Abstract
Gene engineering and combinatorial approaches with other cancer immunotherapy agents may confer capabilities enabling full tumor rejection by adoptive T cell therapy (ACT). The provision of proper costimulatory receptor activity and cytokine stimuli, along with the repression of inhibitory mechanisms, will conceivably make the most of these treatment strategies. In this sense, T cells can be genetically manipulated to become refractory to suppressive mechanisms and exhaustion, last longer and differentiate into memory T cells while endowed with the ability to traffic to malignant tissues. Their antitumor effects can be dramatically augmented with permanent or transient gene transfer maneuvers to express or delete/repress genes. A combination of such interventions seeks the creation of the ultimate bionic T cell, perfected to seek and destroy cancer cells upon systemic or local intratumor delivery.
Keywords: Adoptive cell therapy, T cell engineering, Cancer immunotherapy
Subject terms: Immunotherapy, Cancer immunotherapy
Introduction
Immunotherapy has emerged in recent decades as one of the most powerful strategies for cancer treatment. Among the different types of immunotherapy, immune checkpoint blockers (ICBs), such as PD-1/PD-L1 and CTLA-4 inhibitors, have stood out, showing unprecedented clinical benefit.1 However, low response rates and progression after transient remission still represent major challenges. Along with ICBs, adoptive cell therapy (ACT) represents a cutting-edge cancer immunotherapy approach. ACT consists of the infusion of ex vivo expanded lymphocytes and exploits the antigen specificity of the adaptive immune system to direct T-cell effector functions against cancer cells. ACT strategies include the transfer of tumor-infiltrating lymphocytes (TILs) and the transfer of transgenic T cells engineered to express an exogenous T-cell receptor (TCR) or a chimeric antigen receptor (CAR) based on single-chain antibody recognition of surface proteins on cancer cells.2 Cell therapy strategies based on NK cells are also being intensively investigated and are starting to show exciting preclinical and clinical results.3,4
Clinical trials using autologous TILs to treat metastatic melanoma patients have shown objective response rates of up to 50%.5,6 However, the efficacy of TIL transfer in the treatment of other solid tumors has been more limited. Similar to TIL therapy, TCR-engineered ACT requires antigen presentation by the major histocompatibility complex (MHC); thus, efficacy depends on tumor MHC expression. To bypass MHC-dependent antigen presentation, CAR-T cells have been developed. CARs can virtually target any surface tumor cell antigen. In the clinic, CAR-T cells have shown impressive responses against hematological malignancies, and CD19-targeting CARs are the only ACT-based approach thus far approved by the FDA/EMA.7 The outcome of CAR therapy in hematological malignancies has revolutionized the field, but so far, it has not been successful in the treatment of solid tumors.
ACT is far from perfect. Engineering platforms can be used not only to confer antigen recognition specificity to T cells but also to build “the bionic T cell”, meaning a better, stronger, and faster T cell for ACT.8 This review aims to discuss the basic principles of and barriers to ACT use in solid tumors and the ongoing cell and gene engineering strategies to enhance ACT approaches or limit side effects.
An overview of T-cell-mediated immunity in cancer
CD8+ cytotoxic T lymphocytes (CTLs) play an essential role in cancer immunity by specifically recognizing tumor-associated antigens (TAAs) presented by MHC-I molecules on cancer cells through their TCRs and by mediating cytotoxic responses against malignant cells. The ability of T cells to distinguish between self-antigens and tumor antigens is a hallmark of T cell-based ACT. Upon cognate antigen encounter, CTLs activate and release cytotoxic granules containing granzymes and perforin towards the target cell and produce proinflammatory effector Th1 cytokines such as IL-2, TNF, and IFN-ɣ.
To accomplish its effector function, a CTL should initially be activated in the lymph node (LN), migrate to the tumor, infiltrate the tumor parenchyma, interact with the target tumor cell and directionally degranulate. The presence of CTLs in the tumor microenvironment (TME) is currently explained using a cyclic model in which antigen-presenting cells (APCs), tumor cells, and T cells interact to establish an immune response.9 According to this model, dendritic cells (DCs) infiltrate into the tumor tissue and become activated/mature, causing type I IFN secretion that results in the activation of BATF3-dependent cross-presenting migratory DCs, commonly regarded as type 1 conventional DCs (migratory cDC1s). Migratory DCs uptake TAAs and travel from the TME to the draining LNs, where they cross-present the TAAs to antigen-specific T cells, leading to T cell proliferation, activation and differentiation10 and potentially conferring the ability to migrate to the TME in a CXCL9/10-dependent fashion. In addition to the recruitment of primed T cells from the dLNs to the TME, recent results suggest that tissue memory T cells may also contribute to the pool of T cell infiltration into tumors.11
Even though CTLs infiltrate the TME, T-cell activity is a tightly controlled process in which TAA presentation on MHC molecules by APCs or tumor cells (signal 1) is not enough to mount effective T-cell antitumor responses. Costimulatory signals (signal 2) provided by the B7 and TNFR protein families and cytokine stimulation (signal 3), including IL-12 and type I IFN cytokines, are required to cooperate with TCR signaling for full-blown T-cell activation. In addition, recent studies have reported a role for bystander T cells in antitumor immunity. These T cells are not specific for tumor antigens and may functionally contribute to tumor control.12 Unlike T-cell activation, persistent antigen exposure to specific T cells in the TME causes the attenuation of T-cell effector function and proliferation, a set of features that resembles the T-cell inhibited phenotype previously reported during chronic viral infections.13 Such a dysfunctional state is characterized by the expression of inhibitory receptors (checkpoint receptors) such as PD1, CTLA-4, TIM-3, and LAG-3.14 In addition, the loss of costimulatory molecules on T cells, such as the costimulatory receptor CD226, contributes to a dysfunctional T cell state.15 CD226 acting in competition with the inhibitory checkpoint receptors TIGIT and CD96 expressed on tumor-specific T cells compete for binding to CD155. In fact, CD155 is a surface protein highly expressed on tumor cells. In this sense, the downregulation of CD226 expression unbalances the T cell costimulatory output in the TME and is likely to contribute to T-cell dysfunction.16 This T-cell state has been termed T-cell exhaustion. This state arises as the result of transcriptional and epigenetic changes.17 Checkpoints expressed as a result of exhaustion inhibit T-cell activity and ultimately contribute to cancer progression.
