Highlights
-
•
Low socioeconomic status influence on the risk of stroke mortality.
-
•
Low income and ocuption for stroke mortality is higher than education.
-
•
The heterogeneity of the study was mainly from different SES indicator.
Keywords: Socioeconomic status, Stroke, Mortality, Meta-analysis, Systematic review, Prospective cohort studies
Abstract
Low socioeconomic status appears to be an independent risk factor for stroke mortality in epidemiology studies, but there has been no systematic assessment of this association. We performed a systematic review and meta-analysis evaluating the association between low socioeconomic status and stroke mortality. A systematic review of MEDLINE, EMBASE, and Web of Science for cohort studies that reported low socioeconomic status and stroke mortality was conducted from inception until July 2017. Research information, adjusted risk ratio (RR) estimates and 95% confidence intervals (Cls) were extracted. Estimates were pooled using a random-effects model. Heterogeneity was examined using the Q statistic and I2. Twenty-seven prospective cohort studies (471,354,852 subjects; 429,886 deaths) assessing stroke mortality with low socioeconomic status were identified. Compared with the highest socioeconomic status, overall RR of stroke mortality was 1.39 (95% CI, 1.31–1.48) for those with the lowest after adjustment for confounding factors, but there was substantial heterogeneity between studies (I2 = 89.9%, P = 0.001). Significant relationships were observed between risk of stroke mortality and the lowest education (RR = 1.21, 95% CI 1.11–1.33; I2 = 70.9%, P < 0.001), income (RR = 1.54, 95% CI 1.30–1.82; I2 = 91.6%, P < 0.001), occupation (RR = 1.54, 95% CI 1.35–1.75; I2 = 78.3%, P < 0.001), composite socioeconomic status (RR = 1.37, 95% CI 1.25–1.51; I2 = 69.5%, P = 0.001). After subgroup analysis, it was found that the heterogeneity of each SES indicator mainly came from the follow-up time, study population, stroke type, study area. Patients with low socioeconomic status had a higher risk of stroke mortality. The heterogeneity of income and occupation is larger, and the education and composite SES is smaller.
1. Introduction
Socioeconomic status (SES) is an individual's or family's position relative to others, based on income, education and occupation, which combine economics and sociology. The complexities of SES determine the diversity of its indicator of measurement. Each single indicator has its own limitations and reflects different aspects of SES. While multiple indicators can comprehensively reflect the level of SES and overcome the limitations of a single indicator.
In the past few decades, the incidence and mortality of stroke have been on the decline in high-income countries, most likely due to improvement in primary and secondary prevention as well as acute stroke treatment and rehabilitation (Katan and Luft, 2018). However, stroke remains globally the second leading cause of death (Wang et al., 2015). SES and other factors interact and modify the incidence and prevalence of stroke. Socioeconomic disparities also affect the short-term and long-term outcomes after stroke, especially for stroke mortality (Marshall et al., 2015). Therefore, it is essential to establish the relationship between socioeconomic disparities and stroke mortality. However, studies on association between SES and stroke mortality were inconsistent (Page et al., 2012, McCormick and Chen, 2016, Pan et al., 2016, Shin et al., 2017, Brown et al., 2013, Arrich et al., 2005, Zhou et al., 2006, Lindmark et al., 2014, Chen et al., 2015). Several findings showed that low education, income, occupation increased risk of stroke mortality (Page et al., 2012, McCormick and Chen, 2016, Pan et al., 2016, Shin et al., 2017), but others showed nothing associations (Pan et al., 2016, Arrich et al., 2005, Zhou et al., 2006, Chen et al., 2015). May be due to inconsistencies in the selection of SES indicators or region lead to differences in the relationship between SES and stroke mortality. The evidence showed that increased conventional risk factors explain about half of these relationships between low SES and stroke mortality, such as hypertension, diabetes, hyperlipidemia, smoking, sedentary (McCormick and Chen, 2016, Pan et al., 2016, Shin et al., 2017, Brown et al., 2013). In addition, evidence showed that people with lower SES were less likely to receive good-quality acute hospital and rehabilitation care in many countries (Kapral et al., 2002, Glader et al., 2013, Chen et al., 2014, Heeley et al., 2011). However, the extent of its contribution to increased stroke risk in patients with low SES in other potential mechanisms is not clear. Therefore, given the need to provide more robust estimates regarding the relationship between low SES and stroke mortality after adjusted for confounding factors.
2. Methods
2.1. Data sources and search
The present meta-analysis was conducted following guidelines for Systematic Review and meta-analyses of observational studies (MOOSE) (Stroup et al., 2000). Electronic database searches were conducted using MEDLINE, EMBASE, and Web of Science from their dates of inception to July 2017 using search strategy combined text word and medical subject headings(Supplement 1). The database search was performed by two authors (SW and HZ).
2.2. Study selection
Studies were independently screened by two authors (SW and BS) using the following criteria. Inclusion criteria: (1) human prospective cohort studies; (2) reported the Cause of Death or death certificate register; (3) SES was clearly defined; (4) adjusted odds ratio (OR), relative risk (RR) or hazard risk (HR) were reported together with 95% confidence intervals (CIs) describing the association between low SES and increased risk of stroke mortality; (5) considering that long-term drug and rehabilitation treatment are needed to prevent recurrence or treat sequelae. The endpoint time of follow-up must be more than one year; (6) the study was published in Chinese or English. Exclusion criteria: (1) cross-sectional and case-control studies due to the higher risk of bias than in prospective cohort studies; (2) abstracts, protocols, letters, expert opinions, case reports and reviews.
