Abstract
Current treatments have limited effectiveness in treating tumors. The combination of multiple drugs or treatment strategies is widely studied to improve therapeutic effect and reduce adverse effects of cancer therapy. The codelivery system is the key to realize combined therapies. It is necessary to design and construct different codelivery systems in accordance with the variable structures and properties of cargoes and vectors. This review presented the typical design considerations about codelivery vectors for cancer therapy and described the current state of codelivery systems from two aspects: different types of vectors and collaborative treatment strategies. The commonly used loading methods of cargoes into the vectors, including physical and chemical processes, are discussed in detail. Finally, we outline the challenges and perspectives about the improvement of codelivery systems.
Keywords: Codelivery system, Chemotherapy, Phototherapy, Gene therapy, Immunotherapy
Graphical abstract

1. Introduction
Cancer is one of deadly threats to global human health [1]. Cancer cells are very crafty and possess a variety of defenses against treatments. As a result, cancer is difficult to be cured, which leads to a high fatality rate. Research into the prevention and treatment of cancer is in full swing. The commonly used clinical method of cancer treatments include surgery, chemotherapy, and radiotherapy. Chemotherapy, as a systemic treatment, has a therapeutic effect on some tumors that tend to spread throughout body or have metastasized to advanced tumors [2]. Yet chemotherapy still has some hurdles to overcome. (i) Chemotherapy would cause systemic toxicity, which is often accompanied by obvious side effects such as hair loss, diarrhea, and liver/kidney damage. (ii) Most chemotherapeutic drugs are poorly soluble in aqueous solution and unstable during circulation. (iii) The tumor targeting and cellular uptake of chemotherapeutic drugs need to be improved. (iv) Multidrug resistance (MDR) is an issue that needs to be seriously considered after long-term administration [3]. Clinically, multiple chemotherapeutic drugs are administered in sequence to improve the efficacy of chemotherapy and delay MDR. The collaborative treatment strategy of paclitaxel (PTX) and cisplatin (DDP) is a first-line treatment for lung cancer. Docetaxel (TXT) combined with adriamycin (AMD) in the treatment of breast cancer is an effective and safe neoadjuvant chemotherapy. Various drug delivery systems are also proposed to extend the half-life of drugs, increase drug targeting, improve drug bioavailability, and reduce adverse reactions to chemotherapy drugs [4]. Over the past decade, plenty of effective drug delivery vectors, such as liposomes [5], polymersomes [6,7], nanoparticles [[8], [9], [10]], micelles [[11], [12], [13]], exosomes [14,15], hydrogels [16,17], and drug-polymer conjugates [18,19], have been developed. To further improve the delivery efficiency, delivery vector functionalization is the focus at present [20].
The combination of different therapeutic strategies exhibits ideal therapeutic effects on cancer, meanwhile higher requirements are put forward for the rational design of delivery systems. Vectors that deliver only one type of drug are no longer sufficient for cancer therapy. To realize combination therapy similar to clinical treatments, vectors delivering two or more kinds of reagents, which termed as codelivery vectors, were proposed. For instance, doxorubicin (DOX) and PTX are delivered simultaneously by nanoparticles or micelles for cancer therapy [21,22]. The combination of vincristine sulfate and verapamil hydrochloride by poly(d,l-lactide-co-glycolide acid) nanoparticles increases the sensitivity of tumor cells to chemotherapy drugs [23]. Codelivery vectors can combine gene therapy, phototherapy, or immunotherapy by loading different reagents such as nucleic acid, photosensitive molecules, and quantum dots [24,25]. For example, Zhu et al. [26] combined camptothecin (CPT) and upconversion nanoparticles (NaYF4:Yb/Er) into a hybrid vesicle for synergistic chemotherapy and photodynamic therapy (PDT).
To build effective codelivery systems, the interaction of each component, the balance of underlying conflicting factors, and the synergistic treatment strategies should be considered overall. Besides, in accordance with the typical cancer drug-delivery process of an intravenously administered nanomedicine, which including circulation, accumulation, penetration, internalization, and drug release, it is important to choose the type of vectors and the approach of drug loading in a proper way [27]. All these factors come together to guarantee high overall therapeutic efficiency. Similar to the barrel effect, any weak factor will reduce the performance of the codelivery systems. Although some codelivery systems have been reviewed, these reviews mainly focus on the properties and applications of nanocarriers [[28], [29], [30]]. Few reviews on the structure of codelivery vectors and the loading methods of cargoes have been reported to date. In this review, the design strategies and considerations of codelivery systems are discussed in detail. Then we highlight the latest advances of codelivery systems for cancer therapy. Typical examples about synergistic therapy are also illustrated.
2. Design considerations
The design of codelivery systems requires an overall consideration of the interaction between vectors and cargoes, as well as the synergistic action between the loaded reagents. Plenty of vectors, such as micelles, organic/inorganic nanoparticles, and microgels, have been successfully used for codelivery systems [[31], [32], [33]]. The different structures of drugs and vectors bring a variety of strategies to achieve the purpose of codelivering different drugs. Though all roads lead to Rome, the optimal design route needs to be explored, and some general rules need to be summarized. Although nanocarriers have some advantages owing to their nanoscale, such as the enhanced permeability and retention effect, further modification of the vectors is necessary to enhance the diagnostic and therapeutic effects. For example, targeting molecules are conjugated to the surface of the vectors to enhance the targeting of the vectors to the lesion site [34]; the vectors can be loaded with imaging agents to realize the integration of diagnosis and treatment [35]. As the functionalization of the vectors has been summarized in several excellent reviews in recent years [28,29,36], they will only be briefly described here to cover the subject. In this section, we will mainly focus on the loading methods of drug molecules and collaborative strategies of codelivery systems.
2.1. Loading methods
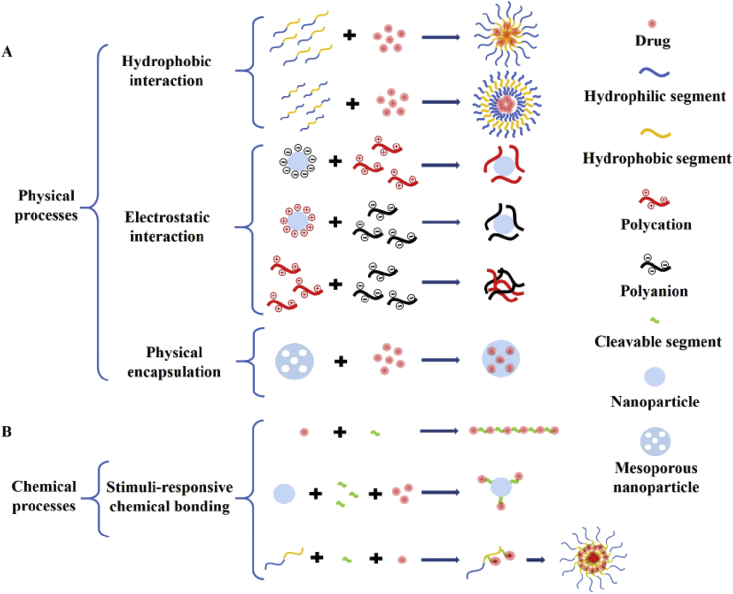
Loading methods can be categorized into physical and chemical processes. The commonly used physical interactions include electrostatic interaction, hydrophobic interaction, and simple encapsulation. Physical process operations are generally straight forward. Simple stirring for a certain period may accomplish the packaging process. However, owing to the unstable encapsulation, premature leakage may occur during the circulation. Chemical processes usually involve multiple chemical reactions and the necessary refinements. Stimuli-responsive bonds or structures should be introduced between the drug molecule and backbone to achieve the controlled release of intact drug molecules. Fig. 1 illustrates the typical loading methods of physical and chemical processes.
Fig. 1.
Illustration of the typical loading methods.
2.1.1. Physical processes
The top three commonly used physical processes are hydrophobic interaction, electrostatic interaction, and simple physical encapsulation. The hydrophobic action is a phenomenon that hydrophobic molecules or segments aggregate to avoid water. Monolayer liposomes and micelles have hydrophobic internal cavities that can hold hydrophobic drugs [37,38]. The bilayer liposomes can be loaded with hydrophobic drugs between the bilayer, and the inner cavity can be loaded with water-soluble drugs. The hydrophilic shell of the vectors increases the biocompatibility and circulation time of the delivery system [39]. Most chemotherapeutic drugs are poorly water-soluble. The first formulation, liposomal DOX, was used clinically in 1995. Since then, many liposomes and lipid-based products have been placed on the market or undergo clinical trials [40]. Liposomes can be designed flexibly [41,42]. As shown in Fig. 2A, Lee et al. [43] prepared a polymer-caged nanobin which comprises a doxorubicin-encapsulated liposomal core protected by a pH-responsive cisplatin prodrug-loaded polymer shell.
Fig. 2.
(A) The liposomes with DOX-encapsulated core and pH-responsive cisplatin prodrug-loaded polymer shell [43]. Copyright 2016 Elsevier B.V. (B) DOX and PTX were simultaneously encapsulated into the TAT/FOL modified micelles (TAT: TAT peptide; FOL: folate) [48]. Copyright 2013, Elsevier B.V. (C) Schematic diagram of drug loading in dendrimer cavities [53]. Copyright 2013, Elsevier Ltd. DOX, doxorubicin; PTX, paclitaxel.
In 1984, polymer micelles were first proposed by Bader and co-workers as drug vectors [44]. Nowadays, different kinds of micelles have been developed as effective vectors with good biodegradability, effective encapsulation, and long circulation in the blood [[45], [46], [47]]. Fig. 2B shows a typical micelle that codeliver DOX and PTX by hydrophobic interaction [48]. The solubility and bioavailability of hydrophobic drugs are improved by this delivery system. Dendrimers possess well-defined, highly branched, and spherical three-dimensional structures [49]. Their hydrophobic interior cavities and large number of reactive end groups enable dendrimers to be a candidate for drug/gene delivery vectors [[50], [51], [52], [53]]. As shown in Fig. 2C, the inner cavity or periphery of the dendrimer can be a suitable host for the guest molecules. Taratula et al. [54] synthesized multifunctional theranostic platforms based on phthalocyanine-loaded dendrimer for image-guided drug delivery and PDT because hydrophobic interactions are non-specific. Unwanted aggregation of hydrophobic molecules may occur during the preparation process, which will reduce the drug loading efficiency and increase the heterogeneity of the formulation [55].
Electrostatic interaction is an equilibrium achieved through the mutual attraction of positive and negative charges. Some drug molecules are electrically charged under physiological conditions. For example, DOX is positively charged under physiological conditions owing to the amino group in its structure [56]. Methotrexate (MTX) is negatively charged in solution because of the ionization of carboxyl groups [57]. Most protein-based reagents are negatively charged in a neutral environment [58]. Some materials also exhibit electrical properties in the physiological environment and can be applied to prepare carriers. Chitosan is electropositive due to the large number of amino groups, and hyaluronic acid is electronegative due to the carboxyl groups. They are widely used in the preparation of biomedical materials [59,60]. Therefore, electrostatic interaction is an alternative intermolecular force that can be used to bind up drug molecules. For instance, as shown in Fig. 3A, the negatively charged new indocyanine green (ICG) (IR820) molecules were trapped on the surface of the cationic micelle [24]. Polyamidoamine dendrimers with positive charges were used to increase the solubility of ketoprofen which is a non-steroidal anti-inflammatory drug [61]. For immunotherapy, the use of electrostatic interaction to deliver antigens is an effective method [58]. In fact, the most widely used area of electrostatic interaction is the preparation of non-viral gene delivery systems [62,63]. The nucleic acids present electronegative due to the dissociation of hydrogen ions of phosphate groups. So nucleic acids can be condensed by positively charged polymer chains or adsorbed on the surface of positively charged nanoparticles. As shown in Fig. 3B, a series of cationic gene carriers were prepared with high transmission efficiency [[62], [63], [64], [65], [66]].
Fig. 3.
(A) The negatively charged new indocyanine green (IR820) molecules are attracted to the cationic micelle carrier for phototherapy and imaging [24]. Copyright 2019, American Chemical Society. (B) Typical cationic gene carriers for cancer therapy. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Drug molecules can also be simply encapsulated in the cavity of the carriers, just like putting the goods into the cardboard box, sealing the box and sending it to the receiving address. Mesoporous silica nanoparticles (MSNs), carbon nanotubes, and other nanoparticles with the suitable pore structures can be used to transport molecules in this way [67,68]. The key to this method is to select suitable lids to plug the cavity. It is necessary to consider a suitable occlusion strategy to prevent the leakage of drugs during the circulation in vivo. As shown in Fig. 4A, core-shell-shellac up-conversion (UC) nanoparticles were designed for simultaneous photo-responsive drug release, PDT, and cell imaging [69]. The nanoparticle consists of an UC core, a silica intermediate shell filled with a photosensitizer, and β-cyclodextrin (β-CD)–gated mesoporous silica outmost shell. Similarly, Liu et al. [70] designed hollow silica mesoporous nanoparticles that facilitate drug delivery by cascading pH stimulation in the tumor microenvironment. The hollow silicon mesoporous nanoparticles are loaded with DOX inside and blocked with PEG-modified β-CD outside as lids. Tactfully, Zeng et al. [71] designed a drug-gated mesoporous antitumor nanoplatform based on pH-sensitive dynamic covalent bonds as shown in Fig. 4B. The doors are blocked by the drug itself through a pH-sensitive dynamic benzoate-imine covalent bond. This strategy cleverly bypasses the use of auxiliary capping agents.
Fig. 4.
(A) Schematic illustration of a core-shell-shell structured nanoparticle which consists of an up-conversion (UC) core, a silica intermediate shell filled with a photosensitizer, and β-cyclodextrin (β-CD)–gated mesoporous silica outmost shell [69]. Copyright 2016, American Chemical Society. (B) Schematic illustration of the DOX-self-gated mesoporous silica nanomaterials with pH-responsive drug release property, and dynamically PEGylated and DOX self-gated mesoporous silica nanomaterials with site-specific drug release and cell uptake at weak acidic tumor tissue/cells [71]. Copyright 2017, WILEY-VCH.