CTL priming bolsters an epigenetic and transcriptional differentiation process in which naïve T cells differentiate into short-lived effector cells (SLECs) or into memory precursor cells (MPECs). Memory T cells have a higher proliferative capacity, longer persistence, and a less differentiated phenotype than terminally differentiated effector T cells.18 Based on the expression of CD45 alternative splicing isoforms RA and RO and homing receptors such as CD62L, CCR7 and CD103, memory T cells are classified as central (TCM), effector (TEM), and tissue-resident (TRM) cells.19 Memory CD8+ T cells are essential for acquired immunity by ensuring effector functions upon antigen restimulation. In this sense, the reprogramming of memory T cells is a source of specific CTLs that can eventually infiltrate the TME.
CD4+ T cells that recognize helper epitopes presented by MHC class II molecules are also required to mount efficient antitumor immunity.20,21 During CTL (CD8+) priming, CD4+ helper T cells contribute to optimal CTL differentiation to effector and memory cells by upregulating activating signals on DCs. These CD4+ helper signals include CD40L-CD40-mediated DC licensing that causes IL-12 and IL-15 cytokine production as well as the upregulation of costimulatory receptors such as CD80 and CD70 on cDC1s.22,23 In addition, CD4+ T cells may also directly contribute to the intrinsic properties of CTLs, such as enhancing their cytotoxic functions and trafficking potential.24 Evidence for melanoma regression upon adoptive transfer of antitumor CD4+ T cells has been reported.25 In this regard, and in line with the reported antitumoral properties of the IL-9-producing Th9 CD4+ T cell subset,26 the adoptive transfer of tumor-specific Th9 cells outperformed Th1 and Th7 subsets in terms of antitumor efficacy and persistence in preclinical mouse models.27
Barriers to T-cell immunity in cancer
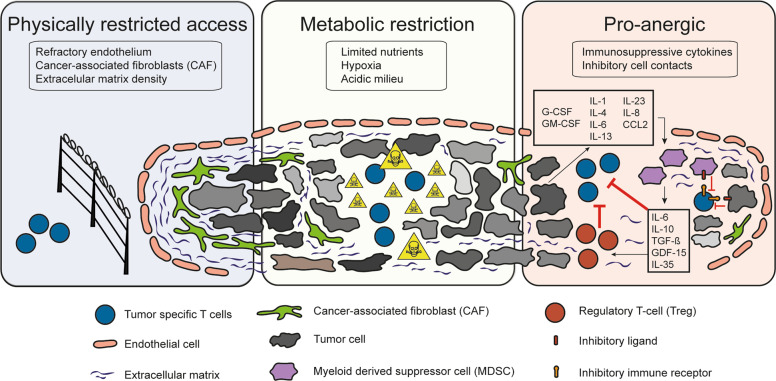
CTLs in solid tumors encounter a hostile tumor environment mainly characterized by (i) a tumor microenvironment physically protected by refractory endothelial cells,28 cancer-associated fibroblasts (CAFs)29 and an extracellular matrix that hinders CTL infiltration; (ii) a metabolically restricted hypoxic and acidic environment that hampers T-cell proliferation; and (iii) a pro-anergic environment caused by immunosuppressive cytokines, such as transforming growth factor β (TGF-β) and IL-10, and inhibitory direct cell contacts that include checkpoint ligation. In addition, intrinsic T-cell factors, such as suboptimal TCR signaling, contribute to the state of peripheral tolerance generation in the TME (Fig. 1). Indeed, tumor-infiltrating T-cells are often described to be in a state of exhaustion driven by epigenetic and transcriptional cues related to chronic antigen exposure and a tissue repair environment.
Fig. 1.
