minimum limit for achieving maximum transfer into the organic phase. Based on the results found here, 100 mM AOT concentration was selected for subsequent experiments.
3.3. Effect of ionic strength on the forward extraction of phytase
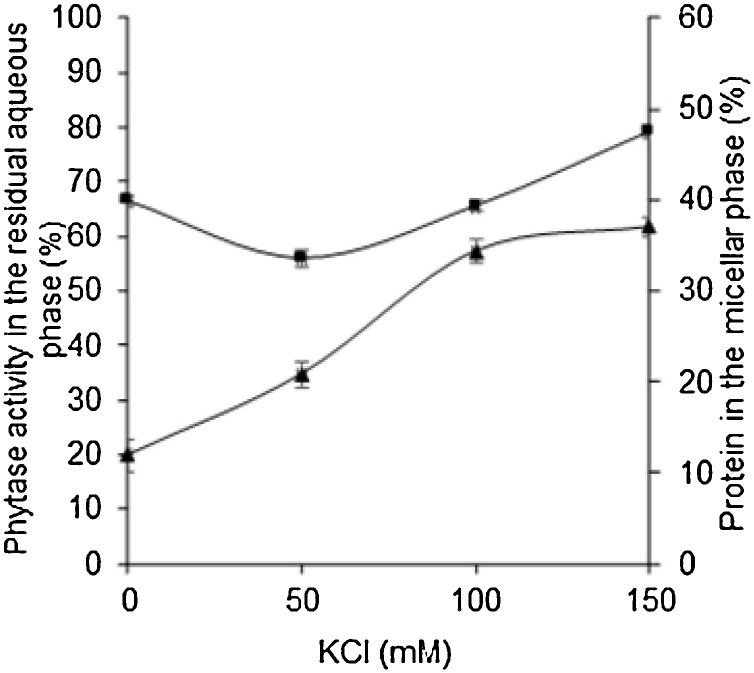
To evaluate the influence of ionic strength of the initial aqueous phase (pH 4.0) on phytase forward extraction to micellar phase (100 mM AOT/isooctane), different concentrations of KCl were analyzed at the range from 0 to 150 mM (Fig. 3). The results obtained showed that the percentage of protein transferred to the micellar phase increased with the increase of KCl concentration up to 150 mM, reaching a maximum of 36.99 ± 1.12 %. A similar case was reported for amoxicillin whose extraction efficiency increased when KCl concentration was increased up to 130 mM (27). The ionic strength is one of the main parameters easily controllable to increase the amount of biomolecule of interest in the micellar system. Its effect is generally explained by the Debye screening effect of electrolyte solutions. According to the Debye–Hückel limiting law as the ionic strength of a solution increases, the activity coefficient of an ion decreases. Therefore, this electrostatic screening effect is also responsible for the decrease of the surfactant head group repulsions, once as salt concentration is increased, the electrical double layers become thinner and allow the potentials to move into closer proximity to each other leading to the formation of smaller reversed micelles for the system used. However the reverse micelle size decreases with increasing salt additions until one reaches a critical concentration, which characterizes the onset of system destabilization [16,28].
Fig. 3.
Effect of KCl concentration on the phytase forward extraction from an initial aqueous phase (CE) at pH 4.0. (▲) % of the protein in the micellar phase, (◼) % of phytase activity in the residual aqueous phase. Extraction condition: 100 mM AOT/isooctane, 200 rpm, 25 °C and 10 min. Each data point is the average of three determinations and the error bars show the standard deviation.
At the same time, the maximum phytase activity obtained in the residual aqueous phase was 79.09 ± 0.89 %, indicating that the increase of ionic strength from 50 to 150 mM KCl favored the migration to the micellar system of a great number of contaminating proteins that tend to invade more rapidly the micelles and little of phytase, limiting so the space inside the micelle for phytase. Consequently, at a moderate concentration of 50 mM KCl, an efficient extraction of ca. 20.79 ± 1.48 % of the total protein of the system was obtained, keeping only the enzyme activity present in the residual aqueous phase at 55.90 ± 1.40 %.
These results indicate that the incorporation of protein with phytase activity into the micelle, was favored by a slight ionic strength, which allowed a most selective migration of phytase to these systems. Therefore, the best KCl concentration was found to be 50 mM, suggesting that ca. 44 % of enzymatic activity and ca. 21.0 % of protein content was transferred from the initial aqueous phase to the micellar phase.