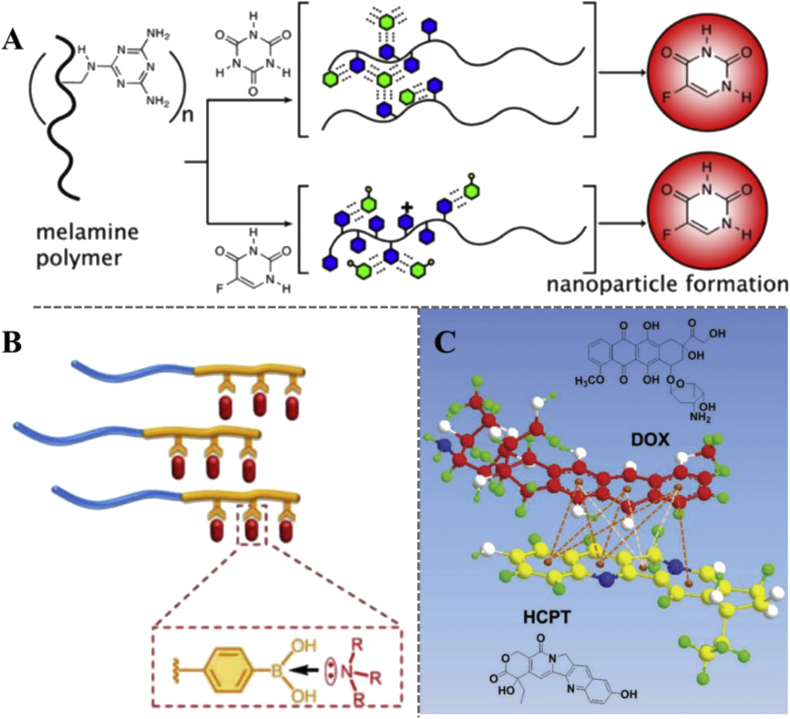
Other physical interactions, such as the hydrogen-bonding [72,73], coordination interaction [74], and π-π stacking [75], are also applied to the preparation of codelivery systems. Because these interactions are directional, special requirements for molecular structure are proposed. For instance, as shown in Fig. 5A, macromolecular assembly with 5-fluorouracil (5-FU) or cyanuric acid (CA) requires specific hydrogen-bonding recognition between 5-FU/CA and polymer-displayed melamine [76]. The coordination interaction is molecular forces between electron donors and acceptors, including π-donor/organometallics or Lewis bases/acids [77]. The bond energy of coordination bonds is generally weaker than covalent bonds but stronger than hydrophobic interactions [78]. Lv et al. [79] prepared a series of micelles with high drug loading capacity by introducing coordination interactions between electron acceptor-containing polymers and electron donor-containing chemotherapy drugs (Fig. 5B). π-π stacking is a special spatial arrangement of aromatic compounds, which refers to a weak interaction that often occurs between aromatic rings. Many chemotherapeutic drugs possess aromatic rings inside the molecules and can be loaded on/in the carrier by π-π stacking interaction [80,81]. As shown in Fig. 5C, Wei et al. [75] conjugated DOX to the amphiphilic polymer chains, and HCPT was loaded along with DOX via π-π stacking interaction.
Fig. 5.
(A) Illustration of assembly into nanoparticles triggered by recognition and encapsulation of cyanuric acid (CA) and 5-fluorouracil (5-FU), shown as green hexagons, by polymer-displayed melamine (blue hexagons) [76]. Copyright 2013, American Chemical Society. (B) Schematic illustration of the encapsulation of drugs via drug-polymer coordination interactions [79]. Copyright 2018, American Chemical Society. (C) Schematic representation of HCPT interact with DOX via π-π stacking interaction [75]. (Light orange dashed lines represent hydrophobic forces by lipophilic moieties. Dark orange dashed lines denote π-π stacking interaction by π-π conjugated architectures.) Copyright 2016, WILEY-VCH. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.1.2. Chemical processes
Chemical processes are performed by the reactions of complementary groups. The functional groups similar to the hydroxyl group (-OH), carboxyl group (-COOH), and amino group (-NH2) could be used to graft drug molecules onto the vectors. It should be noted that, to achieve the controlled release of the carried reagents, cleavable bonds or structures need to be introduced between the drug molecule and the vector. Many indicators in tumor cells are different from normal cells, such as higher reactive oxygen species (ROS) concentration, higher glutathione (GSH) concentration, and relative lower pH [82,83]. The thioketal bond which response to ROS and the disulfide bond which response to GSH have already introduced into some factors for controlled release [84,85]. As shown in Fig. 6, Bai et al. [86] grafted CPT to β-CD with ROS-responsive linker and GSH-responsive linker. The drug release can be controlled by the break of these cleavable bonds. Similarly, Kong et al. [87] combined CPT and NIR fluorophores via disulfide bonds to monitor drug release in vivo. This strategy can use the tumor microenvironment to achieve GSH-responsive release of CPT. High drug loading capacity and precise structures of vectors can be obtained by chemical processes. But, generally, chemical processes are more complex than physical processes. Tedious experimental procedures and purification procedures are required. There is a trade-off between the method and performance.
Fig. 6.
Schematic illustration of the synthesis of highly sensitive prodrug starlike polymer β-CD-b-P(CPTGSH-co-CPTROS-co-OEGMA) (CPGR) based on dual redox-responsive for synergistic chemotherapy [86]. Copyright 2019, American Chemical Society. β-CD, β-cyclodextrin.
2.2. Collaborative strategies
Physical and chemical processes have both strengths and weaknesses. It is necessary to select the appropriate mode or cooperative strategy based on the structure and property of both cargoes and carriers. The collaborative strategies could be a combination of two or more chemotherapeutic drugs, nucleic acids, proteins, and functional molecules. The synergistic action between the loaded reagents needs to be considered in advance. And no matter which combination, there must be no conflict between each component. Proper sequential administration of different drugs may lead to a synergistic effect. For example, the combined therapy of gemcitabine (GEM) and docetaxel (DOC) is order-dependent. The mechanisms of antitumor action and metabolic clearance of DOC and GEM are different. GEM could induce G0/G1 phase block. After 72 h of GEM administration, a large number of tumor cells enter the interphase. A strong synergistic cytotoxic effect by DOC can be obtained. Similarly, combining antimetabolites and taxanes compound in the correct order would transform the antagonistic effect into a synergistic effect [88]. The bonds or structures that could be triggered in the tumor microenvironment needs to be precisely designed. The controlled release of cargoes can be performed in this process. For instance, photosensitizers can be activated to produce ROS for PDT. At the same time, the ROS-responsive bonds that make up the vector could be broken. This cascade will lead to the disintegration of the vectors and realize the following controlled release of the loaded drug molecules [89].
Some components of carriers have special functions. Superparamagnetic nanoparticles, such as Fe3O4 nanoparticles, can be used as contrast agents for magnetic resonance imaging (MRI) or as vectors for the targeted drug delivery [90,91]. Gold nanorods can be used as photothermal agents owing to the photothermal conversion ability. And gold nanoparticles (Au NPs) with high X-ray absorption coefficients are explored as contrast agents for computed tomography (CT) imaging [92]. Using these materials as the matrix, multifunctional vectors, which can be applied for imaging, photothermal therapy (PTT), and PDT, are available. For instance, the combination of gold nanocages with DOX and photosensitizer ICG exerted the simultaneous chemo/photothermal/photodynamic treatment [93]. In general, simply loading multiple cargoes into a carrier is not the goal. The purpose is to amplify the therapeutic effect by collaborating among the components. Besides, the convenience and cost of carrier preparation are also factors to be considered. Table 1 summarized some typical examples of codelivery systems for cancer therapy.
Table 1.
Typical examples of codelivery systems for cancer therapy.
| Vectors | Drug combination | Physical/chemical processes | Therapy model | References |
|---|---|---|---|---|
| Inorganic nanoparticles | DOX & MiR-31 (drug & nucleic acid) | Physical encapsulation, electrostatic interaction | Hela tumor-bearing BALB/c nude mice | [94] |
| Inorganic nanoparticles | Gold nanorod & SF & p53 (photothermal/imaging agent & drug & nucleic acid | Physical encapsulation, electrostatic interaction | Hepatoma-bearing BALB/c nude mice | [95] |
| Inorganic nanoparticles | DOX & TRAIL (drug & cytokine) | π-π stacking interaction, chemical bonding | A549 tumor-bearing BALB/c nude mice | [96] |
| Micelles | CPT & TPP (drug & drug) | Chemical bonding | 4T1 tumor-bearing BALB/c nude mice | [97] |
| Micelles | CPT & IR820 (drug & phototherapy drug) | Chemical bonding, electrostatic interaction | 4T1 tumor-bearing BALB/c nude mice | [24] |
| Micelles | PTX & GFP siRNA (drug & nucleic acid) | Hydrophobic interaction, electrostatic interaction | MDA-MB-435-GFP cells | [98] |
| Micelles | Ir & Cb (drug & drug) | Chemical bonding | MCF-7 tumor-bearing nude mice | [99] |
| Liposomes | DOX & PTX (drug & drug) | Hydrophobic interaction | 4T1 tumor-bearing BALB/c nude mice | [100] |
| Hydrogels | DOX & PTX (drug & drug) | Electrostatic interaction, chemical bonding | B16F10 tumor-bearing mice | [101] |
| Microgels | Au NPs & MTX (photothermal/imaging agent & drug) | Physical encapsulation, chemical bonding | KB cells | [102] |
| Organic nanoparticles | DOX & PTX (drug & drug) | Electrostatic interaction, hydrophobic interaction | A549 tumor-bearing BALB/c nude mice | [33] |
| Organic nanoparticles | SN38&LND (drug & inhibitor) |
Physical encapsulation | 4T1 tumor-bearing BALB/c nude mice | [103] |
| Organic nanoparticles | NY-ESO-1 epitope & CpG (antigen & nucleic acid) | Electrostatic interaction | HLA-A2 transgenic C57BL/6 mice | [58] |
| Organic nanoparticles | DOX & MiR-21 (drug & nucleic acid) | Physical encapsulation, electrostatic interaction | LN229-luc tumor-bearing BALB/c nude mice | [104] |
| Organic nanoparticles | AMD3100 & DNA (antagonist & nucleic acid) | Chemical bonding, electrostatic interaction | CXCR4 & U2OS cells | [105] |
| Organic nanoparticles | Phycocyanin & polypyrrole (photosensitizer & photothermal agent) | Physical encapsulation | MDA-MB-231 & HEK-293 cells | [106] |
Au NPs, gold nanoparticles; DOX, doxorubicin; PTX, paclitaxel; CPT, camptothecin; TPP, triphenylphosphonium bromide; LND, lonidamine.
3. Advances of codelivery systems
Many conventional vectors can be used to build codelivery systems by rational design. The synergistic treatment of two or more drugs could be realized by codelivery systems. This section introduces codelivery systems from two perspectives: different types of vectors and collaborative treatment strategies.
3.1. Different types of vectors
3.1.1. Inorganic nanoparticles
The development of nanotechnology has promoted the production of various inorganic nanoparticles as drug vectors, such as silica nanoparticles [107], iron-based nanoparticles [108], Au NPs [92] and fullerenes [109]. Each nanoparticle has its unique characteristics, which makes the vector versatile.
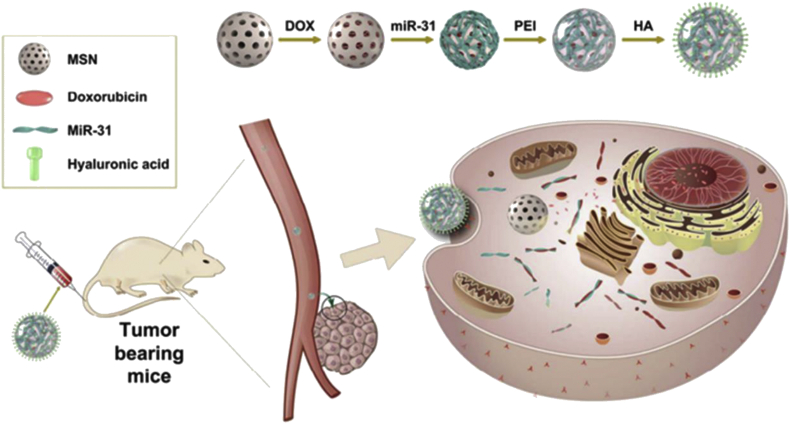
MSNs are used in a variety of nanotechnology applications owing to its high surface area and pore volume [110,111]. The pore structure is orderly, the pore size distribution is single and adjustable, and the mesoporous shape is diverse. A large number of hydroxyl groups on the surface of MSNs can be chemically modified, which provides convenience for subsequent encapsulation of drugs or functionalization. As shown in Fig. 7, a codelivery system based on MSN was reported, where DOX molecules were loaded into the pore of MSNs by physical encapsulation, and microRNAs were conjugated onto the surface via disulfide bonds [94]. MSNs have good biocompatibility but no other special functions. The single function limits the development of MSNs as multifunctional vectors.
Fig. 7.
Schematic diagram of mesoporous silica-based codelivery systems to deliver chemotherapeutic drug and nucleic acid [94]. Copyright 2018, American Chemical Society.