Barriers to endogenous and adoptive T-cell immunity in the tumor microenvironment. Main obstacles to adoptive T-cell therapy efficacy in the treatment of solid malignancies
During tumor development, innate immune subsets comprising myeloid-derived suppressor cells (MDSCs), neutrophils, and macrophages are recruited to the TME and contribute to establishing a hostile immunosuppressive environment that favors tumor progression and impairs antitumoral immunity.30 During their early transformation, tumor cells secrete immunosuppressive factors such as vascular endothelial growth factor (VEGF), TGF-β and indoleamine 2,3-dioxygenase (IDO); myeloid-cell and Treg-recruiting factors such as G-CSF and GM-CSF; the cytokines IL-1, IL-4, IL-6, IL-13, and IL-35; and the chemokines IL-8 and CCL2, among others.31 MDSCs and macrophages, once in the TME, inhibit T cell immunity through a variety of mechanisms. The secretion of immunosuppressive cytokines such as IL-6, IL-10, and TGF-β in the TME directly inhibits T-cell function and recruits regulatory T cells (Tregs). Furthermore, MDSCs curtail the availability of essential amino acids for T-cell function in the TME by expressing IDO or arginase-1 enzymes. The secretion of reactive oxygen species (ROS) disturbs correct TCR function and IFN-γ secretion, while nitric oxide (NO) downregulates IL-2 mRNA stability and inhibits JAK-STAT signaling. In addition, MDSCs directly inhibit T cells by upregulating the expression of checkpoint ligands such as PD-L1.31
Tregs are an immunosuppressive subset of CD4+ T cells involved in maintaining peripheral tolerance that contribute in the TME to the development of CTL anergy. In the TME, Tregs inhibit CTL proliferation by expressing TGF-β, adenosine or IL-35 and hinder CTL homeostasis by consuming high concentrations of IL-2.32 In addition, IL-10 secretion and surface expression of inhibitory ligands such as CTLA-4, LAG-3 or Nrp-1 impede proper T-cell priming by suppressing APC activation.33
An overview of current ACT approaches
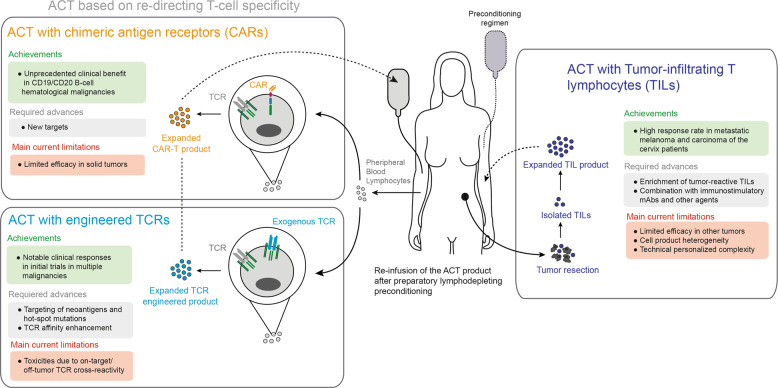
ACT with tumor-infiltrating T lymphocytes (TILs)
ACT with TILs consists of ex vivo expansion of tumor-infiltrating T cells from resected tumor material and transfer in high numbers back into the same patient following a lymphodepletion regime34 (Fig. 2). Lymphodepleting chemotherapy or total body irradiation (TBI) seeks to eliminate competition for homeostatic cytokines such as IL-15 and IL-7. Consistent with the presence of tumor-reactive T cells in the TME,5 ACT with TILs aims to expand the numbers of dysfunctional tumor-reactive T cells and reinvigorate them ex vivo. Moreover, preconditioning depletes Tregs in the patient. The approach initially tested in metastatic melanoma patients by Dr. Rosenberg´s team at the surgery branch of the NCI achieved objective responses in up to 50% of metastatic skin melanoma patients, with all complete responses being remarkably durable.35
Fig. 2.
Current strategies of adoptive T-cell therapy. Main achievements and current limitations in the clinical development of adoptive T-cell therapy
The current standard TIL expansion protocol consists of the initial outgrowth, for approximately two weeks, of the bulk of T cells from the excised tumor material isolated by enzymatic digestion and cultured in the presence of high doses of IL-2. The T cells obtained are then activated/expanded in a rapid expansion protocol (REP) using soluble anti-CD3 mAb, irradiated allogenic or autologous feeder cells, and high concentrations of IL-2. The resulting cells (up to 1 × 1011 T cells) are then infused back into the patient previously treated with a course of nonmyeloablative lymphodepleting chemotherapy consisting of cyclophosphamide and fludarabine.36
The optimization of the standard TIL production protocol is an active field of investigation in which modifications to the different TIL production steps are being proposed.37 Initial selection of tumor-reactive T cells from the bulk T cell population before culture is a promising strategy that has shown tumor regression in patients with epithelial cancers.38 Tumor-cell reactive T cells can be enriched by PD-139 and/or CD137 sorting.40 Additionally, tumor-reactive T cells can also be found in the peripheral blood and identified based on PD-1 expression, thus potentially offering a noninvasive source of tumor-specific T cells for ACT.41,42 Another strategy for preselecting tumor-reactive T cells is based on MHC-multimer staining of previously known reactive peptides presented in an MHC allele-specific manner.43 The benefits of preselecting tumor-reactive T cells include a reduction in the total number of infused cells to achieve responses and the possibility of the use of neoantigen-recognizing T cells. Neoantigens are antigenic peptides that arise from somatic mutations during cancer transformation. These T cells have been found in tumor masses44 or in peripheral blood.41 In contrast to shared tumor antigens, such as cancer-testis or tissue-differentiation antigens, neoantigen-reactive T cells exclusively target tumor cells. Neoantigen-recognizing TCRs are not selected against in the thymus; therefore, high-affinity antigen receptors are present in the repertoire.