3.4. Effect of pH on the backward extraction of phytase
Once established the best condition of forward extraction (pH 4.0 of initial aqueous phase, 100 mM AOT and 50 mM KCl), which led to a higher concentration of phytase in the micellar phase (ca 21.0 %), the next step was to find the pH value of the fresh stripping solution, which favors the migration of the protein of interest from of the micellar phase into the stripping solution. Although being this parameter primarily controlled by a partial protein load of interest, suitable pH value should be found to ensure an adequate recovery. The study of the effect of pH on the phytase backward extraction, solubilized in the micellar phase of forward extraction was carried out using 100 mM fresh stripping solution at pH ranging from 4.0–8.0 with 100 mM KCl (Fig. 4).
Fig. 4.
Fig. 4. Effect of pH on the phytase backward extraction of micellar phase from forward extraction. (▲) % of the protein in the aqueous phase of backward extraction (stripping solution), (◼) % of phytase activity in the stripping solution. Backward extraction condition: fresh stripping solution buffered containing 100 mM KCl, 200 rpm, 25 °C, and 10 min. Each data point is the average of three determinations and the error bars show the standard deviation.
The amount of protein backward extracted into to stripping solution increased up to a pH value of 6.0 (56.77 ± 2.43 %) and after that decreased slightly until pH 7.5 (48.72 ± 1.13 %). At a pH 8.0, only a small amount of protein (24.12 ± 0.39 %) was found. The enzymatic activity increased up at a pH value of 5.5 (31.32 ± 0.49 %) and decreased at higher pH values, corroborating with the same performance observed by Neira-Vielma et al. [3] which evaluated phytase activity from A. niger 7A-1 at different pHs, as well as the one reported as the best pH value (pH 5.6) in the partition of an A. niger NCIM 563 phytase using liquid-liquid extraction by ATPS, which allowed the best purification factor of 2.5 [4]. This behavior on the backward extraction can be attributed both to the migration of other contaminating proteins from the interior of the micellar phase to the stripping solution, as to the activity loss of the phytase due to the pH change. In our work, the highest specific activity (4.96 ± 0.17 U/mg) in the stripping solution was obtained at pH 5.5 which led to a protein recovery of 44.06 ± 2.23 %, achieving a purification factor of 3.28 and suggesting a greater selectivity for the phytase recovery when compared to other similar studies. The effect of the pH of stripping solution for several biomolecules have been reported as an important parameter, since the pH affects the net surface charge of protein, independently of the type of surfactant used in the reversed micellar system. It had been reported for backward extractions of black turtle bean lectin by micelles of AOT/isooctane [20], cellulase by reversed micellar systems formed by biosurfactant rhamnolipid and the nonionic surfactant Tween-20, Tween-40, Tween-60 or Tween-80 [21], amoxicillin by mixed reversed micelles formed by AOT/Tween 85 and with the system AOT/isooctane [27] and bromelain from crude extract aqueous of pineapple wastes by reversed micellar systems of CTAB/isooctane/hexanol/butanol and AOT/isooctane [29]. Therefore, in this present study the pH value of the new stripping solution was fixed at pH 5.5 for the subsequent experiments.
3.5. Effect of the ionic strength on the backward extraction of phytase
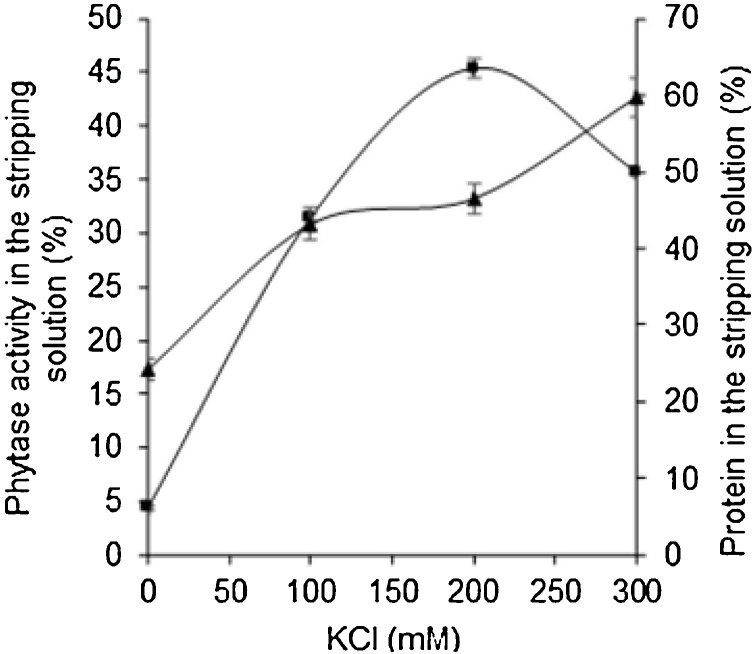
The study of the effect exerted by ionic strength on the backward extraction of phytase, which was solubilized in the micellar phase of forward extraction, was carried out varying the concentration of KCl in the range 0−300 mM, in stripping solution at pH 5.5. From Fig. 5 can be observed an increase of protein in the stripping solution with the increase of KCl molarity obtaining a maximum of 59.67 ± 2.53 % at 300 mM.