Compared with MSNs and organic nanoparticles, metal-based nanoparticles exhibit many special properties due to the metal bonding, small size effect, and surface effect. Iron oxide nanoparticles with a diameter of 50–100 nm have been already developed as contrast agents for MRI [112]. The ultra-small ultra-paramagnetic iron oxide particles with a diameter of about 10 nm can be used to achieve thermotherapy and active targeted treatment of cancer [113]. For example, as shown in Fig. 8, PTX and curcumin (Cur) were codelivered by pluronic-coated Fe3O4 nanoparticles [114]. Au NPs are widely used as drug and gene delivery vectors due to the good biocompatibility and versatility [115]. After modification, Au NPs can also codeliver a variety of reagents. Shiao et al. [116] designed a kind of aptamer-functionalized Au NPs to codeliver DOX and the photosensitizer 5,10,15,20-tetrakis(1-methylpyridinium-4-yl) porphyrin (TMPyP4) for improving the drug effectiveness. It should be noted that with the decrease of the particle size, some metal-based nanoparticles may interfere with the defense mechanism of antioxidants and thus exhibit higher cytotoxicity [117]. Using inorganic nanoparticles as codelivery carriers often require modification of the nanoparticles. Two or more interactions, whether physical or chemical, may be exploited to achieve the codelivery of reagents.
Fig. 8.
Schematic diagram of codelivering PTX and curcumin (Cur) by pluronic-coated Fe3O4 nanoparticles [114].
3.1.2. Micelles
Amphiphilic copolymers are capable of forming micelles by self-assembly in aqueous solutions. Hydrophilic segments, such as PEG, can extend the circulation time in vivo. And hydrophobic segments, such as poly (l-lysine), can provide space for hydrophobic drugs [33]. Micelles have been considered to be one of the potential drug delivery systems [118,119]. Many polymer micelles have been evaluated in vivo and clinical trials. Hydrophobic drugs usually loaded into the micelles via hydrophobic interactions. For instance, Lv et al. [33] designed a series of micelles to codeliver DOX and PTX, where DOX molecules were encapsulated by electrostatic interaction and PTX molecules were encapsulated hydrophobic interaction. Duong and Yung [48] packaged DOX and PTX into one micelle by hydrophobic interaction for synergistic codelivery. The loading capacity of drugs in conventional micelles is usually low, and therefore it is the pursuit to further improve the loading capacity of micelle-based delivery systems [120].
Some kinds of micelles are composed of polyprodrugs. One highlight of the polyprodrugs is the use of the hydrophobicity of the chemotherapeutic drugs to fabricate carriers. The loading capacity and the stability of the drugs would be increased [85,86,121]. For instance, the hydroxyl group on adjacent mitoxantrone (MTO) molecules can be linked by ROS-responsive cleavage linkers to form polyMTO [84]. The polyprodrugs similar to a cluster bomb, which will improve drug accumulation at the tumor site and the reduce side effects along with chemotherapy. As shown in Fig. 9, CPT and triphenylphosphonium bromide (TPP) molecules can be grafted onto the dextran backbone to achieve dual drug delivery. Huang et al. [99] connected hydrophobic anticancer drug chlorambucil (Cb) and hydrophilic anticancer drug irinotecan (Ir) through a hydrolyzable ester bond to make an amphiphilic prodrug, which then formed micelles. This nanoparticle is composed entirely of anticancer drugs. Two kinds of anticancer drugs were delivered together to achieve synergistic treatment and overcome the MDR of tumors. Cong et al. [122] designed a dual sensitive dual drug backboned shattering polymer (DDBSP) which composed of a PP2A inhibitor demethylcantharidin and cisplatin. DDBSP self-assembled micelle can be triggered intracellularly to break down in a chain-shattering manner to release the dual drugs payload (Fig. 10). Using the hydrophobic cavity inside the micelle that structured by polyprodrugs to carry drugs is an alternative way to design codelivery systems [123].
Fig. 9.
Schematic diagram of mitochondrial-targeted camptothecin (CPT) and triphenylphosphonium bromide (TPP) polyprodrug system (MCPS) composed of dextran-P (OEGMA-co-CPT-co-TPP) (DCT) amphiphilic polyprodrug [97]. Copyright 2019, American Chemical Society.
Fig. 10.
Schematic illustration of dual drug backboned shattering polymeric theranostic nanomedicine (DDBSP) for synergistic eradication of patient-derived lung cancer (PDLC) [122]. Copyright 2018, WILEY-VCH.
3.1.3. Liposomes
Liposomes are phospholipid vesicles formed by lipid bilayer membranes. Vesicles have isolated hydrophilic and lipophilic phase spaces. Hydrophilic drugs can be encapsulated in the inner aqueous phase, whereas hydrophobic drugs can be encapsulated in the lipid layer [124,125]. A number of liposome delivery systems are on the market such as conventional doxorubicin liposomes (e.g., Evacet, Myocet), long-cycle doxorubicin liposomes (e.g., Doxil, caelyx), cytarabine liposomes (e.g., Depocyt), and paclitaxel liposomes (e.g., Taxo). Fig. 11 illustrates the structure of a typical liposome drug delivery system [126]. Liu et al. [100] designed a multilayer liposome vesicle that can improve the loading efficiency and sustained release of DOX and PTX, maximizing the combined therapeutic effect and minimizing the systemic toxicity. Liposomes can also be loaded with dyes or imaging agents. Sheng et al. [127] used nanoliposomes to carry perfluorooctyl bromide and ICG for enhanced multimodal imaging-guided phototherapy. This strategy combines CT contrast imaging, PDT, and PTT. Although many liposome drug systems have been proposed, the low drug loading capacity and poor stability hinder the large-scale application of liposomes.
Fig. 11.
Schematic representation of functionalized dual recognition peptide (STP-LS) liposomes encapsulating DOX [119]. Copyright 2016, American Chemical Society.
3.1.4. Hydrogels
Owing to their adjustable chemical and physical properties, hydrogels have been vigorously developed as biomaterials [[128], [129], [130]]. Drugs or reagents can be encapsulated directly in hydrogels. The engineered hydrogels are increasingly considered as promising tools for transporting chemotherapeutic drugs and immunotherapeutics, with decreased systemic toxicities. As shown in Fig. 12, Zhao et al. [101] synthesized an injectable hydrogel with hydrophobic microdomains by glycol chitosan and benzaldehyde capped poly(ethylene glycol)-b-poly(propylene glycol)-b-poly(ethylene glycol) (PEO-PPO-PEO). DOX and PTX are physically encapsulated inside this hydrogel. The tumor microenvironment-responsive crosslinkers gives hydrogels the ability to release drugs in a controlled manner [131]. Zhang et al. [132] prepared various polyplexes with pentablock copolymer micelle (PB) and Pluronic F127 (PL) which can condense plasmid DNA (pDNA) and encapsulate PTX. Then a synthetic barrier gel based on poly(ethylene glycol) diacrylate was developed to enable the released vectors to instantly and continuously transfect cultured cells.
Fig. 12.
Illustration of the injectable hydrogels containing DOX and PTX [101]. Copyright 2011, Elsevier B·V. DOX, doxorubicin; PTX, paclitaxel.
Hydrogels with micron or nanometer size, which termed as microgels and nanogels, also have important applications as vectors [133]. In addition to having the usual properties of hydrogels, microgels and nanogels are more suitable as carriers owing to their small size effect. As shown in Fig. 13, Lu et al. [102] synthesized a kind of polyacrylamide hybrid nanogels for targeted cancer chemotherapy via codelivery of Au NPs and MTX. Au NPs can be easily incorporated into/onto polyacrylamide (PAm) nanogels, whereas MTX was chemically conjugated to and physically adsorbed on Au-PAm hybrid nanogels. Delivery systems derive from microgels and nanogels can combine chemotherapy and gene therapy. Costa et al. [134] developed a new p53-encoded pDNA microgel with porosity, biocompatibility, and photodegradability, which is suitable for the dual release of pDNA and anticancer drug DOX through ultraviolet light irradiation. Microgels and nanogels exhibit outstanding design ability by combining the advantages of the nanocarrier and hydrogel.
Fig. 13.
Schematic illustration of the synthesis of gold/polyacrylamide (Au-Pam) hybrid nanogels and the mechanism of targeted codelivery of Au NPs and drug via receptor-mediated endocytosis [102]. Copyright 2013, Elsevier Inc. (For interpretation of the references to /colour in this figure legend, the reader is referred to the Web version of this article.) Au NPs, gold nanoparticles.
3.1.5. Other organic nanoparticles
In addition to the vectors mentioned previously, many types of organic nanoparticles, such as dendrimers, hyperbranched polymers, polymer mesoporous microspheres, and some complexes, are also developed as delivery systems [[135], [136], [137], [138]]. Dendrimers and hyperbranched polymers have hydrophobic cavities that are suitable for loading cargoes. Plenty of functional groups on the surface provide convenience for the subsequent modification. For example, Qian et al. [104] prepared star-branched amphiphilic copolymers for codelivery of DOX and miRNA (miR-21i) (Fig. 14). DOX was loaded into the hydrophobic core of the branched copolymer through hydrophobic interaction, and the outer layer was provided positive charges by polydimethylaminoethyl methacrylate (PDMAEMA) for binding the miR-21i. Dendrimers are branched macromolecules with many arms protruding from the central core [135]. Although the dendrimer is clear in structure and adjustable in size, its complex preparation process limits its wide application. The construction of codelivery systems using polymer mesoporous microspheres is similar to that of MSNs. Cargoes can be physically encapsulated in pores [139].
Fig. 14.
(A) Illustration of codelivery of DOX and miR-21i by star-branched amphiphilic copolymers comprising polylactic acid (PLA) and polydimethylaminoethyl methacrylate (PDMAEMA) [104]. Copyright 2013, Elsevier Ltd.
Complexes at the nanoscale are usually formed by entanglement of polymer chains. Many polymer gene vectors are formed by the complexation of cationic polymer chains with nucleic acid [140]. The polyelectrolyte complexes protect nucleic acids from degradation and promote transport across cell membranes. To achieve the joint transmission of genes and other reagents, several physical or chemical processes would be applied together in addition to electrostatic interaction. As shown in Fig. 15, Oupický et al. [105] used AMD3100, a cyclam derivative as CXC chemokine receptor 4 (CXCR4) inhibitor, and disulfide-containing bisacrylamide to form cationic polymer chains. Because there are four nitrogen atoms in the center of the ring structure, the polycations, named RPA, can provide positive charges to condense with DNA. This strategy used drugs to structure polycations by a chemical process, and pDNA was loaded by a physical process. Chen et al. [141] synthesized the amphiphilic graft polymer polyethyleneimine-ferrocene (PEI-Fc), which can form micelles in aqueous solution through the hydrophobic side groups of ferrocenes. The oil/water (O/W) method was used to load DOX into micelles. DNA molecule were complexed by PEI through electrostatic interactions. Oh et al. [106] fabricated polypyrrole (PPy) nanoparticles by using bovine serum albumin-phycocyanin (Pc) complex, where PPy and Pc endow PTT and PDT to the nanoparticles respectively.
Fig. 15.
Mechanism of action of dual-function polycations (RPA) as CXCR4 antagonists and gene-delivery vectors [105]. Copyright 2012, WILEY-VCH.
In addition to the conventional vectors mentioned above, some new kinds of vectors have been proposed [142,143]. For example, Gu et al. [144] reported Trojan horse-like injectable engineered adipocytes that can serve as a drug-delivery depot for sustained drug release with suppressed primary tumor growth and postsurgical tumor recurrence. As shown in Fig. 16, the adipocytes were engineered with the encapsulation of rumenic acid, as an anticancer fatty acid, and a ROS-responsive DOX prodrug for chemotherapy and simultaneously inducing an immunogenic tumor phenotype. This section is discussed based on different types of vectors. One drug can be delivered by different types of vectors. To improve the codelivery efficiency of multiple drugs, it is important to select and design the vector reasonably.
Fig. 16.
Adipocytes were engineered for codelivery of rumenic acid and ROS-responsive DOX prodrug [144]. Copyright 2019, Elsevier Inc. ROS, reactive oxygen species.
3.2. Collaborative treatment strategies
In many cases, the purpose of constructing the codelivery systems is to combine different treatment strategies. In addition to chemotherapy, other therapeutic strategies, such as gene therapy, PDT, PTT, and immunotherapy, are also effective in tumor treatment. This requires the vectors can deliver multiple functional reagents, such as antibodies [145], nucleic acids [146], photosensitizer [147]. To observe the interactions between drugs and diseases, it is necessary to bring therapy and diagnosis together. Simultaneously delivering of imaging agents, including optically active small molecules, metals and metal oxides, ultrasonic contrast agents, and radionuclides, is required [148].
3.2.1. Combination of multiple chemotherapy strategies
Since the 1960s, combined chemotherapy with two or more chemotherapeutic drugs has been shown to be an effective strategy for cancer therapy [149]. Goldman et al. [150] demonstrated that two-in-one nanomedicine can terminate the origin of adaptive resistance by ensuring the delivery of two drugs with limited deterministic space to target cells, thereby inducing greater antitumor efficacy. In contrast, free drugs or two nanoparticles (each carry one drug) were less effective at binding because of the random distribution of cells. These findings suggested that two-in-one nanomedicines may become an important strategy for targeting adaptive resistance.
The combination of multiple chemotherapy strategies would reduce the side effects caused by chemotherapy drugs, delay the appearance of MDR, and improve the treatment effect [21]. The strategy for the combined administration of chemotherapeutic drugs depends on the antitumor mechanism of each drug. For example, DOX and PTX codelivery strategies have developed rapidly in recent years. DOX is one of the most effective anthracycline antitumor drugs. It can interfere with DNA by insertion and then induce apoptosis in cancer cells. PTX is a representative antimicrotubule agent [151]. PTX specifically acts on the G2 and M phases of the cell cycle, making microtubules unable to form spindles and spindle filaments during mitosis, and preventing tumor cells from dividing and reproducing. Chemotherapy enhancement was observed by administering PTX and DOX in a reasonable order.