2.3. Data extraction
Information was collected on study characteristics, number of participants, deaths with stroke, endpoint time of follow-up. The study characteristics included year of publication, country, study population, stroke type. The data were extracted for the longest follow-up time in each article. Our study chose the education, income, occupation, and composite SES to measure the SES. Education was considered the number of years of schooling or categorical education levels (Pan et al., 2016, Arrich et al., 2005, Zhou et al., 2006, Lindmark et al., 2014, Chen et al., 2015, Avendano et al., 2004, Ahacic et al., 2012, Andersen et al., 2014, Singh et al., 2015, Belleudi et al., 2016). We can see that although the classification is based on education level or years, there are also differences in grades in each article. Income was assessed with different brackets. According to the average family income per capita per month, taxable income, total annual income or income using country census data (Ahacic et al., 2012, Arrich et al., 2005, Chen et al., 2015, Jakovljević et al., 2001, Kapral et al., 2002, Li et al., 2008, Lindmark et al., 2014, Pan et al., 2016, Singh et al., 2015, Zhou et al., 2006). Occupation was categorized in different ways (Pan et al., 2016, Arrich et al., 2005, Zhou et al., 2006, Chen et al., 2015, Singh et al., 2015, Bennett, 1996, Kunst et al., 1998). The most common is the division of occupations into manual and non-manual, or unemployed, working in own household and retirement. Some studies have more specific occupational classifications, such as unskilled worker, skilled worker, self-employed, white-collar, blue-collar, professional, managerial, non-professional, clerical or sales. Composite SES was measured by more than one individual SES indicator, which is synthetically calculated by mathematical method (Brown et al., 2013, Chen et al., 2014, Heeley et al., 2011, Page et al., 2012, McCormick and Chen, 2016, Pan et al., 2016, Cesaroni et al., 2009, Mariani et al., 2016, Yan et al., 2017). We obtain effect estimates from the adjusted model, and that means adjustment for confounding factor including age, sex, race, smoking, hypertension, diabetes, heart disease and other relative factors. We assessed the quality of cohort studies using the Newcastle-Ottawa Scale (NOS) (Wells et al., 2019).
2.4. Statistical analysis
If stroke mortality probability is small (<10%), OR is close to RR, which we corrected for the case when stroke mortality probability is > 10% using the formula: RR = OR/((1 − P0) + (P0*OR)) (Zhang and Yu, 1998). The HR is often perceived as an RR (Stare and Maucort-Boulch, 2016). The included study estimates were combined using inverse variance in a random-effect model. Heterogeneity was reported using Q statistic and the I2 statistic, with a P value ≤ 0.10 for Q considered to represent statistically significant between study heterogeneity (Doi et al., 2015); for I2 statistic < 50% were classified as low heterogeneity; 50–75%, moderate heterogeneity; and > 75%, high heterogeneity (Higgins and Thompson, 2002). Then the heterogeneity of subgroup analyses was analyzed. Publication bias was evaluated using Begg's and Egger's tests. A P value of < 0.05 was considered statistically significant, and all analysis was performed using Stata 13.0 (Stata Corp LP).
3. Results
3.1. Study selection and characteristics
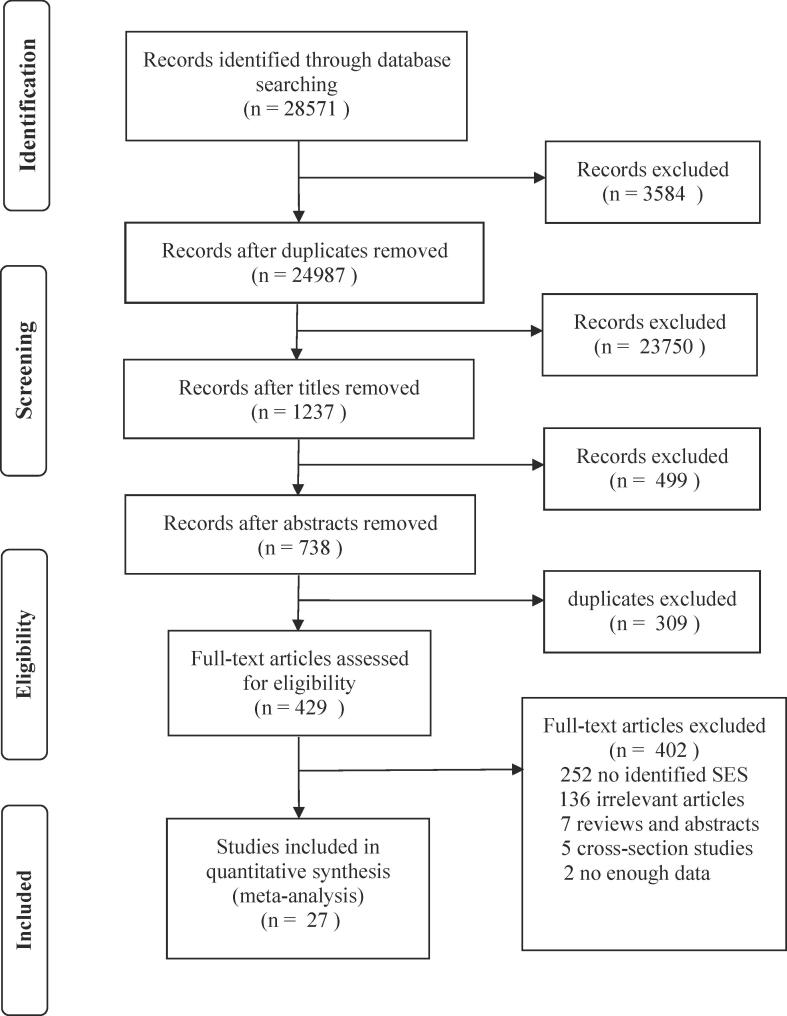
Using this search strategy, 28,571 potentially relevant studies were retrieved from three electronic databases. After eliminating studies based on review of abstracts, titles and duplication, 429 studies remained for assessment. Finally, 27 cohort studies comparing stroke mortality between the lowest and highest SES were included in the meta-analysis (Fig. 1).
Fig. 1.
Flowchart of study identification and selection for meta-analysis.