To avoid excessive differentiation to terminal effector T cells during the REP, the use of cytokines other than IL-2, such as IL-7, is being evaluated.45 In addition, agonist CD137 mAb-mediated costimulation during TIL culture has resulted in better TIL yields with a less differentiated phenotype after REP.46 Type I interferons are also attractive candidates to improve functional TIL culture yields.47
Lymphodepleting preconditioning regimens improve the persistence of the transferred cells by increasing the availability of the homeostatic cytokines IL-7 and IL-15.48 Sustained IL-2 administration post-TIL infusion aims to foster the survival and proliferation of the transferred TILs.49 Even though more aggressive lymphodepleting regimens combining chemotherapy with total body irradiation (TBI) result in higher IL-7 and IL-15 cytokine levels and better overall response rates in a cohort of metastatic melanoma patients, safety problems have been reported.34
Importantly, TIL therapy may benefit from combination strategies with immunostimulatory mAbs. TIL treatment in metastatic melanoma is being tested in combination with anti-PD1, anti-CD137, or anti-CTLA-4 mAbs. Pretreatment with the latter checkpoint inhibitors induces increased TIL cell infiltration, thereby enhancing recovery.37 Immunostimulatory mAbs in ACT conceivably help to bypass the immunosuppressive TME, acting on both transferred and endogenous T-cells. In the case of anti-CTLA-4 mAb, they may also deplete Tregs.50
TIL therapy has been mostly evaluated in metastatic skin melanoma, a tumor frequently characterized by high mutational burdens51 and an abundant presence of neoantigen-reactive T cells.44 However, the efficacy of the approach on other solid tumors is less successful, with the exception of squamous carcinoma of the cervix.52 Difficulties inherent to other tumor types are scant T-cell recovery with poorer tumor cell recognition.53 Beyond metastatic melanoma and cervical carcinoma, TIL-based treatments are being evaluated in other tumors, such as metastatic uveal melanoma,54 breast cancer55 and ovarian cancer.56
ACT with engineered TCRs
A promising strategy to overcome some of the limitations of TIL therapy has been to redirect the specificity of T cells to selected tumor-associated antigens (TAAs) by means of gene transfer of high-affinity TCRs (Fig. 2). Genetic engineering of T cells using viral-based vectors or nonviral gene-editing strategies has been exploited to confer the desired antigen specificities to T cells by inserting genes encoding TCRα and β chains, avoiding mispairing with endogenous TCR chains.57 Advances in sequencing and prediction tools have expanded the possibilities to retrieve the sequence of a tumor-antigen-recognizing TCR derived from TILs or PBMCs from an HLA-matched donor. The TCR sequences are eventually gene transferred to dividing autologous PBLs in culture and infused back into a chemotherapeutically preconditioned patient.2
TAAs are a variable group of antigens presented by MHC molecules expressed on tumor cells. These targetable TAAs can be classified based on their expression selectivity among tissues and individuals. Tissue-differentiation antigens such as MART-1 and gp100 expressed in melanoma cells or CEA in colon cancer are shared antigens among patients and are expressed at least to some extent in nontransformed counterparts. The first clinical trials evaluating ACT with TCRs redirected towards these shared antigens resulted in frequent on-target/off-tumor toxicities as a consequence of the expression of the selected antigens in healthy nontransformed tissue.58,59
Cancer germline antigens have also been found to be shared among tumors in different individuals and are considered tumor-selective to a certain degree. These antigens are expressed by germline cells located in immune-privileged tissues that do not express MHC molecules and cancer cells.60 This type of TAA that belongs to the cancer-testis family includes, among others, NY-ESO1, MAGE, BAGE or GAGE. ACT using NY-ESO1-targeting unmodified TCR T cells has shown responses in patients with melanoma and synovial sarcoma.61 In addition, an affinity-enhanced NY-ESO1 targeting TCR has been evaluated with no reported off-tumor toxicities. Objective responses with no toxicities were also seen when a MAGE-A3 targeting affinity-enhanced TCR was used to treat several types of solid tumors.62 However, in other clinical trials evaluating an affinity-enhanced anti-MAGE-A3 TCR, severe neurological and cardiac toxicities occurred due to cross-reactivity with epitopes present in other proteins expressed in healthy tissues, such as the myocardial protein TITIN63 or MAGE-A12, a protein expressed in a subset of neurons in the human brain.64
Virus-derived antigens such as human papillomavirus (HPV) have also been exploited as targets for TCR redirected ACT approaches.65 Of note, an ACT approach based on transducing a TCR targeting HPV-E7 has entered clinical trials to evaluate the safety and efficacy of the approach against several HPV-associated tumors, including cervical, vulvar, vaginal, penile, anal, and oropharyngeal cancer (NCT02858310).
Neoantigens result from somatic mutations and are thus exclusively expressed by cancer cells. These mutations can affect genes directly related to malignant transformation (driver mutations),66 such as loss-of-function mutations in tumor suppressor genes or gain-of-function mutations in oncogenes. TCRs recognizing hot-spot mutations, prevalently shared among individuals, have been identified, including the mutated forms of p53,67 KRAS66 or BRAF.68 Importantly, TILs recognizing a KRAS mutation showed an outstanding clinical response in a patient with lung cancer.69 These findings stress the clinical potential of TCRs targeting prevalent hot-spot mutations in oncogenes and tumor suppressor genes.70 Serious biotechnological challenges exist for the deployment of the required technologies for such patient-tailored approaches.