Yang et al. [103] synthesized a kind of ROS-sensitive PEGylated bilirubin nanoparticles (BRNPs) to encapsulate dimer-7-ethyl-10-hydroxycamptothecin (d-SN38) and dimer-lonidamine (d-LND). As shown in Fig. 17, dimerization of the drugs significantly increases the drug loading capacity and the encapsulation efficiency of nanoparticles. SN38, which is an active metabolite of CPT in vivo, can act on DNA topoisomerase to kill cancer cells [[152], [153], [154]], whereas hexokinase inhibitor LND exhibits antitumor activity by affecting energy metabolism [155]. This collaborative treatment strategy strongly avoided the non-specific drug action. Similarly, Du et al. [156] fabricated a dual drug-paired polyprodrug nanoparticle (DOX, 25 wt%; cisplatin (CDDP), 12.5 wt%). This work provides an innovative strategy for reversing MDR and combating DOX-resistant breast and CDDP-resistant ovarian cancers. As the mechanism of antitumor action of various drugs becomes clear, more collaborative therapies are proposed, which accelerate the design and preparation process of novel codelivery systems.
Fig. 17.
The schematic illustration of the construction, drug delivery and tumor response of PEGylated bilirubin nanoparticles loaded with dimer-7-ethyl-10-hydroxycamptothecin (d-SN38) and dimer-lonidamine (d-LND) (SL@BRNPs) [103]. Copyright 2019, WILEY-VCH.
3.2.2. Combination of chemotherapy and gene therapy
Gene therapy refers to the introduction of foreign normal genes into target cells to correct or compensate the defective and abnormal genes [[157], [158], [159]]. Therapeutic genes, including pDNA, miRNA, and siRNA, play important roles in the regulation of cell development, differentiation, metabolism, and apoptosis. To realize the codelivery of chemotherapeutic drugs and genes, the commonly used method is to encapsulate chemotherapeutic drugs in the carrier and adsorb genes on the surface [[160], [161], [162], [163]]. Zhu et al. [98] reported on cationic micelles based on well-defined PDMAEMA-PCL-PDMAEMA triblock copolymers for the combinatorial delivery of siRNA and PTX. The zeta potential of the prepared cationic micelles is from +29.3 to +35.5 mV, which is suitable to complex with siRNA. PTX molecules are loaded in the hydrophobic cavity of the micelles. Rattle-structured rough nanocapsules (Au@HSN-PGEA, AHPs) composed of in situformed Au NR cores and polycationic mesoporous silica shells were constructed for trimodal complementary cancer therapy [95]. As shown in Fig. 18, the outer shell of the nanorattles was functionalized with a superior polycation, CD-PGEA (two-armed ethanolamine-functionalized poly(glycidyl methacrylate) with one β-CD core) to carry antioncogene p53 for gene therapy. The interior space around the Au NR cores was reserved for sorafenib (SF, a hydrophobic antiproliferative and antiangiogenic drug) loading. A well-designed combination of chemotherapy and gene therapy can produce synergies to improve the effectiveness of cancer treatment and push back MDR. As more and more therapeutic genes are proposed, the combination of gene therapy with other therapeutic strategies is being widely studied.
Fig. 18.
Schematic diagram of nanocapsules containing antioncogene p53 and sorafenib and the drug/gene codelivery process [95]. Copyright 2018, American Chemical Society.
3.2.3. Combination of chemotherapy and immunotherapy
In recent years, immunotherapy has great prospects for inhibiting metastatic cancer [164]. There were previous reports that the combination of chemotherapy and immunotherapy may result in enhanced anticancer effects and can be used to promote an immunogenic tumor phenotype [[165], [166], [167], [168]]. As shown in Fig. 19, Wang et al. [169] engineered a therapeutic hydrogel that, when formed in situ, allows the local release of GEM and an anti–PD-L1 blocking antibody (aPDL1) with distinct release kinetics. This strategy promotes immune-mediated tumor regression in the tumor-bearing mice, with the prevention of tumor recurrence after primary resection. Similarly, using this therapeutic hydrogel, codelivery of aPD1 and Zebularine (Zeb), an epigenetic modulator which can enhance the antitumor immune response by inducing the expression of tumor-associated antigens, was achieved [170]. The combination of chemotherapy and immunotherapy also increased the therapeutic effect of chemotherapy-insensitive tumors. For example, docetaxel (DTX), a chemotherapeutic agent, and cholesterol-modified Toll-like receptor 9 (TLR9) agonist CpG (cho-CpG) oligonucleotide are coloaded in synthetic high-density lipoprotein nanoparticles (sHDL) nanodiscs for chemoimmunotherapy of colon adenocarcinoma. This collaborative treatment strategy can be used to improve survival outcomes significantly compared with chemotherapy alone.
Fig. 19.
Schematic diagram of in-situ formation of ROS-responsive scaffold for local release of GEM and an anti–PD-L1 blocking antibody (aPDL1) with distinct release kinetics [169]. Copyright 2018, American Association for the Advancement of Science. GEM, gemcitabine; ROS, reactive oxygen species.
3.2.4. Combination of chemotherapy and PTT/PDT
PTT and PDT, are the two most important phototherapy strategies. Copper sulfide NPs [171], gold NPs [172], graphene oxide [173], and carbon nanotubes [174] are commonly used for PTT. Chlorine e6 (Ce6) [175], ICG [176], and rose bengal [177] are widely used for PDT. Under the irradiation of a particular wavelength laser, photosensitizers can convert light energy into thermal energy (>42 °C) or ROS. Tumor cells will be destroyed in this abnormal environment. The combination of phototherapy and chemotherapy has been shown to be feasible and effective. As shown in Fig. 20, Mao et al. [178] developed cyclic cRGDfk peptide and Ce6 conjugated silk fibroin–based nanoparticles for 5-FU delivery and PDT. Chemotherapy and PDT are well combined. Liu et al. [179] constructed an azobenzene (AZO)-containing conjugated polymer-based nanocarrier for codelivery of CPT and Ce6. Under 670 nm laser irradiation, Ce6 generated ROS for PDT and induced tumor hypoxia. The hypoxia-responsive drug release occurred subsequently for chemotherapy. Yu et al. [93] developed a chemotherapeutic agent and photosensitizer corelease system which was constructed by filling the interior of gold nanocages (AuNCs) with DOX, ICG and 1-tetradecanol, and modifying the surface with biotinylated poly (ethylene glycol) via Au-S bonds. Under the irradiation of 808 nm laser, AuNCs will heat up rapidly and release DOX and ICG simultaneously. Some ROS-responsive linkers can be introduced into the carrier to achieve controlled release of cargoes [180].
Fig. 20.
Schematic diagram of cRGDfk and Chlorin e6 (Ce6) conjugated silk fibroin nanoparticles (SF NPs) for 5-FU delivery and PDT [178]. Copyright 2018, Elsevier Ltd.
Chemotherapy can also be used in conjunction with other therapies through codelivery vectors, such as synergistic chemotherapy and protein therapy [181,182]. In addition to chemotherapy, combinations of different treatment strategies, such as PDT/PTT combined therapy, have been shown to be effective [106,183]. More radically, Chang et al. [184] constructed a multifunctional cascade bioreactor based on hollow mesoporous Cu2MoS4 loaded with glucose oxidase for synergetic cancer therapy by chemo-dynamic therapy/starvation therapy/phototherapy/immunotherapy. As can be seen from the aforementioned example, collaborative treatment strategies achieved by codelivery systems are promising treatments for cancer.
4. Conclusions and perspectives
To achieve ideal therapeutic effect of tumor treatment, the combination of several drugs or strategies had been proposed. The codelivery system is the key to realize combined therapies. The different structures of drugs and vectors bring a variety of directions to the design of codelivery systems. The interaction between cargoes and carriers, as well as the synergistic action between loaded reagents should be fully considered. It is necessary to select appropriate modes or cooperative strategies in accordance with the structure and property of cargoes and carriers. Encapsulating of reagents into vectors by physical processes is usually easy to operate. However, the stability of such kind of delivery systems needs to be explored in detail. The delivery systems constructed by chemical processes are relatively stable. Concomitantly, chemical processes usually involve multiple chemical reactions, where the way of drug release has to be precisely designed. Stimuli-responsive chemical bonds or structures should be introduced between drug molecules and backbones to achieve the controlled release of drugs. This review presented the typical design considerations about codelivery vectors for cancer therapy and described their current states from two aspects: different types of vectors and collaborative treatment strategies. As shown in Fig. 21, the first aspect focuses on the design and construct of vectors, whereas the latter focuses on the combined application of different treatment strategies.
Fig. 21.
Codelivery systems and collaborative treatment strategies.
Synergies between different drugs or strategies have been have exhibited excellent therapeutic effects via codelivery systems. The integration of diagnosis and treatment could be realized by carefully selecting constitute substrates. In the meantime, many obstacles still need to overcome. In the design process of the vectors, the advantages and disadvantages of the matrix should be emphasized. For example, although metal-based nanoparticles have appended functions, they are usually modified to serve as vectors, and their biocompatibility needs to be improved. Much attention should be paid to the potential inter-restriction of each part of codelivery systems, including matrixes and cargoes. A delicate equilibrium between each part requires precise regulation. Besides, the degraded components of vectors should have no negative influences on systems. For the future development of codelivery systems, more definite pharmacological actions and new strategies provide infinite possibilities for the design and preparation of codelivery systems.
Based on laboratory research, multiple vectors and strategies are available to prepare codelivery with ideal performances. However, owing to the physiological and pathological differences between humans and rodents, even the difference of social attributes, the treatment plan that relatively effective in rodents may not necessarily efficacy in a human trial. For clinical application, administration methods and many stringent biosafety assessments of vectors need to be assessed. In addition, controlling of production cost and simplifying of preparation processes are also important factors from laboratory to clinic. Therefore, the design of codelivery systems for cancer therapy should be considered holistically. Accompanied by the introduction of novel technologies, such as the application of molecular machines in the field of drug delivery systems, more codelivery systems with promising performances and therapeutic effects will be proposed.
Author contributions
Q.Y.M. and H.L.C. contributed to writing of the manuscript and also in the preparation of the original draft. H.H. and F.J.X. contributed to writing, reviewing, and editing of the manuscript. F.J.X. supervised.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (51703105 and 51829301), Natural Science Foundation of Shandong Province (ZR2017BEM012), China Postdoctoral Science Foundation (2018M630752), Beijing Outstanding Young Scientist Program (BJJWZYJH01201910010024), and Fundamental Research Funds for the Central Universities (XK1802-2).
Contributor Information
H. Hu, Email: huhao@qdu.edu.cn.
F.-J. Xu, Email: xufj@mail.buct.edu.cn.