27 cohort studies involved 471,354,852 subjects, including 429,886 cases of death with stroke (Supplement 2). Two studies did not indicate the number of participants. Fifteen studies were conducted in Europe, five in Asia, three in Oceania, and four in North America. Seventeen studies were population-based and ten were hospital-based. Nineteen studies examined mortality due to stroke; six due to ischemic stroke; and two due to hemorrhagic stroke. Follow-up time ranged from one to 27 years, and most studies did not report follow-up rate. Across the studies, SES was measured in terms of education (12studies), (Pan et al., 2016, Salonen, 1982, Zhou et al., 2006, Lindmark et al., 2014, Chen et al., 2015, Avendano et al., 2004, Ahacic et al., 2012, Andersen et al., 2014, Singh et al., 2015, Belleudi et al., 2016, Arrich et al., 2005, Langagergaard et al., 2011) income (13studies), (Pan et al., 2016, Jakovljević et al., 2001, Li et al., 2008, Salonen, 1982, Zhou et al., 2006, Lindmark et al., 2014, Chen et al., 2015, Kapral et al., 2002, Ahacic et al., 2012, Andersen et al., 2014, Singh et al., 2015, Arrich et al., 2005, Langagergaard et al., 2011) occupation (10studies), (Pan et al., 2016, Arrich et al., 2005, Zhou et al., 2006, Chen et al., 2015, Singh et al., 2015, Bennett, 1996, Kunst et al., 1998, Salonen, 1982, Langagergaard et al., 2011, Virtanen and Notkola, 2002) or a composite SES indicator (10 studies) (Arrich et al., 2005, Brown et al., 2013, Cesaroni et al., 2009, Chen et al., 2014, Heeley et al., 2011, Pan et al., 2016, Shin et al., 2017, Yan et al., 2017). The NOS quality of 25 included studies was more than seven scores. The detailed evaluation of NOS scores was not showed, see Supplement 3.
3.2. Risk of stroke mortality with the lowest SES
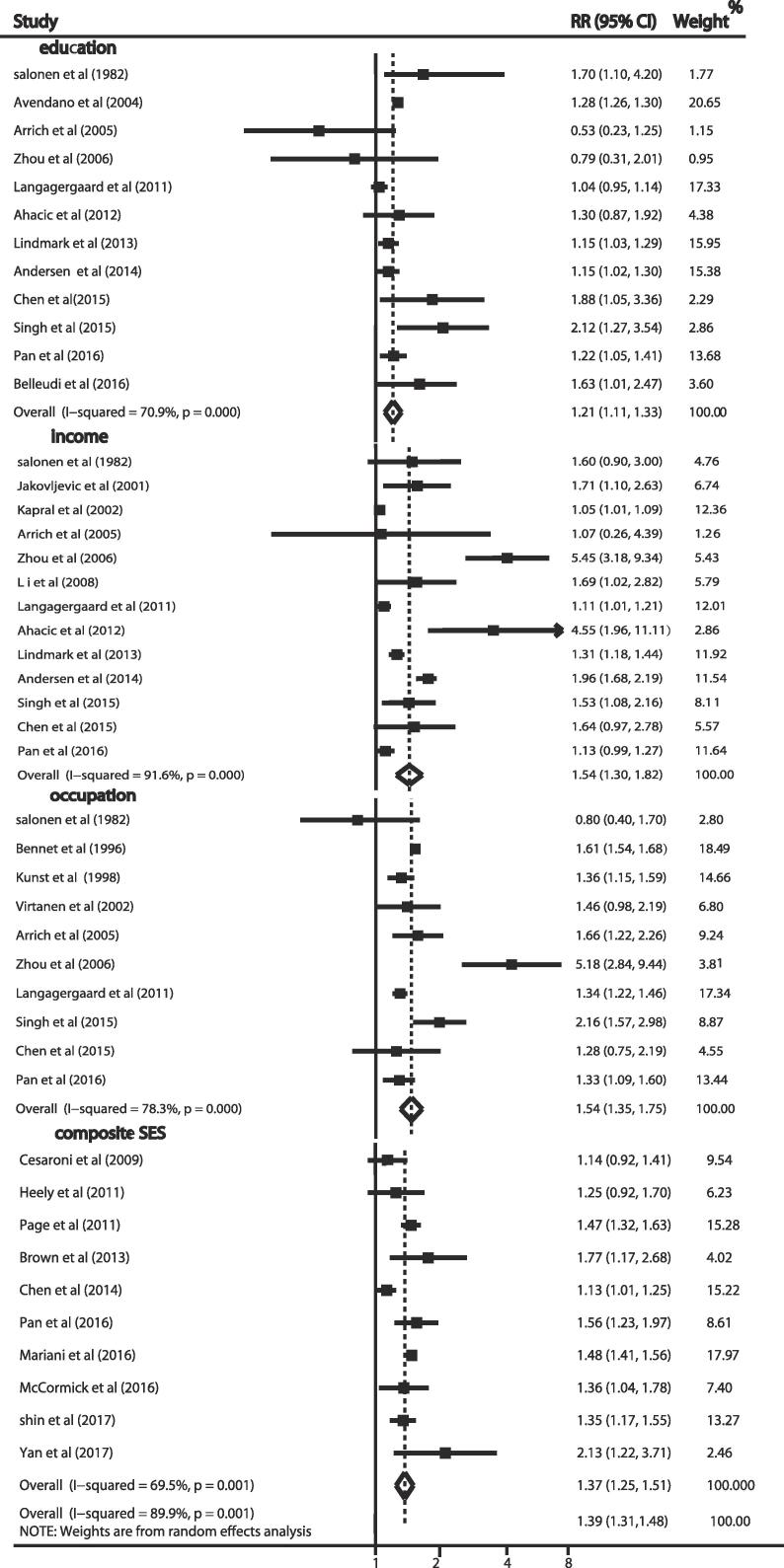
The forest plot in Fig. 2 summarizes the risk of stroke mortality in the lowest SES group compare with the risk in the highest SES group, overall RR was 1.39 (95% CI, 1.31–1.48), but there was substantial heterogeneity between studies (I2 = 89.9%, P = 0.001). Significant relationships were observed between risk of stroke mortality and the lowest education (RR = 1.21, 95% CI 1.11–1.33; I2 = 70.9%, P < 0.001), income (RR = 1.54, 95% CI 1.30–1.82; I2 = 91.6%, P < 0.001), occupation (RR = 1.54, 95% CI 1.35–1.75; I2 = 78.3%, P < 0.001), composite SES (RR = 1.37, 95% CI 1.25–1.51; I2 = 69.5%, P = 0.001).
Fig. 2.
Risk of stroke mortality with the lowest SES compared with the highest SES.
3.3. Subgroup analysis
To further explore the heterogeneity of studies, participants were stratified by follow-up time, study population, stroke type, study area (Table 1).
Table 1.