Fig. 5.
Effect of KCl concentration on the phytase backward extraction of micellar phase from forward extraction. (▲) % of the protein in the aqueous phase of backward extraction (stripping solution), (◼) % of phytase activity in the stripping solution. Backward extraction condition: fresh stripping solution buffered at pH 5.5, 200 rpm, 25 °C, and 10 min. Each data point is the average of three determinations and the error bars show the standard deviation.
The enzymatic activity recovery in the stripping solution increased with the increase of ionic strength up to 200 mM, and decreased at 300 mM KCl, suggesting that the increase of protein concentration was not related to the protein phytase but to the contaminant proteins. It is known that the equilibrium form of reverse micelles is determined by minimizing functional free energy which is strongly affected by the electric double layer (EDL) that is formed in the water core due to surfactant dissociation and from the free energy of intrinsic curvature of the surfactant monolayer [30]. Therefore, this behavior probably can be attributed to the Debye length reduction, defined as the distance over which significant charge separation can occur, promoting interactions between protein molecules and micelles, as well as the salting-out effect generated by high salt concentrations reducing the micellar size, which led to phytase exclusion. Similar cases have been reported for lectin from black turtle bean [20], amoxicillin [27], defatted wheat germ protein [31] and cutinase [8]. Therefore, in this present work we found that the best KCl concentration was 200 mM, presenting 45.35 ± 0.97 % of enzymatic activity recovered, as well as 46.46 ± 1.86 % of protein with a specific activity of 6.41 ± 0.26 U/mg and purification factor (PF) of 4.03. This PF (4.03) achieved in our work was higher when compared with other methods of purification reported in the literature, such as the phytase purification from A. niger ATCC 9142 from a broth fermentation by column chromatography with DEAE-Sepharose CL-6B whose PF was 3.5 [32], for a thermostable phytase from a broth fermentation produced by A. niger UFV-1 purified by ultrafiltration of 30 kDa cut off followed by acid treatment that showed PF 2.33 [33], as well as to the same phytase from A. niger 7A-1 strain from CE obtained by SSF when being purified by ultrafiltration followed by ion-exchange chromatography that exhibited PF 3.5 [3], value 15 % lower than that obtained here by RMS. In addition, these processes usually consume more time and cost. The value of PF obtained in our work was also 1.6 times higher than the purification factor of 2.5 obtained by Bhavsar et al. [4] in the purification of phytase from A. niger NCIM 563 from a crude extract from SSF, by liquid-liquid extraction using ATPS.
3.6. SDS-PAGE and Zymogram analysis
SDS-PAGE allows the separation and identification of proteins. As can be seen in Fig. 6A, there was a reduction of polypeptide bands among the samples, mainly in the backward extraction step, exhibiting the phytase with a single 89 kDa polypeptide band (Lane 4). This value is in agreement with the molecular mass of A. niger 7A-1 phytase purified by ion-exchange chromatography, estimated at 89 kDa, reported by Neira-Vielma et al. [3]. In a similar way, the same value level of the phytases molecular masses produced by SSF from differents isolated A. niger and identified by SDS-PAGE were reported in the literature, for instance, 85 kDa for A. niger 11T53A9 phytase [34], 108 kDa for A. niger FS3 phytase [35], 85 kDa for A. niger NCIM 563 phytase [4] and 66 kDa for A. niger CFR 335 phytase [36].