References
- 1.Siegel R.L., Miller K.D., Fedewa S.A., Ahnen D.J., Meester R.G.S., Barzi A., Jemal A. Colorectal cancer statistics, 2017, Ca-Cancer. J. Clin. 2017;67:177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 2.Ellis C.N., Ellis M.B., Blakemore W.S. Effect of adriamycin on heart mitochondrial DNA. Biochem. J. 1987;245(1):309–312. doi: 10.1042/bj2450309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coates A., Abraham S., Kaye S.B., Sowerbutts T., Tattersall M.H.N. On the receiving end-patient perception of the side-effects of cancer chemotherapy. Eur. J. Cancer Clin. Oncol. 1983;19:203–208. doi: 10.1016/0277-5379(83)90418-2. [DOI] [PubMed] [Google Scholar]
- 4.Torchilin V.P. Structure and design of polymeric surfactant-based drug delivery systems. J. Contr. Release. 2001;73:137–172. doi: 10.1016/S0168-3659(01)00299-1. [DOI] [PubMed] [Google Scholar]
- 5.Centelles M.N., Wright M., So P.W., Amrahli M., Xu X.Y., Stebbing J., Miller A.D., Gedroyc W., Thanou M. Image-guided thermosensitive liposomes for focused ultrasound drug delivery: using NIRF-labelled lipids and topotecan to visualise the effects of hyperthermia in tumours. J. Contr. Release. 2018;280:87–98. doi: 10.1016/j.jconrel.2018.04.047. [DOI] [PubMed] [Google Scholar]
- 6.Zhu D., Fan F., Huang C., Zhang Z., Qin Y., Lu L., Wang H., Jin X., Zhao H., Yang H., Zhang C., Yang J., Liu Z., Sun H., Leng X., Kong D., Zhang L. Bubble-generating polymersomes loaded with both indocyanine green and doxorubicin for effective chemotherapy combined with photothermal therapy. Acta Biomater. 2018;75:386–397. doi: 10.1016/j.actbio.2018.05.033. [DOI] [PubMed] [Google Scholar]
- 7.Sanson C., Schatz C., Le Meins J.F., Soum A., Thévenot J., Garanger E., Lecommandoux S. A simple method to achieve high doxorubicin loading in biodegradable polymersomes. J. Contr. Release. 2010;147:428–435. doi: 10.1016/j.jconrel.2010.07.123. [DOI] [PubMed] [Google Scholar]
- 8.Revia R.A., Zhang M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: recent advances, Mater. Today Off. 2016;19:157–168. doi: 10.1016/j.mattod.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng C., Xu X., Tashi D., Wu Y., Su B., Zhang Q. Co-administration of biocompatible self-assembled polylactic acid–hyaluronic acid block copolymer nanoparticles with tumor-penetrating peptide-iRGD for metastatic breast cancer therapy. J. Mater. Chem. B. 2018;6:3163–3180. doi: 10.1039/C8TB00319J. [DOI] [PubMed] [Google Scholar]
- 10.Rossner C., Zhulina E.B., Kumacheva E. Staged surface patterning and self-assembly of nanoparticles functionalized with end-grafted block copolymer ligands. Angew. Chem. Int. Ed. 2019;58:9269–9274. doi: 10.1002/anie.201904430. [DOI] [PubMed] [Google Scholar]
- 11.Fan W., Zhang L.Y., Li Y.W., Wu H.X. Recent progress of crosslinking strategies for polymeric micelles with enhanced drug delivery in cancer therapy. Curr. Med. Chem. 2019;26:2356–2376. doi: 10.2174/0929867324666171121102255. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y., Zhang L., Liu Y., Tan S., Qu R., Wu Z., Zhou Y., Huang J. Preparation of PGA–PAE-micelles for enhanced antitumor efficacy of cisplatin. ACS Appl. Mater. Interfaces. 2018;10:25006–25016. doi: 10.1021/acsami.8b04259. [DOI] [PubMed] [Google Scholar]
- 13.Guan J., Zhou Z.Q., Chen M.H., Li H.Y., Tong D.N., Yang J., Yao J., Zhang Z.Y. Folate-conjugated and pH-responsive polymeric micelles for target-cell-specific anticancer drug delivery. Acta Biomater. 2017;60:244–255. doi: 10.1016/j.actbio.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 14.Tian T., Zhang H.X., He C.P., Fan S., Zhu Y.L., Qi C., Huang N.P., Xiao Z.D., Lu Z.H., Tannous B.A., Gao J. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137–149. doi: 10.1016/j.biomaterials.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Pitt J.M., André F., Amigorena S., Soria J.C., Eggermont A., Kroemer G., Zitvogel L. Dendritic cell-derived exosomes for cancer therapy. J. Clin. Invest. 2016;126:1224–1232. doi: 10.1172/JCI81137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun W., Jiang H., Wu X., Xu Z., Yao C., Wang J., Qin M., Jiang Q., Wang W., Shi D., Cao Y. Strong dual-crosslinked hydrogels for ultrasound-triggered drug delivery. Nano Res. 2019;12:115–119. doi: 10.1007/s12274-018-2188-4. [DOI] [Google Scholar]
- 17.Cheng J., Amin D., Latona J., Heber-Katz E., Messersmith P.B. Supramolecular polymer hydrogels for drug-induced tissue regeneration. ACS Nano. 2019;13:5493–5501. doi: 10.1021/acsnano.9b00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harmeet K., Sapna D.D., Virender K., Pooja R., Jasbir S. Heterocyclic drug-polymer conjugates for cancer targeted drug delivery, Anti-Cancer Agents. Med. Chem. 2016;16:1355–1377. doi: 10.2174/1871520615666160504094044. [DOI] [PubMed] [Google Scholar]
- 19.Feng Q., Tong R. Anticancer nanoparticulate polymer-drug conjugate. Bioeng. Transl. Med. 2016;1:277–296. doi: 10.1002/btm2.10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho H.S., Dong Z., Pauletti G.M., Zhang J., Xu H., Gu H., Wang L., Ewing R.C., Huth C., Wang F., Shi D. Fluorescent, superparamagnetic nanospheres for drug storage, targeting, and imaging: a multifunctional nanocarrier system for cancer diagnosis and treatment. ACS Nano. 2010;4:5398–5404. doi: 10.1021/nn101000e. [DOI] [PubMed] [Google Scholar]
- 21.Wang H., Wu Y., Zhao R., Nie G. Engineering the assemblies of biomaterial nanocarriers for delivery of multiple theranostic agents with enhanced antitumor efficacy. Adv. Mater. 2013;25:1616–1622. doi: 10.1002/adma.201204750. [DOI] [PubMed] [Google Scholar]
- 22.Wang H., Zhao Y., Wu Y., Hu Y.-l., Nan K., Nie G., Chen H. Enhanced anti-tumor efficacy by co-delivery of doxorubicin and paclitaxel with amphiphilic methoxy PEG-PLGA copolymer nanoparticles. Biomaterials. 2011;32:8281–8290. doi: 10.1016/j.biomaterials.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 23.Xiang R.S., Cai Z., Zheng Y., He G., Cui F.Y., Gong D.Q., Hou S.X., Xiong S.J., Lei X.J., Wei Y.Q. Reversion of multidrug resistance by co-encapsulation of vincristine and verapamil in PLGA nanoparticles. Eur. J. Pharmaceut. Sci. 2009;37:300–305. doi: 10.1016/j.ejps.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 24.Shi X., Ma X., Ren E., Zhang Y., Jia D., Gao Y., Xue P., Kang Y., Liu G., Xu Z. Tumor-microenvironment-activatable nanoreactor based on a polyprodrug for multimodal-imaging-medicated enhanced cancer chemo/phototherapy. ACS Appl. Mater. Interfaces. 2019;11:40704–40715. doi: 10.1021/acsami.9b16054. [DOI] [PubMed] [Google Scholar]
- 25.Aji Alex M.R., Nehate C., Veeranarayanan S., Kumar D.S., Kulshreshtha R., Koul V. Self assembled dual responsive micelles stabilized with protein for co-delivery of drug and siRNA in cancer therapy. Biomaterials. 2017;133:94–106. doi: 10.1016/j.biomaterials.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 26.Zhu K., Liu G., Hu J., Liu S. Near-infrared light-activated photochemical internalization of reduction-responsive polyprodrug vesicles for synergistic photodynamic therapy and chemotherapy. Biomacromolecules. 2017;18:2571–2582. doi: 10.1021/acs.biomac.7b00693. [DOI] [PubMed] [Google Scholar]
- 27.Sun Q., Zhou Z., Qiu N., Shen Y. Rational design of cancer nanomedicine: nanoproperty integration and synchronization. Adv. Mater. 2017;29:1606628. doi: 10.1002/adma.201606628. [DOI] [PubMed] [Google Scholar]
- 28.Li N., Zhao L., Qi L., Li Z., Luan Y. Polymer assembly: promising carriers as co-delivery systems for cancer therapy. Prog. Polym. Sci. 2016;58:1–26. doi: 10.1016/j.progpolymsci.2015.10.009. [DOI] [Google Scholar]
- 29.Mujokoro B., Adabi M., Sadroddiny E., Adabi M., Khosravani M. Nano-structures mediated co-delivery of therapeutic agents for glioblastoma treatment: a review. Mater. Sci. Eng. C. 2016;69:1092–1102. doi: 10.1016/j.msec.2016.07.080. [DOI] [PubMed] [Google Scholar]
- 30.Reza Baradaran E., Niloufar M., Shabnam S., Ali Z., Farid Abedin D. Co-delivery nanosystems for cancer treatment: a review. Pharm. Nanotechnol. 2019;7:90–112. doi: 10.2174/2211738507666190321112237. [DOI] [PubMed] [Google Scholar]
- 31.Xu F., Zhong H., Chang Y., Li D., Jin H., Zhang M., Wang H., Jiang C., Shen Y., Huang Y. Targeting death receptors for drug-resistant cancer therapy: codelivery of pTRAIL and monensin using dual-targeting and stimuli-responsive self-assembling nanocomposites. Biomaterials. 2018;158:56–73. doi: 10.1016/j.biomaterials.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 32.Ma H., He C., Cheng Y., Li D., Gong Y., Liu J., Tian H., Chen X. PLK1shRNA and doxorubicin co-loaded thermosensitive PLGA-PEG-PLGA hydrogels for osteosarcoma treatment. Biomaterials. 2014;35:8723–8734. doi: 10.1016/j.biomaterials.2014.06.045. [DOI] [PubMed] [Google Scholar]
- 33.Lv S., Tang Z., Li M., Lin J., Song W., Liu H., Huang Y., Zhang Y., Chen X. Co-delivery of doxorubicin and paclitaxel by PEG-polypeptide nanovehicle for the treatment of non-small cell lung cancer. Biomaterials. 2014;35:6118–6129. doi: 10.1016/j.biomaterials.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 34.Nie X., Zhang J., Xu Q., Liu X., Li Y., Wu Y., Chen C. Targeting peptide iRGD-conjugated amphiphilic chitosan-co-PLA/DPPE drug delivery system for enhanced tumor therapy. J. Mater. Chem. B. 2014;2:3232–3242. doi: 10.1039/C3TB21744B. [DOI] [PubMed] [Google Scholar]
- 35.Temming K., Schiffelers R.M., Molema G., Kok R.J. RGD-based strategies for selective delivery of therapeutics and imaging agents to the tumour vasculature. Drug Resist. Updates. 2005;8:381–402. doi: 10.1016/j.drup.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Wang M., Wang J., Li B., Meng L., Tian Z. Recent advances in mechanism-based chemotherapy drug-siRNA pairs in co-delivery systems for cancer: a review. Colloids Surf. B. 2017;157:297–308. doi: 10.1016/j.colsurfb.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Fan L., Li F., Zhang H., Wang Y., Cheng C., Li X., Gu C.H., Yang Q., Wu H., Zhang S. Co-delivery of PDTC and doxorubicin by multifunctional micellar nanoparticles to achieve active targeted drug delivery and overcome multidrug resistance. Biomaterials. 2010;31:5634–5642. doi: 10.1016/j.biomaterials.2010.03.066. [DOI] [PubMed] [Google Scholar]
- 38.Bouarab L., Maherani B., Kheirolomoom A., Hasan M., Aliakbarian B., Linder M., Arab-Tehrany E. Influence of lecithin-lipid composition on physico-chemical properties of nanoliposomes loaded with a hydrophobic molecule. Colloids Surf. B. 2014;115:197–204. doi: 10.1016/j.colsurfb.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 39.Kang L., Gao Z., Huang W., Jin M., Wang Q. Nanocarrier-mediated co-delivery of chemotherapeutic drugs and gene agents for cancer treatment. Acta Pharm. Sin. B. 2015;5:169–175. doi: 10.1016/j.apsb.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zou H., Wang Z., Feng M. Nanocarriers with tunable surface properties to unblock bottlenecks in systemic drug and gene delivery. J. Contr. Release. 2015;214:121–133. doi: 10.1016/j.jconrel.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 41.Naseri N., Valizadeh H., Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Adv. Pharmaceut. Bull. 2015;5:305–313. doi: 10.15171/apb.2015.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bozzuto G., Molinari A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015;10:975–999. doi: 10.2147/IJN.S68861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee S.M., O'Halloran T.V., Nguyen S.T. Polymer-caged nanobins for synergistic cisplatin-doxorubicin combination chemotherapy. J. Am. Chem. Soc. 2010;132:17130–17138. doi: 10.1021/ja107333g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones M.C., Leroux J.C. Polymeric micelles-a new generation of colloidal drug carriers. Eur. J. Pharm. Biopharm. 1999;48:101–111. doi: 10.1016/S0939-6411(99)00039-9. [DOI] [PubMed] [Google Scholar]
- 45.Yeh J.C., Yang H.H., Hsu Y.T., Ming C. Synthesis and characteristics of biodegradable and temperature responsive polymeric micelles based on poly(aspartic acid)-g-poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide) Colloids Surf. A. 2013;421:1–8. doi: 10.1016/j.colsurfa.2012.12.014. [DOI] [Google Scholar]
- 46.Ferji K., Hamouda I., Chassenieux C., Nadal B., Dubertret B., Gaillard C., Nicol E. Fast and effective quantum-dots encapsulation and protection in PEO based photo-cross-linked micelles. J. Colloid Interface Sci. 2016;476:222–229. doi: 10.1016/j.jcis.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 47.Yan H., Wei P., Song J., Jia X., Zhang Z. Enhanced anticancer activity in vitro and in vivo of luteolin incorporated into long-circulating micelles based on DSPE-PEG2000 and TPGS. J. Pharm. Pharmacol. 2016;68:1290–1298. doi: 10.1111/jphp.12598. [DOI] [PubMed] [Google Scholar]