ACT with CAR-T cells
An alternative method to redirect T-cell specificity is engineering with chimeric antigen receptors (CARs) (Fig. 2). CARs consist of constructs encompassing an extracellular single-chain variable fragment (scFv) and an intracellular signaling motif from the CD3ξ chain (first-generation CARs) and a costimulatory domain derived from the cytoplasmic tails of CD28, 4-1BB (CD137), OX40 or ICOS (second- and third-generation CARs).71 CARs are designed to confer T cells with antigen specificity in an MHC-independent manner, thus bypassing cancer intrinsic escape mechanisms based on MHC downregulation. In addition, the costimulatory domains ensure proper T-cell activation, proliferation, and persistence upon antigen encounter. In contrast to TCR-engineered T cells, CAR-T cells recognize only extracellular surface-expressed antigens.
The effectiveness and clinical potency of CAR-T cells have been widely evaluated against hematological malignancies in which an extracellular antigen expressed by malignant cells, such as CD19 and BCMA, has been targeted. Currently, CD19-targeting CAR-T cells are the only ACT-based option approved by the FDA.7 Impressive effects against acute lymphoid leukemia and B-cell lymphomas have been reported. Other CAR-T cells targeting plasma cells, such as BCMA,72 are also under advanced clinical investigation. Beyond CD19 targeting, other surface B-cell antigens are being explored, such as CD22 and CD20.73 Despite the clinical success of CARs against hematological malignancies, progression occurs due to targeted antigen loss or toxicities caused by cytokine release syndrome.74
In contrast to the success against hematological malignancies, the efficacy of CAR-T cells in solid tumors has been rather limited. The presence of an immunosuppressive TME and difficulties in finding surface targets exclusively expressed on tumor cells are some of the limiting factors. CAR-T cells targeting mesothelin, CEA, ERBB2/HER2, Glypican-3, IL13R, or EGFRvIII to treat solid tumors have been proposed and are under clinical investigation.75
Enhancing ACT by gene engineering
T-cell gene engineering to overcome the challenges faced by T-cell transfer is a rapidly expanding field of research.74 Exploiting the knowledge of basic T-cell biology and the understanding of the guiding principles in cancer immunotherapy have fostered the development of new technical approaches to modify immune cell function.76 Furthermore, toxicity concerns have led to the development of numerous approaches, including the incorporation of suicide genes, switch-mediated T-cell activation, or combinatorial antigen recognition to temporally, spatially, and functionally control the transferred T cells.77 These gene-editing approaches are used to engineer T cells to modulate specific functions and build powerful “bionic T cells” for cancer treatment.8
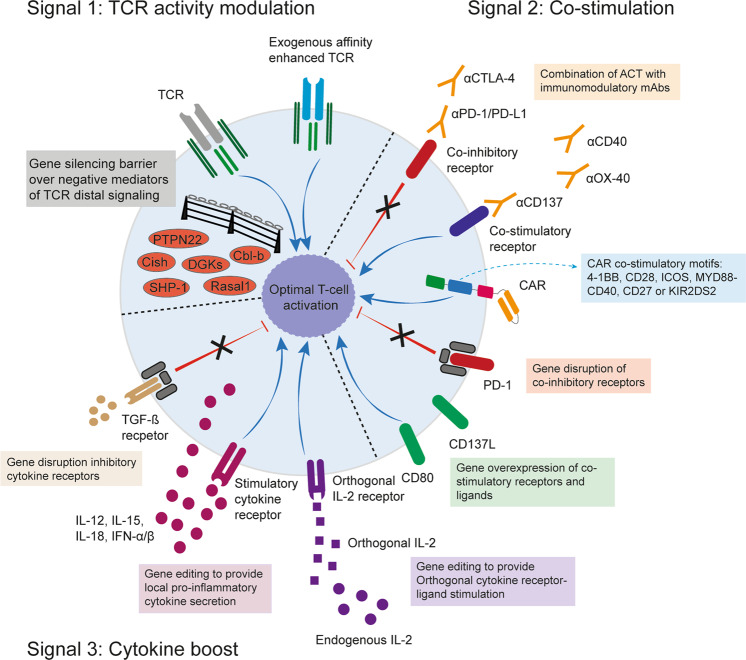
Reinforcing the three signals in ACT
Signal 1—modulating TCR activity for ACT
A limiting factor for antitumor T cell immunity and ACT is the peripheral tolerance-inducing features present in the TME.78 Additionally, T cells with high- to medium-affinity TCRs recognizing TAAs expressed during development or in normal tissues are often deleted during thymic selection or subjected to peripheral tolerance, thus reducing the endogenous tumor-recognizing T repertoire to low-affinity TCRs.79 To improve TCR-based approaches, affinity-augmented antigen receptors have been developed using vaccination strategies in HLA transgenic mice80 or TCR mutagenesis followed by selection in functional screenings.81 However, artificially enhancing TCR affinity can potentially generate unpredicted off-tumor cross-reactive TCRs. In concert, clinical trials evaluating the MAGE-A3 family targeting affinity-enhanced TCR-engineered T cells resulted in severe off-tumor toxicities due to cross-reactivity.63,82 In contrast to TCR affinity enhancement, the selection of neoantigen-targeting T cells for ACT approaches may offer an opportunity to overcome the TCR affinity barrier and, importantly, avoid on-target/off-tumor toxicities.57
Insufficient propagation and amplitude of the TCR signal upon antigen encounter restrains T-cell activation and effector functions. Thus, ways to intensify the TCR signal by modulating distal signaling mediators are being evaluated in ACT (Fig. 3). CISH is a member of the suppressor of cytokine signaling (SOCS) family. Its expression is induced upon TCR stimulation as well as by cytokines such as IL-2. CISH modulates TCR signaling by targeting the TCR intermediate PLC-γ1 for proteasomal degradation.83 Genetic deletion, as well as the knockdown of CISH in tumor-specific CD8+ T cells, has improved T-cell functionality and antitumor immunity upon ACT in preclinical models.83 Additionally, knocking down other TCR signaling mediators in tumor-specific CD8+ T cells, such as SHP-1,84 DGKs,85 PTPN2286 or RASAL1,87 has increased T cell antitumor responses upon ACT in preclinical models. These studies show the therapeutic potential of modulating TCR signaling to increase the functional avidity rather than the chemical affinity in tumor-specific T cells for ACT approaches. CRISPR/Cas9 screenings are used to identify previously unknown factors responsible for the downregulation of T-cell performance.88–91
Fig. 3.