Subgroup analysis of stroke mortality with the lowest SES compared with the highest SES.
| Subgroups | Education RR (95% CI) | I2, P | Income RR (95% CI) | I2, P | OccupationRR (95% CI) | I2, P | Composite SES RR (95% CI) | I2, P |
|---|---|---|---|---|---|---|---|---|
| Overall | 1.21(1.11,1.33) | 70.9%, <0.001 | 1.54(1.30,1.82) | 91.6%, <0.001 | 1.54(1.35,1.75) | 78.3%, <0.001 | 1.39(1.21,1.60) | 39.6%, 0.142 |
| Follow-up time | ||||||||
| <5 years | 1.14(1.02,1.27) | 44.0%, 0.095 | 1.39(1.18, 1.62) | 88.3%, <0.001 | 1.71(1.27,2.30) | 85.4%, <0.001 | 1.36(1.18,1.56) | 85.5%, <0.001 |
| ≥5 years | 1.30(1.14,1.49) | 55.9%, 0.059 | 1.87(1.66, 2.11) | 0.0%, 0.519 | 1.52(1.31,1.77) | 57.1%,0.040 | 1.34(1.21,1.48) | 73.7%, <0.001 |
| Study population | ||||||||
| Population | 1.57(1.17,2.10) | 50.6%, 0.108 | 1.62(1.32, 1.99) | 0.0%, 0.996 | 1.52(1.31,1.77) | 0.0%, 0.040 | 1.34(1.21,1.48) | 73.7%, <0.001 |
| Hospital | 1.14(1.05,1.24) | 36.4%, 0.138 | 1.50(1.22, 1.83) | 94.7%, <0.001 | 1.71(1.27,2.30) | 85.4%, <0.001 | 1.64(1.31,2.05) | 2.1%, 0.312 |
| Stroke type | ||||||||
| Stroke | 1.22(1.10,1.36) | 77.6%, <0.001 | 1.46(1.21, 1.76) | 92.6%, <0.001 | 1.47(1.29,1.68) | 74.4%, 0.001 | 1.48(1.24,1.77) | 36.3%, 0.208 |
| IS | 1.13(0.80, 1.61) | 51.4%, 0.104 | 1.96(0.58, 6.69) | 93.6%, <0.001 | 2.09(1.18,3.70) | 89.0%, <0.001 | 1.36(1.04, 1.78) | – |
| HS | – | – | 1.17(1.11, 2.64) | – | – | – | 1.16(1.06,1.26) | 0.0%, 0.450 |
| Study area | ||||||||
| Europe | 1.18(1.06,1.31) | 77.4%, <0.001 | 1.58(1.25,1.99) | 88.0%, <0.001 | 1.36(1.26,1.46) | 0.0%, 0.420 | 1.16(1.06,1.26) | 0.0%, 0.450 |
| Asia | 1.28(0.94, 1.75) | 31.2%, 0.234 | 2.11(0.85,5.25) | 93.8%, <0.001 | 2.00(0.94,4.26) | 89.0%, <0.001 | 1.48(1.24,1.77) | 36.3%, 0.208 |
| America | 2.12(1.27,3.54) | – | 1.22(0.85,1.74) | 77.7%, 0.034 | 2.16(1.57,2.98) | – | 1.48(1.41,1.56) | 0.0%, 0.401 |
| Oceania | – | – | – | – | 1.61(1.54,1.68) | 78.3%, <0.001 | 1.45(1.31,1.60) | 0.0%, 0.401 |
IS: ischemic stroke; HS: hemorrhagic stroke; RR: relative risk.
An association between stroke mortality and lowest education (RR = 1.14, 95% CI 1.02–1.27; I2 = 44.0%, P = 0.059), income (RR = 1.39, 95% CI 1.18–1.62; I2 = 88.3%, P < 0.001), occupation (RR = 1.71, 95% CI 1.27–2.30; I2 = 85.4%, P < 0.001), and composite SES (RR = 1.36, , 95% CI 1.18–1.56; I2 = 85.5%, P < 0.001) were observed in follow-up < 5 years. The association was also observed in follow-up ≥ 5 years, lowest education (RR = 1.30, , 95% CI 1.14–1.49; I2 = 55.9%, P = 0.059), income (RR = 1.87, 95% CI 1.66–2.11; I2 = 0.0%, P = 0.519), occupation (RR = 1.52, 95% CI 1.31–1.77; I2 = 57.1%, P = 0.040), and SES composite indicator (RR = 1.34, 95% CI 1.21–1.48; I2 = 73.7%, P < 0.001).
The association between stroke mortality and lowest education (RR = 1.57, 95% CI 1.17–2.10; I2 = 50.6%, P = 0.108), income (RR = 1.62, 95% CI 1.32–1.99; I2 = 0.0%, P = 0.996), occupation (RR = 1.52, 95% CI 1.31–1.77; I2 = 0.0%, P = 0.040), and composite SES (RR = 1.34, 95% CI 1.21–1.48; I2 = 73.7%, P < 0.001) were significant based on population, and was observed based on hospital, low education (RR = 1.14, 95% CI 1.05–1.24; I2 = 36.4%, P = 0.138), income (RR = 1.50, 95% CI 1.22–1.83; I2 = 94.7%, P < 0.001), occupation (RR = 1.71, 95% CI 1.27–2.30; I2 = 85.4%, P < 0.001), and composite SES (RR = 1.64, 95% CI 1.31–2.05; I2 = 2.1%, P = 0.312).
An association between stroke mortality and lowest education(RR = 1.22, 95% CI 1.10–1.36; I2 = 77.6%, P < 0.001), income (RR = 1.46, 95% CI 1.21–1.76; I2 = 92.6%, P < 0.001), occupation (RR = 1.47, 95% CI 1.29–1.68; I2 = 74.4%, P = 0.001) were observed, with a higher heterogeneity, but composite SES with a lower heterogeneity (RR = 1.48, 95% CI 1.24–1.77; I2 = 36.3%, P = 0.208). There was an association between ischemic stroke mortality and lowest occupation (RR = 2.09, 95% CI 1.18–3.70; I2 = 89.0%, P < 0.001), with a higher heterogeneity. An association between hemorrhagic stroke and composite SES was observed, with no heterogeneity (RR = 1.16, 95% CI 1.06–1.26; I2 = 0.0%, P = 0.450).