- 48.Duong H., Yung L. Synergistic co-delivery of doxorubicin and paclitaxel using multi-functional micelles for cancer treatment. Int. J. Pharm. 2013;454:486–495. doi: 10.1016/j.ijpharm.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 49.Patri A.K., Kukowska-Latallo J.F., Baker J.R. Targeted drug delivery with dendrimers: comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv. Drug Deliv. Rev. 2005;57:2203–2214. doi: 10.1016/j.addr.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 50.Cong H., Zhou L., Meng Q., Zhang Y., Yu B., Shen Y., Hu H. Preparation and evaluation of PAMAM dendrimer-based polymer gels physically cross-linked by hydrogen bonding. Biomater. Sci. 2019;7:3918–3925. doi: 10.1039/C9BM00960D. [DOI] [PubMed] [Google Scholar]
- 51.Barman S.R., Nain A., Jain S., Punjabi N., Mukherji S., Satija J. Dendrimer as a multifunctional capping agent for metal nanoparticles for use in bioimaging, drug delivery and sensor applications. J. Mater. Chem. B. 2018;6:2368–2384. doi: 10.1039/C7TB03344C. [DOI] [PubMed] [Google Scholar]
- 52.Li J., Liang H., Liu J., Wang Z. Poly (amidoamine) (PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy. Int. J. Pharm. 2018;546:215–225. doi: 10.1016/j.ijpharm.2018.05.045. [DOI] [PubMed] [Google Scholar]
- 53.Wang T., Zhang Y., Wei L., Teng Y.G., Honda T., Ojima I. Design, synthesis, and biological evaluations of asymmetric bow-tie PAMAM dendrimer-based conjugates for tumor-targeted drug delivery. ACS Omega. 2018;3:3717–3736. doi: 10.1021/acsomega.8b00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taratula O., Schumann C., Naleway M.A., Pang A.J., Chon K.J., Taratula O. A multifunctional theranostic platform based on phthalocyanine-loaded dendrimer for image-guided drug delivery and photodynamic therapy. Mol. Pharm. 2013;10:3946–3958. doi: 10.1021/mp400397t. [DOI] [PubMed] [Google Scholar]
- 55.Cai K., He X., Song Z., Yin Q., Zhang Y., Uckun F.M., Jiang C., Cheng J. Dimeric drug polymeric nanoparticles with exceptionally high drug loading and quantitative loading efficiency. J. Am. Chem. Soc. 2015;137:3458–3461. doi: 10.1021/ja513034e. [DOI] [PubMed] [Google Scholar]
- 56.Li M., Song W., Tang Z., Lv S., Lin L., Sun H., Li Q., Yang Y., Hong H., Chen X. Nanoscaled poly(l-glutamic acid)/doxorubicin-amphiphile complex as pH-responsive drug delivery system for effective treatment of nonsmall cell lung cancer. ACS Appl. Mater. Interfaces. 2013;5:1781–1792. doi: 10.1021/am303073u. [DOI] [PubMed] [Google Scholar]
- 57.Yan L., Zhou M., Zhang X., Huang L., Chen W., Roy V.A.L., Zhang W., Chen X. A novel type of aqueous dispersible ultrathin-layered double hydroxide nanosheets for in vivo bioimaging and drug delivery. ACS Appl. Mater. Interfaces. 2017;9:34185–34193. doi: 10.1021/acsami.7b05294. [DOI] [PubMed] [Google Scholar]
- 58.Neek M., Tucker J.A., Kim T.I., Molino N.M., Nelson E.L., Wang S.W. Co-delivery of human cancer-testis antigens with adjuvant in protein nanoparticles induces higher cell-mediated immune responses. Biomaterials. 2018;156:194–203. doi: 10.1016/j.biomaterials.2017.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ida Malarselvi R., Ramachandra Raja C., Priscilla J., Viswanathan K. Influence of bio-based chitosan on properties of 5-nitrosalicylaldehyde-anilinenanocomposites. Mater. Today: SAVE Proc. 2016;3:1444–1450. doi: 10.1016/j.matpr.2016.04.027. [DOI] [Google Scholar]
- 60.Hosseinzadeh H., Atyabi F., Varnamkhasti B.S., Hosseinzadeh R., NasserOstad S., Ghahremani M.H., Dinarvand R. SN38 conjugated hyaluronic acid gold nanoparticles as a novel system against metastatic colon cancer cells. Int. J. Pharm. 2017;526:339–352. doi: 10.1016/j.ijpharm.2017.04.060. [DOI] [PubMed] [Google Scholar]
- 61.Cheng Y., Yang J. Polyamidoamine dendrimers used as solubility enhancers of Ketoprofen. Eur. J. Med. Chem. 2005;40:1390–1393. doi: 10.1016/j.ejmech.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 62.Hu H., Xiu K.M., Xu S.L., Yang W.T., Xu F.J. Functionalized layered double hydroxide nanoparticles conjugated with disulfide-linked polycation brushes for advanced gene delivery. Bioconjugate Chem. 2013;24:968–978. doi: 10.1021/bc300683y. [DOI] [Google Scholar]
- 63.Hu H., Song H.Q., Yu B.R., Cai Q., Zhu Y., Xu F.J. A series of new supramolecular polycations for effective gene transfection. Polym. Chem. 2015;6:2466–2477. doi: 10.1039/C4PY01756K. [DOI] [Google Scholar]
- 64.Hu H., Yuan W., Liu F.S., Cheng G., Xu F.J., Ma J. Redox-responsive polycation-functionalized cotton cellulose nanocrystals for effective cancer treatment. ACS Appl. Mater. Interfaces. 2015;7:8942–8951. doi: 10.1021/acsami.5b02432. [DOI] [PubMed] [Google Scholar]
- 65.Hu H., Hou X.J., Wang X.C., Nie J.J., Cai Q., Xu F.J. Gold nanoparticle-conjugated heterogeneous polymer brush-wrapped cellulose nanocrystals prepared by combining different controllable polymerization techniques for theranostic applications. Polym. Chem. 2016;7:3107–3116. doi: 10.1039/C6PY00251J. [DOI] [Google Scholar]
- 66.Wang R., Hu H., Cai Q., Zhao N., Zhu Y., Xu F. Versatile functionalization of amylopectin for effective biomedical applications. Sci. China Chem. 2015;58:1461–1470. doi: 10.1007/s11426-015-5327-8. [DOI] [Google Scholar]
- 67.Liu W., Xin W., Gao X., Chen X., Yu X., Wang H., Deng X. Biotemplated multichannel mesoporous bioactive glass microtubes as a drug carrier. Ceram. Int. 2013;39:8521–8526. doi: 10.1016/j.ceramint.2013.03.034. [DOI] [Google Scholar]
- 68.Chen C., Sun W., Wang X., Wang Y., Wang P. pH-responsive nanoreservoirs based on hyaluronic acid end-capped mesoporous silica nanoparticles for targeted drug delivery. Int. J. Biol. Macromol. 2018;111:1106–1115. doi: 10.1016/j.ijbiomac.2018.01.093. [DOI] [PubMed] [Google Scholar]
- 69.Wang H., Han R.l., Yang L.M., Shi J.H., Liu Z.J., Hu Y., Wang Y., Liu S.J., Gan Y. Design and synthesis of core-shell-shell upconversion nanoparticles for NIR-induced drug release, photodynamic therapy, and cell imaging. ACS Appl. Mater. Interfaces. 2016;8:4416–4423. doi: 10.1021/acsami.5b11197. [DOI] [PubMed] [Google Scholar]
- 70.Liu J., Luo Z., Zhang J., Luo T., Zhou J., Zhao X., Cai K. Hollow mesoporous silica nanoparticles facilitated drug delivery via cascade pH stimuli in tumor microenvironment for tumor therapy. Biomaterials. 2016;83:51–65. doi: 10.1016/j.biomaterials.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 71.Zeng X., Liu G., Tao W., Ma Y., Zhang X., He F., Pan J., Mei L., Pan G. A drug-self-gated mesoporous antitumor nanoplatform based on pH-sensitive dynamic covalent bond. Adv. Funct. Mater. 2017;27:1605985. doi: 10.1002/adfm.201605985. [DOI] [Google Scholar]
- 72.Safdari F., Raissi H., Shahabi M., Zaboli M. DFT calculations and molecular dynamics simulation study on the adsorption of 5-fluorouracil anticancer drug on graphene oxide nanosheet as a drug delivery vehicle. J. Inorg. Organomet. Polym. Mater. 2017;27:805–817. doi: 10.1007/s10904-017-0525-9. [DOI] [Google Scholar]
- 73.Rezaei M., Abbasi A., Dinarvand R., Jeddi-Tehrani M., Janczak J. Design and synthesis of a biocompatible 1D coordination polymer as anti-breast cancer drug carrier, 5-Fu: In vitro and in vivo studies. ACS Appl. Mater. Interfaces. 2018;10:17594–17604. doi: 10.1021/acsami.8b03111. [DOI] [PubMed] [Google Scholar]
- 74.Bünzli J.C.G. Benefiting from the unique properties of lanthanide ions. Acc. Chem. Res. 2006;39:53–61. doi: 10.1021/ar0400894. [DOI] [PubMed] [Google Scholar]
- 75.Wei X., Wang Y., Xiong X., Guo X., Zhang L., Zhang X., Zhou S. Codelivery of a π–π stacked dual anticancer drug combination with nanocarriers for overcoming multidrug resistance and tumor metastasis. Adv. Funct. Mater. 2016;26:8266–8280. doi: 10.1002/adfm.201603336. [DOI] [Google Scholar]
- 76.Zhou Z., Bong D. Small-molecule/polymer recognition triggers aqueous-phase assembly and encapsulation. Langmuir. 2013;29:144–150. doi: 10.1021/la304457y. [DOI] [PubMed] [Google Scholar]
- 77.Galbraith E., James T.D. Boron based anion receptors as sensors. Chem. Soc. Rev. 2010;39:3831–3842. doi: 10.1039/B926165F. [DOI] [PubMed] [Google Scholar]
- 78.Webber M.J., Appel E.A., Meijer E.W., Langer R. Supramolecular biomaterials. Nat. Mater. 2015;15:13–26. doi: 10.1038/nmat4474. [DOI] [PubMed] [Google Scholar]
- 79.Lv S., Wu Y., Cai K., He H., Li Y., Lan M., Chen X., Cheng J., Yin L. High drug loading and sub-quantitative loading efficiency of polymeric micelles driven by donor-receptor coordination interactions. J. Am. Chem. Soc. 2018;140:1235–1238. doi: 10.1021/jacs.7b12776. [DOI] [PubMed] [Google Scholar]
- 80.Yang X., Wang Y., Huang X., Ma Y., Huang Y., Yang R., Duan H., Chen Y. Multi-functionalized graphene oxide based anticancer drug-carrier with dual-targeting function and pH-sensitivity. J. Mater. Chem. 2011;21:3448–3454. doi: 10.1039/C0JM02494E. [DOI] [Google Scholar]
- 81.Varghese O.P., Liu J., Sundaram K., Hilborn J., Oommen O.P. Chondroitin sulfate derived theranostic nanoparticles for targeted drug delivery. Biomater. Sci. 2016;4:1310–1313. doi: 10.1039/C6BM00335D. [DOI] [PubMed] [Google Scholar]
- 82.Zhai S., Hu X., Hu Y., Wu B., Xing D. Visible light-induced crosslinking and physiological stabilization of diselenide-rich nanoparticles for redox-responsive drug release and combination chemotherapy. Biomaterials. 2017;121:41–54. doi: 10.1016/j.biomaterials.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 83.Luo C.Q., Zhou Y.X., Zhou T.J., Xing L., Cui P.F., Sun M., Jin L., Lu N., Jiang H.L. Reactive oxygen species-responsive nanoprodrug with quinone methides-mediated GSH depletion for improved chlorambucil breast cancers therapy. J. Contr. Release. 2018;274:56–68. doi: 10.1016/j.jconrel.2018.01.034. [DOI] [PubMed] [Google Scholar]
- 84.Xu X., Saw P.E., Tao W., Li Y., Ji X., Bhasin S., Liu Y., Ayyash D., Rasmussen J., Huo M. ROS-responsive polyprodrug nanoparticles for triggered drug delivery and effective cancer therapy. Adv. Mater. 2017;29:1700141. doi: 10.1002/adma.201700141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu X.D., Cheng Y.J., Wu J., Cheng H., Cheng S.X., Zhuo R.X., Zhang X.Z. Smart and hyper-fast responsive polyprodrug nanoplatform for targeted cancer therapy. Biomaterials. 2016;76:238–249. doi: 10.1016/j.biomaterials.2015.10.056. [DOI] [PubMed] [Google Scholar]
- 86.Bai S., Ma X., Shi X., Shao J., Zhang T., Wang Y., Cheng Y., Xue P., Kang Y., Xu Z. Smart unimolecular micelle-based polyprodrug with dual-redox stimuli response for tumor microenvironment: enhanced in vivo delivery efficiency and tumor penetration. ACS Appl. Mater. Interfaces. 2019;11:36130–36140. doi: 10.1021/acsami.9b13214. [DOI] [PubMed] [Google Scholar]
- 87.Kong F., Liang Z., Luan D., Liu X., Xu K., Tang B. A glutathione (GSH)-responsive near-infrared (NIR) theranostic prodrug for cancer therapy and imaging. Anal. Chem. 2016;88:6450–6456. doi: 10.1021/acs.analchem.6b01135. [DOI] [PubMed] [Google Scholar]
- 88.Dumez H., Louwerens M., Pawinsky A., Planting A., de Jonge M., Van Oosterom A., Highley M., Guetens G., Mantel M., De Boeck G. The impact of drug administration sequence and pharmacokinetic interaction in a phase I study of the combination of docetaxel and gemcitabine in patients with advanced solid tumors. Anti Canc. Drugs. 2002;13:583–593. doi: 10.1097/00001813-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 89.Chen Y., Zhang F., Wang Q., Tong R., Lin H., Qu F. Near-infrared light-mediated LA-UCNPs@SiO2-C/HA@mSiO2-DOX@NB nanocomposite for chemotherapy/PDT/PTT and imaging. Dalton Trans. 2017;46:14293–14300. doi: 10.1039/C7DT02529G. [DOI] [PubMed] [Google Scholar]
- 90.Corot C., Robert P., Idée J.M., Port M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv. Drug Deliv. Rev. 2006;58(14):1471–1504. doi: 10.1016/j.addr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 91.Wang G., Qian K., Mei X. A theranostic nanoplatform: magneto-gold@fluorescence polymer nanoparticles for tumor targeting T1&T2-MRI/CT/NIR fluorescence imaging and induction of genuine autophagy mediated chemotherapy. Nanoscale. 2018;10:10467–10478. doi: 10.1039/C8NR02429D. [DOI] [PubMed] [Google Scholar]
- 92.Zhang T., Wang W., Zhang D., Zhang X., Ma Y., Zhou Y., Qi L. Biotemplated synthesis of gold nanoparticle-bacteria cellulose nanofiber nanocomposites and their application in biosensing. Adv. Funct. Mater. 2010;20:1152–1160. doi: 10.1002/adfm.200902104. [DOI] [Google Scholar]