Reinforcing the three signals in adoptive T-cell therapy. Potential mechanisms of intervention to make the most of the ACT by manipulating the to-be-infused cell product
Most of the gene-editing strategies used on T cells for ACT are based on knocking down the expression of candidate genes using short hairpin RNAs to achieve constitutive gene silencing. However, disrupting mutations of genes encoding TCR signaling mediators are often found in autoimmune conditions.89 Thus, constitutive silencing of some of these targets on T cells may result in the development of unintended autoimmune side effects. As an alternative, transient modulation of T-cell function using siRNAs has been evaluated.92 Cbl-b is an E2 ubiquitin ligase that functions as a central modulator of T-cell responses by inhibiting signaling pathways including TCR and CD28.93 Transient silencing of Cbl-b gene expression in tumor-specific T cells using siRNAs in preclinical ACT settings has shown increased antitumor activity.94 This approach is currently being tested in a phase I clinical trial (NCT03087591).
In CAR-T cell approaches, affinity towards the targeted tumor antigen can be tuned by generating different scFvs.71 Of note, it has recently been reported that low-affinity scFvs in CARs against tumors with high surface expression of the antigen are sufficient to enable robust expansion of the CAR-T cells, with less serious adverse events.95,96
Signal 2—providing costimulation to transferred T cells
A major disadvantage of ACT in solid tumors is the presence of co-inhibitory ligands and poor costimulatory signals in the TME that result in T-cell dysfunction and, ultimately, treatment failure. In the case of second- and third-generation CAR-T cells, the requirement for external T-cell costimulation for proper T-cell activation is met by the costimulatory motif within the CAR construct. The impact of several costimulatory signals has been evaluated in CAR-T cells, including FDA/EMA-approved CARs containing CD28 or CD137/4-1BB motifs as well as ICOS, MYD88-CD40, CD27 or KIR2DS2 signaling motifs (reviewed in refs. 71,97).
The success of ICB therapy using mAbs depends on a pre-existing antitumor CD8+ T cell infiltrate,98 a feature that can be circumvented in ACT approaches where T cells are administered exogenously. This suggests a potential synergistic combination between ICB therapy and ACT. T cells, when infused in ACT strategies, upregulate multiple inhibitory and costimulatory receptors. Blocking the PD-1/PD-L1 axis in ACT strategies enhanced the antitumor activity and increased the migration of the transferred cells to the TME in murine preclinical models.99 Similarly, CTLA-4 blockade alone100 or in combination with PD-1/PD-L1 blockade101 improved T-cell therapy. As an alternative to ICB, agonistically triggering costimulatory receptors has also been evaluated in ACT settings. Preclinically, combinatorial strategies using CD137/4-1BB,102 OX40,103 GITR104 or CD40105 targeting agonistic mAbs and ACT have resulted in increased antitumor T-cell responses in mice. Currently, multiple clinical trials are evaluating the combination of different ACT approaches and immune-stimulatory mAbs.
An alternative approach to providing T cell costimulation is to modify the transferred cells by genetic engineering strategies. These strategies aim to supply cells with intrinsic stimulatory capacities to make the use of systemic immunomodulatory mAbs unnecessary, thereby minimizing treatment-related toxicities (Fig. 3). In the case of PD-1, its expression has been modulated in CAR-T cells by transducing a truncated form of the receptor to act as a dominant-negative (DN) receptor or by shRNA-mediated silencing.106 In addition, CRISPR-Cas9 deletion of PD-1 has also been used to improve CAR-T cell performance in antitumor responses preclinically.107 This latter approach of using CRISPR-Cas9 technology to delete PD-1 has been used with NY-ESO1 TCR transduced T cells in a phase I clinical trial. Intriguingly, in this setting, PD-1 silencing did not result in increased T cell persistence, but the clinical feasibility of the gene-editing approach was convincingly shown.108 Regarding T cell-intrinsic costimulation, PSMA-targeting CAR-T cells have been transfected to overexpress CD80 and CD137L, resulting in increased antitumor activity in an autocrine and/or paracrine manner.109 Furthermore, the retroviral gene delivery strategy to provide a T cell with CD137L overexpression is being evaluated in a phase I clinical trial using CD19-targeting CAR-T cells (NCT03085173).