Fig. 6.
(A) SDS-PAGE of the purification steps of phytase from Aspergillus niger 7A-1 by reversed micelles. The protein bands were stained with Coomassie Brilliant Blue. Lane 1: Molecular weight markers, Lane 2: sample from initial aqueous phase (CE); Lane 3 – sample from the residual aqueous phase of forward extraction; Lane 4: sample from stripping solution (Phytase purified). (B) Zymogram developed for phytase activity purified by reversed micelles. The activity band was visualized by immersing of gel in cobalt chloride/ammonium molybdate/ammonium vanadate (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Zymogram assays has been used widely to identify proteins activity. By zymogram analysis (Fig. 6B) it is possible to observe that the polypeptide band was able to produce a clear band, a positive result, thus confirming the phytase activity. These results confirm the effectiveness of reversed micelles system constituted by AOT/isooctane in the purification of proteins, proving to be a promising method especially when concerning to the time of the purification process attained by conventional methods
4. Conclusion
Liquid-liquid extraction by reversed micelles of AOT / Isooctane showed to be a viable alternative for phytase purification from A. niger, produced by SSF, with simple two steps and a short process time of only about 40 min. Extraction and recovery of phytase by reversed micelles system were affected by aqueous solution pH, ionic strength, and AOT concentration. In our best knowledge, this is the first report on phytase purification using RMS and the results suggest its use as a food supplement for feed of non-ruminant animals to improve the efficiency of phosphorus intake and reduce the amount of phosphorus in the environment.
CRediT authorship contribution statement
Alberto A. Neira-Vielma: Investigation, Methodology, Writing - original draft. Anna Iliná: Data curation, Formal analysis. Georgina Michelena Álvarez: Investigation, Methodology. Cynthia O. Nascimento: Investigation, Methodology, Supervision. Cristóbal Noé Aguilar: Conceptualization, Funding acquisition, Project administration, Resources, Software, Validation, Visualization, Writing - review & editing. José Luis Martínez-Hernández: Conceptualization, Funding acquisition, Resources, Software, Validation, Visualization, Writing - review & editing. Maria das Graças Carneiro-da-Cunha: Conceptualization, Formal analysis, Funding acquisition, Resources, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
A.A. Neira-Vielma is PhD student, recipient of a scholarship from Consejo Nacional de Ciencia y Tecnología México (CONACYT), to whom he extends its thanks. M.G.Carneiro-da-Cunha express her gratitude to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for research grants and fellowship. The authors acknowledge CNPq for financial support.
References
- 1.Song H.-Y., Sheikha A.F.E., Hu D.-M. The positive impacts of microbial phytase on its nutritional applications. Trends Food Sci. Technol. 2019;86:553–562. doi: 10.1016/j.tifs.2018.12.001. [DOI] [Google Scholar]
- 2.Kumar V., Sinha A.K., Kajbaf K. Phytic acid and phytase enzyme. Cap 18, pp 467-483. In: Johnson Jodee, Wallace Taylor C., editors. Whole Grains and Their Bioactives: Composition and Health. first edition. © 2019 JohnWiley & Sons Ltd. Published 2019 by JohnWiley & Sons Ltd; 2019. [Google Scholar]
- 3.Neira-Vielma A.A., Aguilar C.N., Ilyina A., Contreras-Esquivel J.C., Carneiro-da-Cunha M.G., Michelena-Álvarez G., Martínez-Hernández J.L. Purification and biochemical characterization of an Aspergillus niger phytase produced by solid-state fermentation using triticale residues as substrate. Biotechnol. Rep. 2018;17:49–54. doi: 10.1016/j.btre.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhavsar K., Kumar V.R., Khire J.M. Downstream processing of extracellular phytase from Aspergillus Niger: Chromatography process vs. aqueous two phase extraction for its simultaneous partitioning and purification. Process Biochem. 2012;47:1066–1072. doi: 10.1016/j.procbio.2012.03.012. [DOI] [Google Scholar]