- 93.Yu Y., Zhang Z., Wang Y., Zhu H., Li F., Shen Y., Guo S. A new NIR-triggered doxorubicin and photosensitizer indocyanine green co-delivery system for enhanced multidrug resistant cancer treatment through simultaneous chemo/photothermal/photodynamic therapy. Acta Biomater. 2017;59:170–180. doi: 10.1016/j.actbio.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 94.Wang F., Zhang L., Bai X., Cao X., Jiao X., Huang Y., Li Y., Qin Y., Wen Y. Stimuli-responsive nanocarrier for co-delivery of MiR-31 and doxorubicin to suppress high MtEF4 cancer. ACS Appl. Mater. Interfaces. 2018;10:22767–22775. doi: 10.1021/acsami.8b07698. [DOI] [PubMed] [Google Scholar]
- 95.Chen X., Zhang Q., Li J., Yang M., Zhao N., Xu F.J. Rattle-structured rough nanocapsules with in-situ-formed gold nanorod cores for complementary gene/chemo/photothermal therapy. ACS Nano. 2018;12:5646–5656. doi: 10.1021/acsnano.8b01440. [DOI] [PubMed] [Google Scholar]
- 96.Jiang T., Sun W., Zhu Q., Burns N.A., Khan S.A., Mo R., Gu Z. Furin-mediated sequential delivery of anticancer cytokine and small-molecule drug shuttled by graphene. Adv. Mater. 2015;27:1021–1028. doi: 10.1002/adma.201404498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang Y., Zhang T., Hou C., Zu M., Lu Y., Ma X., Jia D., Xue P., Kang Y., Xu Z. Mitochondria-specific anticancer drug delivery based on reduction-activated polyprodrug for enhancing the therapeutic effect of breast cancer chemotherapy. ACS Appl. Mater. Interfaces. 2019;11:29330–29340. doi: 10.1021/acsami.9b10211. [DOI] [PubMed] [Google Scholar]
- 98.Zhu C., Jung S., Luo S., Meng F., Zhu X., Park T.G., Zhong Z. Co-delivery of siRNA and paclitaxel into cancer cells by biodegradable cationic micelles based on PDMAEMA-PCL-PDMAEMA triblock copolymers. Biomaterials. 2010;31:2408–2416. doi: 10.1016/j.biomaterials.2009.11.077. [DOI] [PubMed] [Google Scholar]
- 99.Huang P., Wang D., Su Y., Huang W., Zhou Y., Cui D., Zhu X., Yan D. Combination of small molecule prodrug and nanodrug delivery: amphiphilic drug-drug conjugate for cancer therapy. J. Am. Chem. Soc. 2014;136:11748–11756. doi: 10.1021/ja505212y. [DOI] [PubMed] [Google Scholar]
- 100.Liu Y., Fang J., Kim Y.-J., Wong M.K., Wang P. Codelivery of doxorubicin and paclitaxel by cross-linked multilamellar liposome enables synergistic antitumor activity. Mol. Pharm. 2014;11:1651–1661. doi: 10.1021/mp5000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhao L., Zhu L., Liu F., Liu C., Shan D., Wang Q., Zhang C., Li J., Liu J., Qu X., Yang Z. pH triggered injectable amphiphilic hydrogel containing doxorubicin and paclitaxel. Int. J. Pharm. 2011;410:83–91. doi: 10.1016/j.ijpharm.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 102.Lu S., Neoh K.G., Huang C., Shi Z., Kang E.T. Polyacrylamide hybrid nanogels for targeted cancer chemotherapy via co-delivery of gold nanoparticles and MTX. J. Colloid Interface Sci. 2013;412:46–55. doi: 10.1016/j.jcis.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 103.Yang X., Hu C., Tong F., Liu R., Zhou Y., Qin L., Ouyang L., Gao H. Tumor microenvironment-responsive dual drug dimer-loaded PEGylated bilirubin nanoparticles for improved drug delivery and enhanced immune-chemotherapy of breast cancer. Adv. Funct. Mater. 2019;29:1901896. doi: 10.1002/adfm.201901896. [DOI] [Google Scholar]
- 104.Qian X., Long L., Shi Z., Liu C., Qiu M., Sheng J., Pu P., Yuan X., Ren Y., Kang C. Star-branched amphiphilic PLA-b-PDMAEMA copolymers for co-delivery of miR-21 inhibitor and doxorubicin to treat glioma. Biomaterials. 2014;35:2322–2335. doi: 10.1016/j.biomaterials.2013.11.039. [DOI] [PubMed] [Google Scholar]
- 105.Li J., Zhu Y., Hazeldine S.T., Li C., Oupický D. Dual-function CXCR4 antagonist polyplexes to deliver gene therapy and inhibit cancer cell invasion. Angew. Chem. 2012;124:8870–8873. doi: 10.1002/ange.201203463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bharathiraja S., Manivasagan P., Santha Moorthy M., Bui N.Q., Jang B., Phan T.T.V., Jung W.K., Kim Y.M., Lee K.D., Oh J. Photo-based PDT/PTT dual model killing and imaging of cancer cells using phycocyanin-polypyrrole nanoparticles. Eur. J. Pharm. Biopharm. 2018;123:20–30. doi: 10.1016/j.ejpb.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 107.Xu C., He Y., Li Z., Ahmad Nor Y., Ye Q. Nanoengineered hollow mesoporous silica nanoparticles for the delivery of antimicrobial proteins into biofilms. J. Mater. Chem. B. 2018;6:1899–1902. doi: 10.1039/C7TB03201C. [DOI] [PubMed] [Google Scholar]
- 108.Yan C., Zhang N., Guan P., Chen P., Ding S., Hou T., Hu X., Wang J., Wang C. Drug-based magnetic imprinted nanoparticles: enhanced lysozyme amyloid fibrils cleansing and anti-amyloid fibrils toxicity. Int. J. Biol. Macromol. 2020;153:723–735. doi: 10.1016/j.ijbiomac.2020.03.061. [DOI] [PubMed] [Google Scholar]
- 109.Rajagopalan P., Wudl F., Schinazi R.F., Boudinot F.D. Pharmacokinetics of a water-soluble fullerene in rats. Antimicrob. Agents Chemother. 1996;40:2262–2265. doi: 10.1128/AAC.40.10.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang Y., Zhao Q., Han N., Bai L., Li J., Liu J., Che E., Hu L., Zhang Q., Jiang T., Wang S. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2015;11:313–327. doi: 10.1016/j.nano.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 111.Slowing I., Trewyn B.G., Lin V.S.Y. Effect of surface functionalization of MCM-41-type mesoporous silica nanoparticles on the endocytosis by human cancer cells. J. Am. Chem. Soc. 2006;128:14792–14793. doi: 10.1021/ja0645943. [DOI] [PubMed] [Google Scholar]
- 112.Stark D.D., Weissleder R., Elizondo G., Hahn P.F., Saini S., Todd L.E., Wittenberg J., Ferrucci J.T. Superparamagnetic iron oxide: clinical application as a contrast agent for MR imaging of the liver. Radiology. 1988;168:297–301. doi: 10.1148/radiology.168.2.3393649. [DOI] [PubMed] [Google Scholar]
- 113.Ito A., Shinkai M., Honda H., Kobayashi T. Medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng. 2005;100:1–11. doi: 10.1263/jbb.100.1. [DOI] [PubMed] [Google Scholar]
- 114.Hiremath C.G., Heggnnavar G.B., Kariduraganavar M.Y., Hiremath M.B. Co-delivery of paclitaxel and curcumin to foliate positive cancer cells using Pluronic-coated iron oxide nanoparticles. Prog. Biomater. 2019;8:155–168. doi: 10.1007/s40204-019-0118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ghosh P., Han G., De M., Kim C.K., Rotello V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008;60:1307–1315. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 116.Shiao Y.S., Chiu H.H., Wu P.H., Huang Y.F. Aptamer-functionalized gold nanoparticles as photoresponsive nanoplatform for co-drug delivery. ACS Appl. Mater. Interfaces. 2014;6:21832–21841. doi: 10.1021/am5026243. [DOI] [PubMed] [Google Scholar]
- 117.Schrand A.M., Rahman M.F., Hussain S.M., Schlager J.J., Smith D.A., Syed A.F. Metal-based nanoparticles and their toxicity assessment. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 2010;2:544–568. doi: 10.1002/wnan.103. [DOI] [PubMed] [Google Scholar]
- 118.Rafi M., Cabral H., Kano M.R., Mi P., Iwata C., Yashiro M., Hirakawa K., Miyazono K., Nishiyama N., Kataoka K. Polymeric micelles incorporating (1,2-diaminocyclohexane)platinum (II) suppress the growth of orthotopic scirrhous gastric tumors and their lymph node metastasis. J. Contr. Release. 2012;159:189–196. doi: 10.1016/j.jconrel.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 119.Yi Y., Lin G., Chen S., Liu J., Zhang H., Mi P. Polyester micelles for drug delivery and cancer theranostics: current achievements, progresses and future perspectives. Mater. Sci. Eng. 2018;83:218–232. doi: 10.1016/j.msec.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 120.Gao X., Wang B., Wei X., Rao W., Ai F., Zhao F., Men K., Yang B., Liu X., Huang M., Gou M., Qian Z., Huang N., Wei Y. Preparation, characterization and application of star-shaped PCL/PEG micelles for the delivery of doxorubicin in the treatment of colon cancer. Int J Nanomedicine. 2013;8:971–982. doi: 10.2147/IJN.S39532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Um W., Park J., Ko H., Lim S., Yoon H., Shim M., Lee S., Ko Y., Kim M., Park J., Lim D., Byun Y., Kwon I.C., Kim K. Visible light-induced apoptosis activatable nanoparticles of photosensitizer-DEVD-anticancer drug conjugate for targeted cancer therapy. Biomaterials. 2019;224:119494. doi: 10.1016/j.biomaterials.2019.119494. [DOI] [PubMed] [Google Scholar]
- 122.Cong Y., Xiao H., Xiong H., Wang Z., Ding J., Li C., Chen X., Liang X.J., Zhou D., Huang Y. Dual drug backboned shattering polymeric theranostic nanomedicine for synergistic eradication of patient-derived lung cancer. Adv. Mater. 2018;30:1706220. doi: 10.1002/adma.201706220. [DOI] [PubMed] [Google Scholar]
- 123.Li Y., Li Y., Ji W., Lu Z., Liu L., Shi Y., Ma G., Zhang X. Positively charged polyprodrug amphiphiles with enhanced drug loading and reactive oxygen species-responsive release ability for traceable synergistic therapy. J. Am. Chem. Soc. 2018;140:4164–4171. doi: 10.1021/jacs.8b01641. [DOI] [PubMed] [Google Scholar]
- 124.Kraft J., Freeling J., Wang Z., Ho R. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J. Pharm. Sci. 2014;103:29–52. doi: 10.1002/jps.23773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tardi P., Johnstone S., Harasym N., Xie S., Harasym T., Zisman N., Harvie P., Bermudes D., Mayer L. In vivo maintenance of synergistic cytarabine:daunorubicin ratios greatly enhances therapeutic efficacy. Leuk. Res. 2009;33:129–139. doi: 10.1016/j.leukres.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 126.Han Q., Wang W., Jia X., Qian Y., Li Q., Wang Z., Zhang W., Yang S., Jia Y., Hu Z. Switchable liposomes: targeting-peptide-functionalized and ph-triggered cytoplasmic delivery. ACS Appl. Mater. Interfaces. 2016;8:18658–18663. doi: 10.1021/acsami.6b05678. [DOI] [PubMed] [Google Scholar]
- 127.Sheng D., Liu T., Deng L., Zhang L., Li X., Xu J., Hao L., Li P., Ran H., Chen H., Wang Z. Perfluorooctyl bromide & indocyanine green co-loaded nanoliposomes for enhanced multimodal imaging-guided phototherapy. Biomaterials. 2018;165:1–13. doi: 10.1016/j.biomaterials.2018.02.041. [DOI] [PubMed] [Google Scholar]
- 128.Cohn D., Sosnik A., Garty S. Smart hydrogels for in situ generated implants. Biomacromolecules. 2005;6:1168–1175. doi: 10.1021/bm0495250. [DOI] [PubMed] [Google Scholar]
- 129.Langer R. Drugs on target. Science. 2001;293:58–59. doi: 10.1126/science.1063273. [DOI] [PubMed] [Google Scholar]
- 130.Yallapu M., Jaggi M., Chauhan S. Design and engineering of nanogels for cancer treatment. Drug Discov. Today. 2011;16:457–463. doi: 10.1016/j.drudis.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chacko R., Ventura J., Zhuang J., Thayumanavan S. Polymer nanogels: a versatile nanoscopic drug delivery platform. Adv. Drug Deliv. Rev. 2012;64:836–851. doi: 10.1016/j.addr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang B., Jia F., Fleming M., Mallapragada S. Injectable self-assembled block copolymers for sustained gene and drug co-delivery: an in vitro study. Int. J. Pharm. 2012;427:88–96. doi: 10.1016/j.ijpharm.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 133.Peppas N., Hilt J., Khademhosseini A., Langer R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv. Mater. 2006;18:1345–1360. doi: 10.1002/adma.200501612. [DOI] [Google Scholar]
- 134.Costa D., Valente A., Miguel M., Queiroz J. Plasmid DNA microgels for a therapeutical strategy combining the delivery of genes and anticancer drugs. Macromol. Biosci. 2012;12:1243–1252. doi: 10.1002/mabi.201200096. [DOI] [PubMed] [Google Scholar]
- 135.Gillies E., Fréchet J. Dendrimers and dendritic polymers in drug delivery. Drug Discov. Today. 2005;10:35–43. doi: 10.1016/S1359-6446(04)03276-3. [DOI] [PubMed] [Google Scholar]
- 136.Chen S., Zhang X.Z., Cheng S.X., Zhuo R.X., Gu Z.W. Functionalized amphiphilic hyperbranched polymers for targeted drug delivery. Biomacromolecules. 2008;9:2578–2585. doi: 10.1021/bm800371n. [DOI] [PubMed] [Google Scholar]
- 137.Varde N., Pack D. Microspheres for controlled release drug delivery. Expert Opin. Biol. Ther. 2004;4:35–51. doi: 10.1517/14712598.4.1.35. [DOI] [PubMed] [Google Scholar]