The association between stroke mortality and lowest education (RR = 1.18, 95% CI 1.06–1.31; I2 = 77.4%, P < 0.001), income (RR = 1.58, 95% CI 1.25–1.99; I2 = 88.0%, P < 0.001) was significant in Europe, with a higher heterogeneity. An association between stroke mortality and lowest occupation (RR = 1.36, 95% CI 1.26–1.46; I2 = 0.0%, P = 0.420) and composite SES (RR = 1.16, 95% CI 1.06–1.26; I2 = 0.0%, P = 0.450) were observed in Europe, with no heterogeneity. There association between stroke mortality and lowest composite SES in Asia (RR = 1.48, 95% CI 1.24–1.77; I2 = 36.3%, P = 0.208), America (RR = 1.48, 95% CI 1.41–1.56; I2 = 0.0%, P = 0.401), and Oceania (RR = 1.45, 95% CI 1.31–1.60; I2 = 0.0%, P = 0.401), with low heterogeneity.
3.4. Publication bias
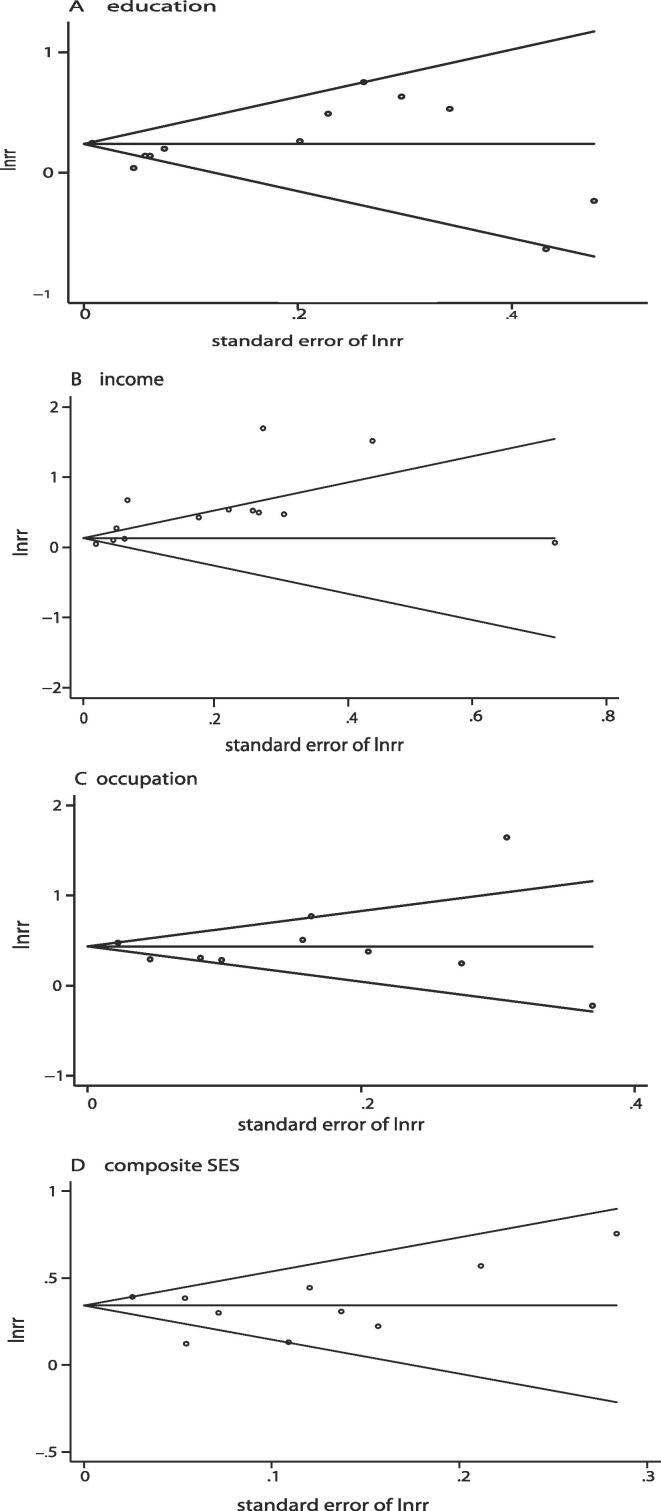
Fig. 3, funnel plots assessed using Begg's and Egger's tests did not indicate evidence of publication bias for data assessing SES in terms of education (Begg's P = 0.58, Egger's P = 0.44), occupation (Begg's P = 0.65, Egger's P = 0.90), or a composite SES indicator (Begg's P = 0.53 Egger's P = 0.70). However, there was publication bias for data assessing SES in terms of income (Begg's P = 0.46, Egger's P = 0.01), the absence of statistically significant results of Egger's and Begg's test could be due to low statistical power of these tests.
Fig. 3.
Funnel plots assessing potential publication bias according to different SES indices. (A: education indicator; B: income indicator; C: occupation indicatior; D: composite indicator.)
