Abstract
Background
Transdiaphragmatic intercostal hernias are extremely rare. Their physiopathology is different from traumatic diaphragmatic ruptures, and their clinical presentation and management strategies place them in a different category than abdominal intercostal hernias.
Case presentation
A 56 yo female presented to the outpatient trauma clinic with a symptomatic, subacute left sided transdiaphragmatic intercostal hernia secondary to a motor vehicle crash almost 3 months prior to presentation. The injury was managed with a combined thoracoscopic and laparoscopic approach, only the second time ever this has been reported. She was discharged on POD#3, and after 6 months of follow up continues to do well, without clinical evidence of hernia recurrence.
Conclusion
Minimally invasive management of this rare pathology is possible and should be encouraged.
Keywords: Traumatic abdominal wall hernia, TAWH, Transdiaphragmatic intercostal hernia, Traumatic diaphragmatic rupture, Intercostal pleuroperitoneal hernia, Laparoscopic bioprosthetic hernioplasty
Introduction
The practice of acute care surgery in rural environments in the United States of America in 2020 is challenging in several aspects: these are well established “surgical deserts” [1] were expertise in trauma care and minimally invasive techniques is lacking. In addition, there is an ongoing shortage of general surgeons in these areas, compounding the problem [2]. One of the most popular solutions has been regionalization of surgical and trauma care [3], in which a trauma referral network directs patients to regional hospitals (designated as Level I), were the necessary specialized care is readily available. The downside of this model is that most times these centers are many miles away from the patient's community. This produces disruptions in continuity of care, severely limits patient's choice and satisfaction with their care and bring difficult complications to the local practitioners, who are ill-prepared to deal with them [2,3].
When the disease is a rare one, with potential for severe, life threatening complications, and when the optimal management requires significant expertise, the situation becomes more difficult for the patient. Below you will find a real-life example of this issue.
Case report
A 56-year-old female presented to the Trauma outpatient clinic, referred by a local general surgeon. As the patient related, she was admitted to a regional Level I trauma center 75 days before our evaluation. The patient was the front passenger (wearing her seatbelt) during a high-speed motor vehicle crash in which the other car veered into incoming traffic and struck her vehicle in a T-bone fashion on her side. She experienced loss of consciousness of undetermined duration. After a prolonged extrication she was airlifted from the scene to the regional Level I trauma center, located close to 200 miles from her place of residence. During her primary survey her airway was secured with an endotracheal tube. Imaging obtained during her secondary survey showed intra-abdominal contents in her left hemithorax. Her catalog of injuries also included a left clavicle fracture. She was admitted to the trauma ICU, and her diaphragmatic rupture was managed non-operatively. She did receive, however, open reduction and internal fixation of her left clavicle fracture, which has healed as expected. Upon discharge (after a 9-day hospital stay) she was advised to follow up, however no appointments were made at the time, there were no phone calls placed from the outpatient clinic, and she decided to seek local options for her continued care.
Her current symptoms included shortness of breath during walking and mild exercise (worsening compared with before the accident), and one episode of moderate-to-severe abdominal pain, for which she was seen in one of the community ED's (and discharged to follow up with the local referring surgeon). Her past medical history was significant for hypertension, atrial fibrillation, obstructive sleep apnea, chronic obstructive pulmonary disease and non-insulin dependent diabetes. The patient admitted to smoking about half a pack per day for over 20 years.
Significant findings stemming from the clinical examination included a rather large (greater than 20 cm) new bulging on the patient's left lower rib cage, partially reducible, with borborygmi, consistent with an intercostal hernia. Respiratory sounds were diminished over the anterior left lower hemithorax. Her clavicle plating wound was dry and clean. In addition to the above, she was morbidly obese (228 lb, BMI 37.9). The rest of her physical examination was within normal limits. We ordered an outpatient CT scan of the chest, abdomen and pelvis (Fig. 1, Fig. 2, Fig. 3), since we were unable to review the actual images obtained at the previous local ED or the Level I trauma center (only the interpretation of the radiologic studies, her H&P, operative report and discharge summary). After reviewing our images with the patient, she was scheduled for surgery.