- 138.Luo Y., Wang Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2013;64:353–367. doi: 10.1016/j.ijbiomac.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 139.Freiberg S., Zhu X. Polymer microspheres for controlled drug release. Int. J. Pharm. 2004;282:1–18. doi: 10.1016/j.ijpharm.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 140.Kwoh D., Coffin C., Lollo C., Jovenal J., Carlo D. Stabilization of poly-L-lysine/DNA polyplexes for in vivo gene delivery to the liver. Biochim. Biophys. Acta. 1999;1444:171–190. doi: 10.1016/S0167-4781(98)00274-7. [DOI] [PubMed] [Google Scholar]
- 141.Chen L., Wang Y., Zhu Y., Sun Y., Wang Y. Stimulus-responsive polyplexes with drug and gene co-delivery. Chem. Res. Chin. Univ. 2013;34:720–725. doi: 10.7503/cjcu20120563. [DOI] [Google Scholar]
- 142.Yang P., Song H., Qin Y., Huang P., Zhang C., Kong D., Wang W. Engineering dendritic-cell-based vaccines and PD-1 blockade in self-assembled peptide nanofibrous hydrogel to amplify antitumor T-cell immunity. Nano Lett. 2018;18:4377–4385. doi: 10.1021/acs.nanolett.8b01406. [DOI] [PubMed] [Google Scholar]
- 143.Wang Q., Cheng H., Peng H., Zhou H., Li P.Y., Langer R. Non-genetic engineering of cells for drug delivery and cell-based therapy. Adv. Drug Deliv. Rev. 2015;91:125–140. doi: 10.1016/j.addr.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 144.Wen D., Wang J., Van Den Driessche G., Chen Q., Zhang Y., Chen G., Li H., Soto J., Liu M., Ohashi M., Wang Z., Abdou P., Hu Q., Dotti G., Li S., Fourches D., Gu Z. Adipocytes as anticancer drug delivery depot. Matter. 2019;1:1203–1214. doi: 10.1016/j.matt.2019.08.007. [DOI] [Google Scholar]
- 145.Chen M., Ouyang H., Zhou S., Li J., Ye Y. PLGA-nanoparticle mediated delivery of anti-OX40 monoclonal antibody enhances anti-tumor cytotoxic T cell responses, Cell. Immunol. 2014;287:91–99. doi: 10.1016/j.cellimm.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 146.Opalinska J.B., Gewirtz A.M. Nucleic-acid therapeutics: basic principles and recent applications. Nat. Rev. Drug Discov. 2002;1:503–514. doi: 10.1038/nrd837. [DOI] [PubMed] [Google Scholar]
- 147.Shibu E.S., Hamada M., Murase N., Biju V. Nanomaterials formulations for photothermal and photodynamic therapy of cancer. J. Photochem. Photobiol., A C. 2013;15:53–72. doi: 10.1016/j.jphotochemrev.2012.09.004. [DOI] [Google Scholar]
- 148.Janib S.M., Moses A.S., MacKay J.A. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev. 2010;62:1052–1063. doi: 10.1016/j.addr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kline I., Venditti J.M., Mead J.A.R., Tyrer D.D., Goldin A. The antileukemic effectiveness of 5-fluorouracil and methotrexate in the combination chemotherapy of advanced leukemia L1210 in mice. Cancer Res. 1966;26:848–852. https://cancerres.aacrjournals.org/content/26/5/848.long [PubMed] [Google Scholar]
- 150.Goldman A., Kulkarni A., Kohandel M., Pandey P., Rao P., Natarajan S.K., Sabbisetti V., Sengupta S. Rationally designed 2-in-1 nanoparticles can overcome adaptive resistance in cancer. ACS Nano. 2016;10:5823–5834. doi: 10.1021/acsnano.6b00320. [DOI] [PubMed] [Google Scholar]
- 151.Murphy C.G., Seidman A.D. Evolving approaches to metastatic breast cancer previously treated with anthracyclines and taxanes. Clin. Breast Canc. 2009;9:S58–S65. doi: 10.3816/CBC.2009.s.006. [DOI] [PubMed] [Google Scholar]
- 152.Guo Y., Jiang K., Shen Z., Zheng G., Fan L., Zhao R., Shao J. A small molecule nanodrug by self-assembly of dual anticancer drugs and photosensitizer for synergistic near-infrared cancer theranostics. ACS Appl. Mater. Interfaces. 2017;9:43508–43519. doi: 10.1021/acsami.7b14755. [DOI] [PubMed] [Google Scholar]
- 153.Pei Q., Hu X., Zheng X., Liu S., Li Y., Jing X., Xie Z. Light-activatable red blood cell membrane-camouflaged dimeric prodrug nanoparticles for synergistic photodynamic/chemotherapy. ACS Nano. 2018;12:1630–1641. doi: 10.1021/acsnano.7b08219. [DOI] [PubMed] [Google Scholar]
- 154.Zheng X., Wang L., Liu S., Zhang W., Liu F., Xie Z. Nanoparticles of chlorin dimer with enhanced absorbance for photoacoustic imaging and phototherapy. Adv. Funct. Mater. 2018;28:1706507. doi: 10.1002/adfm.201706507. [DOI] [Google Scholar]
- 155.Nath K., Guo L., Nancolas B., Nelson D.S., Shestov A.A., Lee S.C., Roman J., Zhou R., Leeper D.B., Halestrap A.P., Blair I.A., Glickson J.D. Mechanism of antineoplastic activity of lonidamine. Biochim. Biophys. Acta Rev. Canc. 2016;1866:151–162. doi: 10.1016/j.bbcan.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Du C., Ding Y., Qian J., Zhang R., Dong C.M. Dual drug-paired polyprodrug nanotheranostics reverse multidrug resistant cancers via mild photothermal-cocktail chemotherapy. J. Mater. Chem. B. 2019;7:5306–5319. doi: 10.1039/C9TB01368G. [DOI] [PubMed] [Google Scholar]
- 157.Zuckerman J.E., Davis M.E. Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat. Rev. Drug Discov. 2015;14:843–856. doi: 10.1038/nrd4685. [DOI] [PubMed] [Google Scholar]
- 158.Teo P.Y., Cheng W., Hedrick J.L., Yang Y.Y. Co-delivery of drugs and plasmid DNA for cancer therapy. Adv. Drug Deliv. Rev. 2016;98:41–63. doi: 10.1016/j.addr.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 159.Dai X., Tan C. Combination of microRNA therapeutics with small-molecule anticancer drugs: mechanism of action and co-delivery nanocarriers. Adv. Drug Deliv. Rev. 2015;81:184–197. doi: 10.1016/j.addr.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 160.Qin S.Y., Feng J., Rong L., Jia H.Z., Chen S., Liu X.J., Luo G.F., Zhuo R.X., Zhang X.Z. Theranostic GO-based nanohybrid for tumor induced imaging and potential combinational tumor therapy. Small. 2014;10:599–608. doi: 10.1002/smll.201301613. [DOI] [PubMed] [Google Scholar]
- 161.Yin F., Yang C., Wang Q., Zeng S., Hu R., Lin G., Tian J., Hu S., Lan R.F., Yoon H.S., Lu F., Wang K., Yong K.T. A light-driven therapy of pancreatic adenocarcinoma using gold nanorods-based nanocarriers for co-delivery of doxorubicin and siRNA. Theranostics. 2015;5:818–833. doi: 10.7150/thno.11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Heger Z., Polanska H., Krizkova S., Balvan J., Raudenska M., Dostalova S., Moulick A., Masarik M., Adam V. Co-delivery of VP-16 and Bcl-2-targeted antisense on PEG-grafted oMWCNTs for synergistic in vitro anti-cancer effects in non-small and small cell lung cancer. Colloids Surf., B. 2017;150:131–140. doi: 10.1016/j.colsurfb.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 163.He C., Lu K., Liu D., Lin W. Nanoscale metal-organic frameworks for the co-delivery of cisplatin and pooled sirnas to enhance therapeutic efficacy in drug-resistant ovarian cancer cells. J. Am. Chem. Soc. 2014;136:5181–5184. doi: 10.1021/ja4098862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pohlmann P.R., Mernaugh R.L., Goff L.W. Chemotherapy and immunotherapy in metastatic colorectal cancer. N. Engl. J. Med. 2009;360:2134–2136. doi: 10.1056/NEJMc090489. https://www.nejm.org/doi/full/10.1056/NEJMc090489 [DOI] [PubMed] [Google Scholar]
- 165.Tang H., Wang Y., Chlewicki L.K., Zhang Y., Guo J., Liang W., Wang J., Wang X., Fu Y.-X. Facilitating T cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Canc. Cell. 2016;29:285–296. doi: 10.1016/j.ccell.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Galluzzi L., Buqué A., Kepp O., Zitvogel L., Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017;17:97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 167.Cook A.M., Lesterhuis W.J., Nowak A.K., Lake R.A. Chemotherapy and immunotherapy: mapping the road ahead. Curr. Opin. Immunol. 2016;39:23–29. doi: 10.1016/j.coi.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 168.Lin E., Yang C., Lin C., Huang B., Lai W., Tseng Y., Yang P. Priming PD-L1 expression by chemotherapeutic agents in non-small cell lung cancers. J. Clin. Oncol. 2017;35 doi: 10.1200/JCO.2017.35.15_suppl.e20087. [DOI] [Google Scholar]
- 169.Wang C., Wang J., Zhang X., Yu S., Wen D., Hu Q., Ye Y., Bomba H., Hu X., Liu Z., Dotti G., Gu Z. In situ formed reactive oxygen species–responsive scaffold with gemcitabine and checkpoint inhibitor for combination therapy. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aan3682. eaan3682. [DOI] [PubMed] [Google Scholar]
- 170.Ruan H., Hu Q., Wen D., Chen Q., Chen G., Lu Y., Wang J., Cheng H., Lu W., Gu Z. A dual-bioresponsive drug-delivery depot for combination of epigenetic modulation and immune checkpoint blockade. Adv. Mater. 2019;31:1806957. doi: 10.1002/adma.201806957. [DOI] [PubMed] [Google Scholar]
- 171.Wang S., Riedinger A., Li H., Fu C., Liu H., Li L., Liu T., Tan L., Barthel M.J., Pugliese G., De Donato F., Scotto D'Abbusco M., Meng X., Manna L., Meng H., Pellegrino T. Plasmonic copper sulfide nanocrystals exhibiting near-infrared photothermal and photodynamic therapeutic effects. ACS Nano. 2015;9:1788–1800. doi: 10.1021/nn506687t. [DOI] [PubMed] [Google Scholar]
- 172.Lin M., Guo C., Li J., Zhou D., Liu K., Zhang X., Xu T., Zhang H., Wang L., Yang B. Polypyrrole-coated chainlike gold nanoparticle architectures with the 808 nm photothermal transduction efficiency up to 70% ACS Appl. Mater. Interfaces. 2014;6:5860–5868. doi: 10.1021/am500715f. [DOI] [PubMed] [Google Scholar]
- 173.Robinson J.T., Tabakman S.M., Liang Y., Wang H., Sanchez Casalongue H., Vinh D., Dai H. Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J. Am. Chem. Soc. 2011;133:6825–6831. doi: 10.1021/ja2010175. [DOI] [PubMed] [Google Scholar]
- 174.Liang C., Diao S., Wang C., Gong H., Liu T., Hong G., Shi X., Dai H., Liu Z. Tumor metastasis inhibition by imaging-guided photothermal therapy with single-walled carbon nanotubes. Adv. Mater. 2014;26:5646–5652. doi: 10.1002/adma.201401825. [DOI] [PubMed] [Google Scholar]
- 175.Huang S., Duan S., Wang J., Bao S., Qiu X., Li C., Liu Y., Yan L., Zhang Z., Hu Y. Folic-acid-mediated functionalized gold nanocages for targeted delivery of anti-mir-181b in combination of gene therapy and photothermal therapy against hepatocellular carcinoma. Adv. Funct. Mater. 2016;26:2532–2544. doi: 10.1002/adfm.201504912. [DOI] [Google Scholar]
- 176.Luo S., Zhang E., Su Y., Cheng T., Shi C. A review of NIR dyes in cancer targeting and imaging. Biomaterials. 2011;32:7127–7138. doi: 10.1016/j.biomaterials.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 177.Devulapally R., Foygel K., Sekar T.V., Willmann J.K., Paulmurugan R. Gemcitabine and antisense-microRNA co-encapsulated PLGA-PEG polymer nanoparticles for hepatocellular carcinoma therapy. ACS Appl. Mater. Interfaces. 2016;8:33412–33422. doi: 10.1021/acsami.6b08153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mao B., Liu C., Zheng W., Li X., Ge R., Shen H., Guo X., Lian Q., Shen X., Li C. Cyclic cRGDfk peptide and Chlorin e6 functionalized silk fibroin nanoparticles for targeted drug delivery and photodynamic therapy. Biomaterials. 2018;161:306–320. doi: 10.1016/j.biomaterials.2018.01.045. [DOI] [PubMed] [Google Scholar]
- 179.Zhang X., Wu M., Li J., Lan S., Zeng Y., Liu X., Liu J. Light-enhanced hypoxia-response of conjugated polymer nanocarrier for successive synergistic photodynamic and chemo-therapy. ACS Appl. Mater. Interfaces. 2018;10:21909–21919. doi: 10.1021/acsami.8b06491. [DOI] [PubMed] [Google Scholar]
- 180.Dai L., Yu Y., Luo Z., Li M., Chen W., Shen X., Chen F., Sun Q., Zhang Q., Gu H., Cai K. Photosensitizer enhanced disassembly of amphiphilic micelle for ROS-response targeted tumor therapy in vivo. Biomaterials. 2016;104:1–17. doi: 10.1016/j.biomaterials.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 181.Song C., Xiao Y., Ouyang Z., Shen M., Shi X. Efficient co-delivery of microRNA 21 inhibitor and doxorubicin to cancer cells using core-shell tecto dendrimers formed via supramolecular host-guest assembly. J. Mater. Chem. B. 2020;8:2768–2774. doi: 10.1039/D0TB00346H. [DOI] [PubMed] [Google Scholar]
- 182.Gao M., Deng J., Liu F., Fan A., Wang Y., Wu H., Ding D., Kong D., Wang Z., Peer D., Zhao Y. Triggered ferroptotic polymer micelles for reversing multidrug resistance to chemotherapy. Biomaterials. 2019;223:119486. doi: 10.1016/j.biomaterials.2019.119486. [DOI] [PubMed] [Google Scholar]
- 183.Liu X., Su H., Shi W., Liu Y., Sun Y., Ge D. Functionalized poly(pyrrole-3-carboxylic acid) nanoneedles for dual-imaging guided PDT/PTT combination therapy. Biomaterials. 2018;167:177–190. doi: 10.1016/j.biomaterials.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 184.Chang M., Wang M., Wang M., Shu M., Ding B., Li C., Pang M., Cui S., Hou Z., Lin J. A multifunctional cascade bioreactor based on hollow-structured Cu2MoS4 for synergetic cancer chemo-dynamic therapy/starvation therapy/phototherapy/immunotherapy with remarkably enhanced efficacy. Adv. Mater. 2019;31:1905271. doi: 10.1002/adma.201905271. [DOI] [PubMed] [Google Scholar]