Signal 3—cytokine boost in ACT
In addition to the insufficient costimulatory ligands in the TME, poor immune-stimulatory cytokine support limits the differentiation, expansion, and persistence of the transferred T cells. Standard TIL treatment protocols generally include exogenous administration of high doses of IL-2 to support the expansion and activity of the TIL product. However, IL-2 administration is associated with serious toxicities, including capillary leak syndrome and off-target deleterious consequences, such as the expansion of endogenous Tregs.110 Furthermore, the short half-life of exogenously given recombinant cytokines, including IL-2, IL-7, IL-15 or IL-21, which are evaluated in ACT approaches, requires repeated high-dose administration to achieve clinical activity, often resulting in serious toxicities.111 Of note, such toxicities are related to maximal concentrations administered intravenously, and lower concentrations via subcutaneous administration are being tested.111
To circumvent these limitations, different gene-editing strategies to provide more restricted cytokine stimulation to T cells are being developed in preclinical studies (Fig. 3). Genetic engineering of T cells with orthogonal IL-2 receptor pairs that do not interact with the natural cytokine was used to selectively deliver IL-2 stimulation to the transferred cells.112 The approach aims to render exclusive orthogonal IL-2 sensitivity to the engineered T cells and to avoid systemic IL-2 detection, thereby resulting in no toxicities and enhanced antitumor efficacy in mouse models. Moreover, CAR-T cells engineered with a constitutive signaling IL-7 receptor showed improved antitumor activity and longer persistence in vivo in mouse models.113
Alternatively, genetic modifications of T cells to locally secrete cytokines are being evaluated. IL-12 is a Th1-promoting cytokine physiologically secreted by activated APCs that principally leads to IFN-γ secretion by T cells. Despite the potent antitumor activity of IL-12, the development of IL-12-based therapies has been overshadowed and limited by toxic side effects.114 To confine controlled and restricted IL-12 secretion to tumor-specific T cells, such T cells have been retrovirally transduced with a gene fragment consisting of a single-chain IL-12 variant under the control of an NFAT-binding promoter.115 This construct restricts IL-12 secretion to tumor-specific transferred cells undergoing TCR-mediated antigen stimulation. In mouse models of melanoma, the approach showed impressive antitumor efficacy with no apparent toxicity. However, in the subsequent clinical trial using TILs transduced with the inducible IL-12 construct, life-threatening toxicities and fatalities were reported that were attributable to leaky expression of IL-12.116 To further limit exposure to IL-12 only in the TME and to avoid systemic cytokine exposure, tumor-specific T cells have been transfected with a T cell activation-induced membrane-anchored version of IL-12.117 The membrane-anchored IL-12-producing T cells showed comparable antitumor efficacy to the secreted version with no systemic cytokine exposure and no toxicity, at least in mouse models. Beyond IL-12 genetically engineered T cells, in an attempt to overcome toxicity limitations, inducible IL-18 secreting tumor-specific T cells have been tested in mouse models showing safety and enhanced antitumor responses.118 In the CAR-T cell setting, multiple cytokine-producing CARs, so-called “armored” CAR-T cells, including IL-12,119 IL-15120 and IL-18,121 are being developed. Notably, in one study, CAR-T cells engineered to secrete IL-23 outperformed both IL-15- and IL-18-secreting CAR-T cells in terms of antitumor efficacy and better safety profiles.122
Recently, the idea of conferring transient IL-12 and CD137L expression by means of mRNA electroporation into tumor-specific CD8+ T cells has been tested.123 This approach works well in mouse models, contingent on repeated administration. Importantly, the transient engineering strategy offers obvious safety advantages since it is extinguished over time.
Furthermore, the presence of immunosuppressive cytokines such as IL-4 or TGF-β in the TME limits the effective effector function of ACT cells, particularly in solid tumors. To reverse the inhibitory stimulus provided by IL-4, a chimeric cytokine receptor consisting of the extracellular motifs of the IL-4 receptor fused to the intracellular domain of the IL- 7 receptor has been developed.124 In a subsequent study, a CAR-T cell with the IL-4R/IL-7R chimeric cytokine receptor was additionally provided with another chimeric construct recognizing TGF-β fused to 4-1BB intracellular signaling domains. This combination reverses the effect of the inhibitory cytokine and turns a negative factor into a costimulatory one.125 Another approach to confer TGF-β resistance to the transferred cells has been to express a dominant-negative TGF-β receptor II in CAR-T cells126 or to knock down an endogenous TGF-β receptor II chain by CRISPR/Cas9.127 Observations from the group of Richard Flavell in transgenic mice are very supportive of this approach.128,129
Turning down T-cell differentiation for ACT
T-cell differentiation status correlates with the outcome of ACT therapies. The infusion of highly differentiated effector cells with reduced proliferative capacity results in low T-cell persistence and poor memory formation that limits long-lasting antitumor activity.130
Gene engineering strategies to obtain less differentiated T cells with self-renewal capacity and effector functions for ACT are intensively being investigated (Fig. 4a). In line with this, retroviral transduction of c-Myb, a master regulator of T-cell stemness and memory differentiation, conferred transferred T cells a long life with a TCM phenotype that resulted in greater antitumor control in mouse models.131 Gene engineering expression of a membrane-anchored form of IL-15 in CAR-T cells also contributed to the development of long-term persisting T cells with a memory stem-cell phenotype and increased antitumor activity.120
Fig. 4.