- 5.El Aferni A., Guettari M. T. determination of the water/AOT/isooctane reverse micelles size parameters from their refractive index datatajouri. J. Solution Chem. 2017 doi: 10.1007/s10953-016-0563-x. [DOI] [Google Scholar]
- 6.Asgaria S., Saberib A.H., McClements D.J., Lina M. Microemulsions as nanoreactors for synthesis of biopolymer nanoparticles. Trends Food Sci. Technol. 2019;86:118–130. doi: 10.1016/j.tifs.2019.02.008. [DOI] [Google Scholar]
- 7.Sun X., Bandara N. Applications of reverse micelles technique in food science: a comprehensive review. Trends Food Sci. Technol. 2019;91:106–115. doi: 10.1016/j.tifs.2019.07.001. [DOI] [Google Scholar]
- 8.Carneiro-da-Cunha M.G., Cabral J.M.S., Aires-Barros M.R. Studies on the extraction and back-extraction of a recombinant cutinase in a reversed micellar extraction process. Bioprocess Eng. 1994;11:203–208. [Google Scholar]
- 9.Melo E., Aires-Barros M., Cabral J.M. Reverse micelles and protein biotechnology. Biotechnol. Annu. Rev. 2001;7:87–129. doi: 10.1016/s1387-2656(01)07034-x. [DOI] [PubMed] [Google Scholar]
- 10.Zhao X., Liu H., Zhang X., Zhu H., Ao Q. Surface structure and volatile characteristic of peanut proteins obtained through AOT reverse micelles. Colloid. Surf. B: Biointerfaces. 2019;173:860–868. doi: 10.1016/j.colsurfb.2018.10.070. [DOI] [PubMed] [Google Scholar]
- 11.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 12.Pires M.J., Prazeres D.M.F., Cabral J.M.S. Protein assay in reversed micelle solutions. Biotechnol. Tech. 1993;7:293–294. doi.10.1007/BF00150901. [Google Scholar]
- 13.Harland B.F., Harland J. Fermentative reduction of Phytate in rye, white, and whole wheat breads. Cereal Chem. 1980;57:226–229. [Google Scholar]
- 14.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Bae H.D., Yanke L.J., Cheng K.-J., Selinger L.B. A novel staining method for detecting phytase activity. J. Microbiol. Meth. 1999;39:17–22. doi: 10.1016/s0167-7012(99)00096-2. [DOI] [PubMed] [Google Scholar]
- 16.Monteiro T.I.R.C., Porto T.S., Carneiro-Leão A.M.A., Silva M.P.C., Carneiro-da-Cunha M.G. Reversed micellar extraction of an extracellular protease from Nocardiopsis sp. fermentation broth. Biochem. Eng. J. 2005;24:87–90. doi: 10.1016/j.bej.2005.01.010. [DOI] [Google Scholar]
- 17.Porto T.S., Monteiro T.I.R., Moreira K.A., Lima-Filho J.L., Silva M.P.C., Porto A.L.F., Carneiro-da-Cunha M.G. Liquid–liquid extraction of an extracellular alkaline protease from fermentation broth using aqueous two-phase and reversed micelles systems. World J. Microbiol. Biotechnol. 2005;21:655–659. doi: 10.1007/s11274-004-3570-9. [DOI] [Google Scholar]
- 18.Gaikaiwari R.P., Wagh S.A., Bhaskar D., Kulkarni B.D. Efficient lipase purification using reverse micellar extraction. Bioresour. Technol. 2012;108:224–230. doi: 10.1016/j.biortech.2011.11.126. [DOI] [PubMed] [Google Scholar]
- 19.Nascimento C.O., Coelho L.C.B.B., Correia M.T.S., Carneiro-da-Cunha M.G. Liquid-liquid extraction of lectin from Cratylia mollis seeds using reversed micelles. Biotechnol. Lett. 2002;24:905–907. doi: 10.1023/A:1015557802326. [DOI] [Google Scholar]
- 20.He S., Shi J., Walid E., Zhang H., Ma Y., Xue S.J. Reverse micellar extraction of lectin from black turtle bean (Phaseolus vulgaris): optimisation of extraction conditions by response surface methodology. Food Chem. 2015;166:93–100. doi: 10.1016/j.foodchem.2014.05.156. [DOI] [PubMed] [Google Scholar]