4. Discussions
The result of the study has high heterogeneity. The lowest education, income, occupation and composite SES was associated with increased risk of stroke mortality. The risk of stroke mortality in patients with lower income and occupation is higher(RRincome = RRoccupation). While the contribution of the lowest education for risk of stroke mortality is lower. After subgroup analysis, it was found that the heterogeneity was mainly from income and occupation, and education and composite indicators was small, may be due to the difference of classification of SES. Heterogeneity of each SES indicator mainly came from the follow-up time, study population, stroke type, study area. For education, the studies of follow-up time ≥ 5 years, based-population, and Europe have a higher heterogeneity. May be there is a big difference in education among European populations. Stroke treatment and rehabilitation is a long-term process, and patients with high educational level may have an effective management of stroke. For income, the source of heterogeneity is the same as occupation, and opposite of education. May be the indicator of income and occupation are unstable indicators and vulnerable to the development of society and economy, especially for low-and -middle income countries. Previous study has shown that risk of stroke mortality was higher in low-and -middle income countries than in high-income countries. (Yusuf et al., 2014) For composite SES, the source of heterogeneity is follow-up time, based-population, may be due to the difference of calculation method of composite SES. Based on available evidence, we also found that the risk of stroke mortality in patients with the lowest education, income, and occupation in Europe was lower than Asia, America, and Oceania. This may be due to socioeconomic inequalities is smaller, and the effective stroke management was carried in Europe. (Mikulik et al., 2017) In addition, this analysis was limited due to lack of data reported on specific stroke subtype in the majority of studies. Five studies reported that the association between occupation, composite SES and increased risk of ischemic stroke mortality. (Pan et al., 2016, Shin et al., 2017, Zhou et al., 2006, Belleudi et al., 2016, Yan et al., 2017) Two studies reported that there was an association between lower income or composite SES and increased risk of hemorrhagic stroke. McCormick and Chen, 2016, Jakovljević et al., 2001
Although no association between lowest occupation and stroke mortality was not observed in three original studies from high income countries(Finland and USA), (Singh et al., 2015, Salonen, 1982, Virtanen and Notkola, 2002) the manual is still associated with increased stroke mortality in meta-analysis, this is consistent with most findings of studies. A large based-population study indicated higher stroke mortality in men with manual among 30–64 years compared with non-manual in several countries of the European Union as well as in the USA. (Kunst et al., 1998) This discrepancy was considerably large in England, Wales, Ireland, and Finland. Australia, which is not part of continental Europe, found similar results, men aged 25–64 in manual occupations were at least 60% more likely to die from stroke. (Bennett, 1996) Similarly, A research reported that unskilled workers, skilled workers, patients with lower income, early retired patients had a higher stroke mortality in Austria. (Arrich et al., 2005) Previous study from Finland showed that working conditions explained a relatively small portion of socioeconomic inequalities in stroke mortality among men, and association between inequalities and occupation were more attributable to varying levels of education and income. (Virtanen and Notkola, 2002) The reason for this relationship may be the most influential job exposures in men, such as appeared to be shift work, low control, diesel exhaust, or they had the bad behavior of excessive alcohol consumption. (Virtanen and Notkola, 2002, Hart et al., 1999)
We found that most studies measured SES using education and income. A study from Sweden reported that low income was associated with higher 28-day and 1-year fatality rates in men in aged 40 to 65 years, but not in Women. (Li et al., 2008) But a result of study was observed that education and income had only a limited effect on fatality in acute phase (Lindmark et al., 2014) In Italy, elementary education level was inversely associated with mortality both in acute and post-acute phases after ischemic stroke. (Belleudi et al., 2016) In a the 9.5 years follow-up study from Denmark showed that survival of patients with low income was reduced by 30%. (Andersen et al., 2014) This showed that the effect of SES on post-stroke death was on late, and the survival inequality after stroke increased markedly over time. Large socioeconomic discrepancies in long-term survival after stroke may exist also in income inequity countries. But in subgroups analysis we found that short-term stroke mortality with lowest education was higher than long-term, probably those with lowest education lack of awareness of stroke onset and missed the best treatment time contributing to poor outcome. (Ramírezmoreno et al., 2016, Liu et al., 2011) However, long-term risk of stroke mortality with lowest income was higher than short-term, may be due to lowest income did not receive effective rehabilitation or subsequent treatment. (Langagergaard et al., 2011, Huang et al., 2013) Data from China National Stroke Registry (CNSR) showed that patients with low education, manual laboring, and low income had a higher stroke mortality. (Pan et al., 2016) However, the association in patients with lower occupation and income, (Chen et al., 2015) or educational level (Zhou et al., 2006) was not confirmed in other studies from Nanjing, China. Arrich et al reported that income and occupation affected stroke mortality, but education did not. (Arrich et al., 2005) Differences in results may be due to different classifications of SES indicators, and most studies in China was mainly based on hospital, the samples were not sufficiently representative, resulting in negative results.
We found that the low composite SES increased stroke mortality. In UK, there were two based-population studies showed that patients with SES deprivation using carstairs scores have a higher risk of stroke mortality. (McCormick and Chen, 2016, Chen et al., 2014) Similar evidence indicated that people living in areas that were relatively more deprived in socioeconomic conditions experienced higher rates of stroke. (Heeley et al., 2011) Previous study from in Korea reported that for the patients with low income in advantaged regions had a high mortality compared to the others in disadvantaged regions. (Shin et al., 2017) While living in a socioeconomically disadvantaged neighborhood is associated with higher stroke mortality at one year in the United States. (Brown et al., 2013) Interestingly, a study reported that differences in mortality rates between lower and higher SES groups narrowed for stroke from 1979 to 2006, may be changes in the health care system increased health care support for patients with low SES. (Page et al., 2012)
There are several strengths of the present meta-analysis. We included the single, and composite SES indicator to analyze, not only focusing on different aspects of SES but also the overall level of SES. The relatively large number of studies was included. Therefore, the association between SES and stroke mortality could be estimated with a relatively high accuracy. In addition, inclusion of prospective studies can avoid the recall or selection bias of case-control studies. Our meta-analysis has several limitations. First, income, education, occupation and composite SES were classified differently, which may lead to measurement bias. But it is inevitable, because the choice of SES indicator is associated with the social and cultural background at that time. Second, there are two small sample studies from China, (Zhou et al., 2006, Yan et al., 2017) which may contribute to selection bias. Although the sample size of some studies is small, it still contributes to the overall pooled analysis and increase the overall sample size. Third, except for several articles only adjusted for age, (Avendano et al., 2004, Bennett, 1996, Cesaroni et al., 2009) sex, (Mariani et al., 2016) ethnicity (Jakovljević et al., 2001) or place of residence, (Page et al., 2012, Kunst et al., 1998) most article adjusted demographic, cardiovascular, clinical factors, the results will overestimate the effect of socioeconomic status on stroke mortality, due to patients received receive stroke unit care, secondary prevention factors, revascularisation treatment have a better survival. May be patients with low SES or socioeconomic deprivation are less likely to receive stroke unit care, imaging examination, medication, and rehabilitation after stroke, which lead to higher risk of stroke mortality. (Kapral et al., 2002, Huang et al., 2013, Chen et al., 2014) Forth, data of >1 year were included for analysis, not included short-term mortality trends in this study. Finally, studies on the SES impact of stroke mortality are more common in high-income countries and not be sufficient in low-and middle-income countries, so analysis of country of origin was not conducted.
5. Conclusions
Te results of this study suggest that low income, education, occupation and composite SES were associated with increased risk of stroke mortality. However, the heterogeneity between the studies is larger, mainly due to the difference of SES indicator including choice and classification, follow-up time, study population, stroke type, study area.
6. Ethics statements
None.