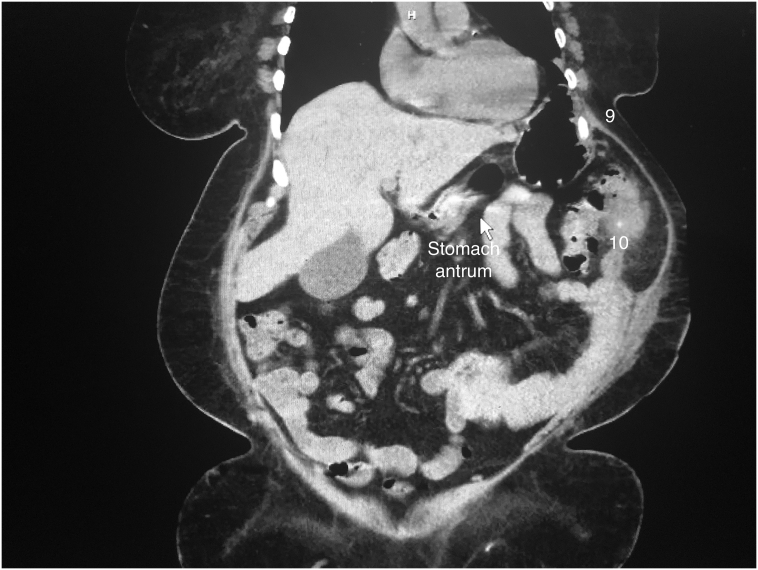
Fig. 1.
Coronal reconstruction of the preoperative axial computed tomography of the patient, showing the injury. The arrow points to the antrum, that can be followed herniating into the chest. 9 and 10 are the ninth and tenth ribs respectively. The splenic flexure can be seen herniating into the 9th intercostal space.
Fig. 2.
Another more posterior coronal reconstruction of the preoperative axial computed tomography of the patient, showing the injury. The gastric fundus is highlighted, in close apposition to the pericardium and the pericardial fatpad. Close adherences were found in the operation.
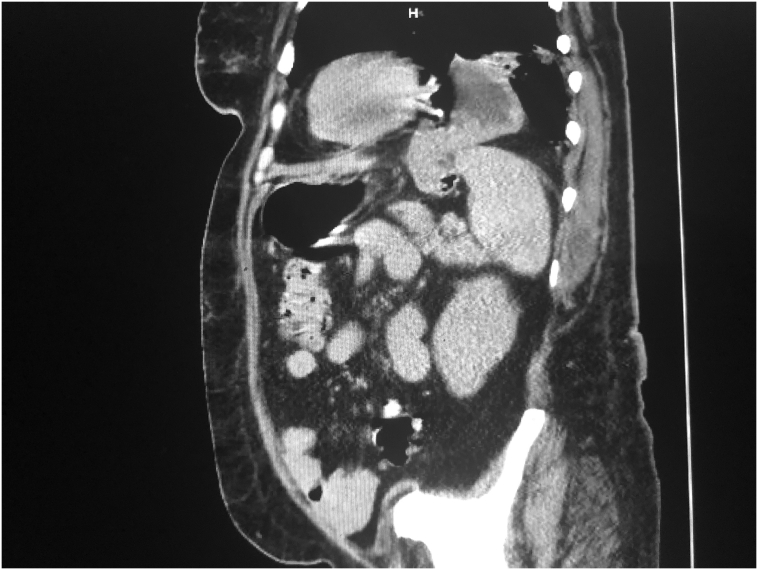
Fig. 3.
Sagittal reconstruction of the preoperative axial computed tomography of the patient, showing the diaphragmatic component of the injury in relation to the anterior mediastinum. The gastric fundus is seen herniating and bringing in the upper pole of the spleen into the chest.