- 21.Peng X., Xu H., Yuan X., Leng L., Meng Y. Mixed reverse micellar extraction and effect of surfactant chain length on extraction efficiency. Sep. Purif. Technol. 2016;160:117–122. doi: 10.1016/j.seppur.2016.01.022. [DOI] [Google Scholar]
- 22.Wan J., Guo J., Miao Z., Guo W. Reverse micellar extraction of bromelain from pineapple peel – effect of surfactant structure. Food Chem. 2016;197:450–456. doi: 10.1016/j.foodchem.2015.10.145. [DOI] [PubMed] [Google Scholar]
- 23.Lei X.G., Weaver J.D., Mullaney E., Ullah A.H., Azain M.J. Phytase, a new life for an “old” enzyme. Annu. Rev. Anim. Biosci. 2013;1:283–309. doi: 10.1146/annurev-animal-031412-103717. [DOI] [PubMed] [Google Scholar]
- 24.Pandey A., Szakacs G., Soccol C.R., Rodriguez-Leon J.A., Soccol V.T. Production, purification and properties of microbial phytases. Bioresour. Technol. Rep. 2001;77:203–214. doi: 10.1016/s0960-8524(00)00139-5. [DOI] [PubMed] [Google Scholar]
- 25.Eskici G., Axelsen P.A. The Size of AOT Reverse Micelles. J. Phys. Chem. B. 2016;120:11337–11347. doi: 10.1021/acs.jpcb.6b06420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishiki T., Nakamura K., Kato D. Forward and backward extraction rates of amino acid in reversed micellar extraction. Biochem. Eng. J. 2000;4:189–195. [Google Scholar]
- 27.Chuo S.C., Mohd-Setapar S.H., Mohamad-Aziz S.N., Starov V.M. A new method of extraction of amoxicillin using mixed reverse micelles. Colloids Surf. A Physicochem. Eng. Asp. 2014;460:137–144. doi: 10.1016/j.colsurfa.2014.03.107. [DOI] [Google Scholar]
- 28.Fathi H., Kelly J.P., Vasquez V.R., Graeve O.A. Ionic concentration effects on reverse micelle size and stability: implications for the synthesis of nanoparticles. Langmuir. 2012;28:9267–9274. doi: 10.1021/la300586f. [DOI] [PubMed] [Google Scholar]
- 29.Hebbar U.H., Sumana B., Raghavarao K.S.M.S. Use of reverse micellar systems for the extraction and purification of bromelain from pineapple wastes. Bioresour. Technol. Rep. 2008;99:4896–4902. doi: 10.1016/j.biortech.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 30.Tovstun S.A., Razumo V.F. Symmetry and stability of AOT reverse micelles: poisson–boltzmann calculations. J. Mol. Liq. 2019;275:578–585. doi: 10.1016/j.molliq.2018.11.117. [DOI] [Google Scholar]
- 31.Sun X.-H., Zhu K.-X., Hui-Ming Zhou H.-M. Optimization of a novel backward extraction of defatted wheat germ protein from reverse micelles. Innov. Food Sci. Emerg. Technol. 2009;10:328–333. doi: 10.1016/j.ifset.2009.01.006. [DOI] [Google Scholar]
- 32.Casey A., Walsh G. Purification and characterization of extracellular phytase from Aspergillus niger ATCC 9142. Bioresour. Technol. Rep. 2003;86:183–188. doi: 10.1016/s0960-8524(02)00145-1. [DOI] [PubMed] [Google Scholar]
- 33.Monteiro P.S., Guimarães V.M., Melo R.R., Rezende S.T. Isolation of a thermostable acid phytase from Aspergillus niger UFV-1 with strong proteolysis resistance. Braz. J. Microbiol. 2015;46:251–260. doi: 10.1590/S1517-838220120037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greiner R., Silva L.G., Couri S. Purification and characterization of an extracellular phytase from Aspergillus niger 11T53A9. Braz. J. Microbiol. 2009;40:795–807. doi: 10.1590/S1517-83822009000400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spier M.R., Fendrich R.C., Almeida P.C., Noseda M., Greiner R., Konietzny U., Woiciechowski A.L., Soccol V.T. Phytase produced on citric byproducts: purification and characterization. World J. Microbiol. Biotechnol. 2011;27:267–274. doi: 10.1007/s11274-010-0455-y. [DOI] [Google Scholar]
- 36.Gunashree B.S., Venkateswaran G., Gunashree B.S., Venkateswaran G. Extracellular phytase from Aspergillus niger CFR 335: purification and characterization. J. Food Sci. Technol. 2015;52:4558–4564. doi: 10.1007/s13197-014-1304-z. [DOI] [PMC free article] [PubMed] [Google Scholar]