Funding
This work was funded by Science and Technology Planning Project of Guangdong Province (Grant number: 2017 A070706023).
CRediT authorship contribution statement
Siping Wang: Conceptualization, Data curation, Methodology, Supervision. Huiying Zhai: Retrieving literature, Extracting information Vector drawings. Lin Wei: Review, Revision. Binyan Shen: Retrieving literature, Extracting information. Juan Wang: Project administration, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2020.101124.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ahacic K., Trygged S., Kåreholt I. Income and education as predictors of stroke mortality after the survival of a first stroke. Stroke Res Treat. 2012;2012 doi: 10.1155/2012/983145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K.K., Dalton S.O., Steding-Jessen M., Olsen T.S. Socioeconomic Position and Survival After Stroke in Denmark 2003 to 2012 Nationwide Hospital-Based Study. Stroke. 2014;45:3556–3560. doi: 10.1161/strokeaha.114.007046. [DOI] [PubMed] [Google Scholar]
- Arrich J., Lalouschek W., Mullner M. Influence of socioeconomic status on mortality after stroke: retrospective cohort study. Stroke. 2005;36:310–314. doi: 10.1161/01.STR.0000152962.92621.b5. [DOI] [PubMed] [Google Scholar]
- Avendano M., Kunst A.E., Huisman M., Van Lenthe F., Bopp M., Borrell C. Educational level and stroke mortality: a comparison of 10 European populations during the 1990s. Stroke. 2004;35:432–437. doi: 10.1161/01.str.0000109225.11509.ee. [DOI] [PubMed] [Google Scholar]
- Belleudi V., Sciattella P., Agabiti N., Di Martino M., Di Domenicantonio R., Davoli M. Socioeconomic differences in one-year survival after ischemic stroke: the effect of acute and post-acute care-pathways in a cohort study. BMC Public Health. 2016;16:408. doi: 10.1186/s12889-016-3019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett S. Socioeconomic inequalities in coronary heart disease and stroke mortality among Australian men, 1979–1993. Int. J. Epidemiol. 1996;25:266–275. doi: 10.1093/ije/25.2.266. [DOI] [PubMed] [Google Scholar]
- Brown A.F., Liang L.J., Vassar S.D., Merkin S.S., Longstreth W.T., Ovbiagele B. Neighborhood socioeconomic disadvantage and mortality after stroke. Neurology. 2013;80:520–527. doi: 10.1212/WNL.0b013e31828154ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesaroni G., Agabiti N., Forastiere F., Perucci C.A. Socioeconomic Differences in Stroke Incidence and Prognosis Under a Universal Healthcare System. Stroke. 2009;40:2812–2819. doi: 10.1161/strokeaha.108.542944. [DOI] [PubMed] [Google Scholar]
- Chen R., McKevitt C., Rudd A.G., Wolfe C.D.A. Socioeconomic deprivation and survival after stroke: findings from the prospective South London Stroke Register of 1995 to 2011. Stroke. 2014;45:217–223. doi: 10.1161/strokeaha.113.003266. [DOI] [PubMed] [Google Scholar]
- Chen R., McKevitt C., Crichton S.L., Rudd A.G., Wolfe C.D.A. Socioeconomic deprivation and provision of acute and long-term care after stroke: the South London Stroke Register cohort study. J. Neurol. Neurosurg. Psychiatry. 2014;85:1294–1300. doi: 10.1136/jnnp-2013-306413. [DOI] [PubMed] [Google Scholar]
- Chen R., Hu Z., Chen R.-L., Zhang D., Xu L., Wang J. Socioeconomic deprivation and survival after stroke in China: a systematic literature review and a new population-based cohort study. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2014-005688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi Suhail A.R., Barendregt Jan J., Khan Shahjahan, Thalib Lukman, Williams Gail M. Advances in the meta-analysis of heterogeneous clinical trials I: The inverse variance heterogeneity model. Contempor. Clin. Trials. 2015;45:130–138. doi: 10.1016/j.cct.2015.05.009. [DOI] [PubMed] [Google Scholar]
- Glader E.L., Edlund H., Sukhova M., Asplund K., Norrving B., Eriksson M. Reduced inequality in access to stroke unit care over time: A 15-year follow-up of socioeconomic disparities in Sweden. Cerebrovasc. Dis. 2013;36:407–411. doi: 10.1159/000355497. [DOI] [PubMed] [Google Scholar]
- Hart C.L., Smith G.D., Hole D.J., Hawthorne V.M. Alcohol consumption and mortality from all causes, coronary heart disease, and stroke: results from a prospective cohort study of scottish men with 21 years of follow up. BMJ. 1999;318(7200):1725–1729. doi: 10.1136/bmj.318.7200.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeley E.L., Wei J.W., Carter K., Islam M.S., Thrift A.G., Hankey G.J. Socioeconomic disparities in stroke rates and outcome: pooled analysis of stroke incidence studies in Australia and New Zealand. Med. J. Aust. 2011;195:10–14. doi: 10.5694/j.1326-5377.2011.tb03180.x. [DOI] [PubMed] [Google Scholar]
- Higgins J., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Huang K., Khan N., Kwan A., Fang J., Yun L., Kapral M.K. Socioeconomic status and care after stroke: results from the Registry of the Canadian Stroke Network. Stroke. 2013;44:477–482. doi: 10.1161/STROKEAHA.112.672121. [DOI] [PubMed] [Google Scholar]
- Jakovljević D., Sivenius J., Sarti C., Torppa J., Mähönen M., Immonen-Räihä P. Socioeconomic inequalities in the incidence, mortality and prognosis of subarachnoid hemorrhage: The FINMONICA Stroke Register. Cerebrovasc. dis. 2001;12:7–13. doi: 10.1159/000047674. [DOI] [PubMed] [Google Scholar]
- Kapral M.K., Wang H., Mamdani M., Tu J.V. Effect of socioeconomic status on treatment and mortality after stroke. Stroke. 2002;33:268–273. doi: 10.1161/hs0102.101169. [DOI] [PubMed] [Google Scholar]
- Katan M., Luft A. Global burden of stroke. Sem. Neurol. 2018;38(02):208–211. doi: 10.1055/s-0038-1649503. [DOI] [PubMed] [Google Scholar]