In the operating room a double lumen endotracheal tube was placed, and she was positioned in the right lateral semi-decubitus position, with the head of the bed elevated, the shoulders oriented at 60° and the hips at 30°. This position allowed for access to both left thorax and the abdomen. A moldable beanbag with suction and vacuum creation (McKeeson Surgical, Elgin IL) was used to maintain patient position during the procedure. Access was obtained at Palmer's point with the aid of a Verres needle (C2202, Applied Medical, Rancho Santa Margarita, CA), that was later upsized to a 12 mm balloon-type Hasson cannula (Applied Medical, Rancho Santa Margarita, CA). Three additional 5 mm ports were placed in the left upper quadrant and the left flank. An extensive adhesiolysis with the use of LigaSure Retractable L-hook Laparoscopic Sealer/Divider (Medtronics, Minneapolis, MN) took place until the injury came into view (Fig. 4, Fig. 5): the diaphragmatic defect was measured to 140 mm, adjacent to a 9th intercostal space hernia with a very displaced 10th rib fracture and an associated disruption of the costochondral cartilage, that avulsed the diaphragm from the abdominal wall. The fracture has already healed in this malposition (inferiorly and outwardly displaced) and had callus, to which the bowel was adherent. More anteriorly the muscular layers of the abdominal wall were also torn, giving the intercostal hernia a component of abdominal wall disruption in continuity. The visceral content of the intercostal hernia was the splenic flexure of the colon. The stomach was visualized entering the chest through the diaphragmatic defect, and manual traction of the organ failed to obtain reduction.
Fig. 4.
Caudal laparoscopic view of the injury after adhesiolysis. The thoracic port is visualized through the diaphragmatic defect.
Fig. 5.
Medial closeup laparoscopic view of the injury after adhesiolysis. 9 and 10 are the ninth and tenth ribs. Note the angle of separation between the ribs, marking the beginning of the hernia defect in the chest. The fat remnants on the intercostal space are omental leftovers after adhesiolysis.
A secondary access was obtained at the 6th intercostal space and the anterior axillary line, were another balloon-type Hasson cannula was placed (to avoid losing pneumoperitoneum). Thoracoscopic instruments and the LigaSure were then brought in to release the omental adhesions of the stomach to the anterior pleural wall, the mediastinum, the pericardium and the pericardial fat pad, and the pleural side of the diaphragm until the stomach was successfully evicted from the chest. Care was taken not to injure the upper pole of the spleen which was insinuating itself into the chest. Additional attachments of the lung to the injury sites were lysed until the pleural cavity was fully deloculated. Primary closure of the defect was then attempted with the aid of the EndoStitch (Medtronics, Minneapolis, MN) device, but a very fibrous scar-type ring was found at the edges of the diaphragm that precluded sufficient mobilization. A 20 × 20 cm “thin” Strattice (Allergan, Madison NJ) bioprosthesis was then brought in to perform a bridging (intraperitoneal onlay) reconstruction. It was then sutured intracorporeally using #5 Ethibond (Ethicon, Somerville NJ) to the borders of the diaphragmatic defect. The anterolateral abdominal wall reconstruction was completed using transfascial sutures of #5 Ethibond, placed with the aid of the Carter-Thomason (Cooper Surgical, Trumbull CT) device. A 32 F curved basilar chest tube completed the operation.
The patient was extubated postop and remained in the ICU overnight. Her chest tube was kept on suction for 4 h, and after being on water seal for over 12 h was removed on POD#1. She was discharged home on POD#2. Over the course of the first 2 months postop the patient developed a rather large seroma on the intercostal hernia, that was managed expectantly. A follow up CT scan (Fig. 6) was obtained, that showed no evidence of recurrence, and seroma resolution. As of her 6th month of follow up the patient remains asymptomatic.
Fig. 6.
Coronal reconstruction of the postoperative axial computed tomography (4 months postop), showing satisfactory reconstruction of the left costodiaphragmatic recess, without lung herniation and resolution of the seroma. Arrows point to the bioprosthesis.
Discussion
The first description of what is known today as transdiaphragmatic intercostal hernia (TDIH) can be traced to Croce et al. [4], although 4 possible cases of the disease were included in an earlier report by Maurer et al. [5]. The modern term was coined by Cole [6], typifying a pathological process related to a lower (i.e. seventh to tenth) rib fracture occurring during violent fits of cough. This in fact has been the underlying cause of most of the cases reported since in the literature. In those patients the approach has been consistently through a thoracotomy or a thoracoabdominal incision (to properly repair the thoracic wall defect) and included the reapproximation of the intercostal space through the use of pericostal sutures [[4], [5], [6], [7]], or more recently osteosynthesis titanium plates for the ribs [8].
The occurrence of this type injury during a traumatic event is exceedingly uncommon. As of 2012 less than 40 cases of TDIH following a blunt traumatic injury have been reported in the available English biomedical world literature [9]. It is important to differentiate this process from isolated traumatic diaphragmatic rupture (TDR); an injury found in between 0.8 and 5% of all trauma admissions [10]. While the occurrence, mechanism and management of TDR during blunt trauma is well understood [11,12], TDIH appears to be more akin to intercostal hernias in mechanism [13,14], the forceful tearing of the intercostal muscles (frequently involving fracture of the involved rib) as well as the costal attachments of the diaphragm (as opposed to a sudden increase in the intra-abdominal pressure postulated for TDR [11,12]) creating two distinct defects that superimpose to create the so-called intercostal pleuroperitoneal tunnel. The diaphragmatic component in this presentation is both a function of the location and a distinct clinical entity (that differentiates it from simple abdominal intercostal hernias) [7,13,15,16] as well as a marker for the energy dissipated during the injury [16]. In addition, while TDR occurs predominantly in the left side [11,12], TDIH is equally common on both sides [10,14], as the mechanism of injury is different. Putative risk factors include morbid obesity and the use of seatbelts [16]. When encountered by trauma surgeons the approach is usually a laparotomy [9,10,18], and the intercostal component is usually deal with sutures (not pericostal), prosthetic mesh [9] or ignored altogether (thus neglecting the reduction of the intercostal defect and the reconstruction of the Sibson's fascia), a likely cause for recurrences [10].
While the first laparoscopic repair of a traumatic diaphragmatic defect was reported by Frantzides in 1994 [17], laparoscopic techniques have been much slower to adopt in trauma surgery than in other fields. To the best of our knowledge, this is only the second case ever reported of TDIH [16] managed with minimally invasive techniques. Abdominal intercostal hernia repairs have been reported before as being approached via endoscopic means [15,18], but the addition of the diaphragmatic component adds another layer of complexity to the operation. Since this patient presented past the acute phase, her tissues were much less compliant and fibrosis precluded altogether a primary suture repair (as its was first reported [16]), requiring a bridging approach for the diaphragm and the use of transfascial sutures to reduce the intercostal component and to address the abdominal wall defect, a very high level of laparoscopic technical expertise. Without this step the defect is bound to recur. As an added benefit, this allows the seroma to partially drain into the pleural cavity and into the basilar chest tube, preventing the infection that occurred in the first case reported, while reducing the likelihood of a pulmonary hernia. Bringing this level of expertise in the management of emergency surgical pathologies to a community is another benefit of the addition of a local Trauma Center, a phenomenon known as the “Halo Effect” [19].
Funding
None.
Declaration of competing interest
None.
Acknowledgements
The author gratefully acknowledges Dr Kulsoom Laeeq MBBS for her support with the clinical management of the patient, and Dr Izaskun Iglesias MD MBA for her assistance during the writing of the manuscript.