- Kunst A.E., del Rios M., Groenhof F., Mackenbach J.P. Socioeconomic inequalities in stroke mortality among middle-aged men: an international overview. Stroke. 1998;29:2285–2291. doi: 10.1161/01.str.29.11.2285. [DOI] [PubMed] [Google Scholar]
- Langagergaard V., Palnum K.H., Mehnert F., Ingeman A., Krogh B.R., Bartels P. Socioeconomic Differences in Quality of Care and Clinical Outcome After Stroke A Nationwide Population-Based Study. Stroke. 2011;42:2896–2902. doi: 10.1161/strokeaha.110.611871. [DOI] [PubMed] [Google Scholar]
- Li C., Hedblad B., Rosvall M., Buchwald F., Khan F.A., Engstrom G. Stroke incidence, recurrence, and case-fatality in relation to socioeconomic position: a population-based study of middle-aged Swedish men and women. Stroke. 2008;39:2191–2196. doi: 10.1161/strokeaha.107.507756. [DOI] [PubMed] [Google Scholar]
- Lindmark A., Glader E.L., Asplund K., Norrving B., Eriksson M. Riks-Stroke Collaboration. Socioeconomic disparities in stroke case fatality-Observations from Riks-Stroke, the Swedish stroke register. Int. J. Stroke. 2014;9:429–436. doi: 10.1111/ijs.12133. [DOI] [PubMed] [Google Scholar]
- Liu Q., Wang M., Guo J., Li J., Li C., Qian M. Effect of socioeconomic status on secondary prevention of stroke. Int. J. Qual. Health Care. 2011;23:405–412. doi: 10.1093/intqhc/mzr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani J., Monsalvo M., Prieto A.F., Macchia A. Premature death from stroke and socioeconomic status in Argentina. Rev. Argent Cardiol. 2016;84:114–119. doi: 10.7775/rac.v84.i2.8146. [DOI] [Google Scholar]
- Marshall I.J., Wang Y., Crichton S., McKevitt C., Rudd A.G., Wolfe C.D.A. The effects of socioeconomic status on stroke risk and outcomes. Lancet Neurol. 2015;14:1206–1218. doi: 10.1016/S1474-4422(15)00200-8. [DOI] [PubMed] [Google Scholar]
- McCormick J., Chen R. Impact of socioeconomic deprivation on mortality in people with haemorrhagic stroke: a population-based cohort study. Postgrad. Med. J. 2016;92:501–505. doi: 10.1136/postgradmedj-2015-133663. [DOI] [PubMed] [Google Scholar]
- Mikulik R., Caso V., Wahlgren N. Past & Future of Stroke Care in Europe. Oruen–CNS J. 2017;2:19–26. [Google Scholar]
- Page A., Lane A., Taylor R., Dobson A. Trends in socioeconomic inequalities in mortality from ischaemic heart disease and stroke in Australia, 1979–2006. Eur. J. Prev. Cardiol. 2012;19:1281–1289. doi: 10.1177/1741826711427505. [DOI] [PubMed] [Google Scholar]
- Pan Y., Song T., Chen R., Li H., Zhao X., Liu L. Socioeconomic deprivation and mortality in people after ischemic stroke: The China National Stroke Registry. Int. J. Stroke. 2016;11:557–564. doi: 10.1177/1747493016641121. [DOI] [PubMed] [Google Scholar]
- Ramírezmoreno J.M., Alonsogonzález R., Peral P.D., Millán-Nuñez M.V., Roa-Montero A., Constantino-Silva A.B. Effect of socioeconomic level on knowledge of stroke in the general population: A social inequality gradient. Neurologia. 2016;31:24–32. doi: 10.1016/j.nrl.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Salonen J.T. Socioeconomic status and risk of cancer, cerebral stroke, and death due to coronary heart disease and any disease: a longitudinal study in eastern Finland. J. Epidemiol. Community Health. 1982;36:294–297. doi: 10.1136/jech.36.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J., Choi Y., Kim S.W., Lee S.G., Park E.C. Cross-level interaction between individual socioeconomic status and regional deprivation on overall survival after onset of ischemic stroke: National health insurance cohort sample data from 2002 to 2013. J. Epidemiol. 2017;27:381–388. doi: 10.1016/j.je.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G.K., Siahpush M., Azuine R.E., Williams S.D. Increasing Area Deprivation and Socioeconomic Inequalities in Heart Disease, Stroke, and Cardiovascular Disease Mortality Among Working Age Populations, United States, 1969–2011. Int. J. MCH AIDS. 2015;3:119–133. [PMC free article] [PubMed] [Google Scholar]
- Stare J., Maucort-Boulch D. Odds Ratio, Hazard Ratio and Relative Risk. Metodoloski zvezki. 2016;13:59–67. [Google Scholar]
- Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Virtanen S.V., Notkola V. Socioeconomic inequalities in cardiovascular mortality and the role of work: a register study of Finnish men. Int. J. Epidemiol. 2002;31:614–621. doi: 10.1093/ije/31.3.614. [DOI] [PubMed] [Google Scholar]
- Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet 2016; 388: 1459-1544.doi: 10.1016/S0140-6736(16)31012-1Get rights and content. [DOI] [PMC free article] [PubMed]
- Wells GA., Shea B., O’Connell D., Peterson J., Welch V., Losos M. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Clinical Epidemiology. 2019 http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. [Google Scholar]
- Yan H., Liu B., Meng G., Shang B., Jie Q., Wei Y. The influence of individual socioeconomic status on the clinical outcomes in ischemic stroke patients with different neighborhood status in Shanghai, China. Int. J. Med. Sci. 2017;14:86–96. doi: 10.7150/ijms.17241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf S., Rangarajan S., Teo K., Islam S., Li W., Liu L. Cardiovascular Risk and Events in 17 Low-, Middle-, and High-Income Countries. N. Engl. J. Med. 2014;371:818–827. doi: 10.1056/NEJMoa1311890. [DOI] [PubMed] [Google Scholar]
- Zhang J., Yu K.F. What's the relative risk?: A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- Zhou G., Liu X., Xu G., Liu X., Zhang R., Zhu W. The effect of socioeconomic status on three-year mortality after first-ever ischemic stroke in Nanjing, China. BMC Public Health. 2006;6:227. doi: 10.1186/1471-2458-6-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.