References
- 1.Uribe-Leitz T., Esquivel M.M., Garland N.Y. Surgical deserts in California: an analysis of access to surgical care. J. Surg. Res. Mar 2018;223:102–108. doi: 10.1016/j.jss.2017.10.014. (Epub 2017 Nov 15) [DOI] [PubMed] [Google Scholar]
- 2.Bernard A., Staudenmayer K., Minei J.P. Macroeconomic trends and practice models impacting acute care surgery. Trauma Surg. Acute Care Open. Apr 11 2019;4(1) doi: 10.1136/tsaco-2018-000295. eCollection 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chhabra K.R., Dimick J.B. Strategies for improving surgical care: when is regionalization the right choice? JAMA Surg. Nov 1 2016;151(11):1001–1002. doi: 10.1001/jamasurg.2016.1059. [DOI] [PubMed] [Google Scholar]
- 4.Croce E.J., Mehta V.A. Intercostal pleuroperitoneal hernia. J. Thorac. Cardiovasc. Surg. Jun 1979;77(6):856–857. [PubMed] [Google Scholar]
- 5.Maurer E., Blades B. Hernia of the lung. J. Thorac. Surg. Apr 1946;15:77–98. [PubMed] [Google Scholar]
- 6.Cole F.H., Jr., Miller M.P., Jones C.V. Transdiaphragmatic intercostal hernia. Ann. Thorac. Surg. May 1986;41(5):565–566. doi: 10.1016/s0003-4975(10)63045-7. [DOI] [PubMed] [Google Scholar]
- 7.Chaar C., Attanasio P., Detterbeck F. Disruption of the costal margin with transdiaphragmatic abdominal herniation induced by coughing. Am. Surg. Apr 2008;74(4):350–353. [PubMed] [Google Scholar]
- 8.Aladaileh M., O’Driscoll-Collins A., O’Keeffe F. Traumatic thoracoabdominal hernia repair using a novel chest-wall reconstruction technique: a case report. Ann. R. Coll. Surg. Engl. Jan 2020;102(1):e4–e6. doi: 10.1308/rcsann.2019.0120. (Epub 2019 Sep 11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarkar D., Warta M., Solomon J. Transdiaphragmatic intercostal herniation following blunt trauma. Case Rep. Radiol. 2012;2012:502765. doi: 10.1155/2012/502765. (Epub 2012 Nov 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma O.P., Duffy B. Transdiaphragmatic intercostals hernia: review of the world literature and presentation of a case. J. Trauma. 2001;50:1140–1143. doi: 10.1097/00005373-200106000-00026. [DOI] [PubMed] [Google Scholar]
- 11.Asensio J.A., Demetriades D., Rodriguez A. Injury to the diaphragm. In: Feliciano D.V., Moore E.E., Mattox K.L., editors. Trauma. 3rd edn. Appleton and Lange; Stanford: 1996. pp. 474–477. [Google Scholar]
- 12.Petrone P., Asensio J.A., Marini C.P. Diaphragmatic injuries and post-traumatic diaphragmatic hernias. Curr. Probl. Surg. Jan 2017;54(1):11–32. doi: 10.1067/j.cpsurg.2016.11.001. (Epub 2016 Nov 29) [DOI] [PubMed] [Google Scholar]
- 13.Gooseman M.R., Rawashdeh M., Mattam K. Unifying classification for transdiaphragmatic intercostal hernia and other costal margin injuries. Eur. J. Cardiothorac. Surg. Jul 1 2019;56(1):150–158. doi: 10.1093/ejcts/ezz020. [DOI] [PubMed] [Google Scholar]
- 14.Chapman A.A., Duff S.B. A case of spontaneous transdiaphragmatic intercostal hernia with contralateral injury, and review of the literature. Case Rep. Surg. 2017;2017 doi: 10.1155/2017/7416092. (Epub 2017 Feb 23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalles V., Dasiou M., Doga G. Posttraumatic transdiaphragmatic intercostal hernia: report of a case and review of the literature. Int. Surg. Mar 2015;100(3):444–449. doi: 10.9738/INTSURG-D-13-00272.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siow S.L., Wong C.M., Hardin M., Sohail M. Successful laparoscopic management of combined traumatic diaphragmatic rupture and abdominal wall hernia: a case report. J. Med. Case Rep. Jan 18 2016;10:11. doi: 10.1186/s13256-015-0780-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frantzides C.T., Carlson M.A. Laparoscopic repair of a penetrating injury to the diaphragm: a case report. J. Laparoendosc. Surg. Apr 1994;4(2):153–156. doi: 10.1089/lps.1994.4.153. [DOI] [PubMed] [Google Scholar]
- 18.Abunnaja S., Chysna K., Shaikh I., Tripodi G. Acquired abdominal intercostal hernia: a case report and literature review. Case Rep. Surg. 2014;2014:456053. doi: 10.1155/2014/456053. (Epub 2014 Aug 17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagarajan N., Selvarajah S., Gani F. “Halo effect” in trauma centers: does it extend to emergent colectomy? J. Surg. Res. Jun 1 2016;203(1):231–237. doi: 10.1016/j.jss.2016.01.037. (Epub 2016 Feb 4) [DOI] [PubMed] [Google Scholar]