Modulating T cell differentiation, metabolism, and trafficking to enhance the efficacy of adoptive T-cell therapy. Immunobiological functions and features considered exploitable to enhance the efficacy of ACT by controlling a T-cell differentiation, b T-cell metabolism, and c T-cell trafficking and migration
An additional strategy to modulate T-cell differentiation involves the disruption of the transcriptional factors implicated in T-cell dysfunction and terminal differentiation. In broad terms, T-cell dysfunction is characterized by excessive effector differentiation, the upregulation of multiple inhibitory receptors, and diminished capacity to produce IL-2 and IFN-γ upon antigen re-encounter. Multiple functional screening strategies knocking down candidate genes for T-cell activity or transcriptomic comparison analysis revealed potential targets involved in T-cell dysfunction. The silencing of these newly identified candidates in several ACT approaches, including NR4A,132 TOX, and TOX2,133 TET2,134 Regnase1135 or PTPN22,136 has resulted in enhanced T cell function and long-lasting antitumor activity in preclinical models. Additionally, to avoid T-cell dysfunction, the overexpression of c-Jun caused reduced expression of markers of dysfunction and the upregulation of genes related to T-cell memory in CAR-T cells.137
In CAR-T cell settings, the intracellular motif of the CAR construct modulates the differentiation status of the T cells. Intracellular CD137/4-1BB-mediated costimulation leads to longer CAR-T cell persistence and memory differentiation in CD8+ T cells, whereas CD28 signaling results in more effector but shorter-lived antitumor activity.138
Aside from gene engineering, the preselection of T cells with stem-cell-like properties might yield greater persistence.130
Optimizing T-cell metabolism for ACT
The metabolic profile of T cells adapts to the cell-intrinsic requirements during the different phases of T-cell differentiation and activation. Naive and memory T cells rely on mitochondrial respiration (OXPHOS) and fatty acid oxidation (FAO) for homeostatic maintenance. In contrast, activated T cells upregulate aerobic glycolytic pathways to support the high energy demands necessary for proliferation and effector functions.139 In cancer immunotherapy, T-cell differentiation status, function, and persistence correlate with cell metabolic fitness.140 In solid tumors, the metabolically deprived TME characterized by low glucose availability, hypoxia, and acidic pH hinders T-cell activity and compromises antitumor responses. In this regard, gene engineering strategies to reprogram and optimize the metabolic activity of the transferred T cells to protect them from the metabolically hostile TME have only recently come under investigation (Fig. 4b).
PKC1 is a carboxykinase that produces phosphoenolpyruvate, a metabolite of the glycolytic pathway that, in the presence of glucose, sustains intracellular Ca2+ and NFAT signaling upon TCR activation. To circumvent the glucose-deprived TME that limits aerobic glycolysis on activated T cells, retroviral overexpression of PKC1 on tumor-specific T cells results in the potentiation of antitumor responses against tumor models that reduce glucose tissue availability.141
In T cells, HIF-1α is the main transcriptional factor involved in the response to hypoxia, and its downstream target Glut1 facilitates glucose uptake, thus allowing glycolytic metabolism. In a hypoxic and glucose-deprived TME, T cells are found in a stressed metabolic state in which HIF-1α may counterproductively promote glycolysis and inhibit OXPHOS. Although conditional genetic deletion of HIF-1α in T cells leads to accelerated tumor growth and T-cell response impairment,142 in an ACT approach, shRNA-mediated downregulation of HIF-1α in TAA-specific CD8+ cells reduced glycolysis and promoted FAO, resulting in enhanced antitumor activity of the T cells upon transfer to treat a melanoma preclinical model.143
In addition, TILs show a progressive reduction in mitochondrial mass and function mediated by the downregulation of PGC1α, the main transcriptional factor involved in orchestrating mitochondrial biogenesis. Retroviral transduction of TAA-specific T cells to overexpress PGC1α for ACT showed an enhanced mitochondrial mass and activity that resulted in higher memory formation, persistence, and antitumor immunity in vivo.144,145
Notably, CAR-T cells stimulated by a CD28 intracellular motif rely predominantly on glucose-based fast energy via anaerobic glycolysis and are reprogrammed towards effector differentiation. In contrast, CAR-T cells stimulated by the CD137/4-1BB motif upregulate FAO and mitochondrial respiration, which provide a sustained source of energy under mitochondrial stress conditions that support central memory differentiation, resulting in longer persistence.146 This parallels reported functions for CD137 costimulation in nontransduced T cells.147,148
Stimulating epitope spreading in ACT
The loss of the target antigen or the downregulation of MHC-I expression by tumor cells represent cancer escape mechanisms hindering CAR-T and transgenic TCR cell-based therapies, in which a single antigen is targeted. Antigen escape may be prevented by engineering CARs that target multiple antigens.149 In addition, boosting endogenous epitope spreading is a possibility. Epitope spreading is a phenomenon in which endogenous T cells recognizing epitopes different from those targeted by the transferred cells are expanded. This phenomenon provides another tool to prevent tumor escape by antigen loss. In line with this, constitutive expression of CD40L in CAR-T cells increased dendritic cell licensing and generated endogenous tumor-recognizing T cells that prevented the outgrowth of targeted antigen-negative tumor cells.150 The approach resulted in greater antitumor efficacy in mouse tumor models and highlighted the importance of APC licensing and efficient cross-presentation processes151 to recruit endogenous immune effectors and mount long-lasting antitumor responses (Fig. 4c). In the case of T cells transiently engineered to express IL-12 that were intratumorally injected, clear evidence for epitope spreading was documented.123 This mechanism ensured long-term tumor control and was mediated by cDC1 cells known to excel in antigen cross-presentation to CD8+ T cells.151
Directing T-cell homing to cancer tissue in ACT
In solid tumors, the standard protocol for ACT approaches is based on intravenous administration of T cells, in which the transferred cells have to migrate from the blood to the TME. One of the factors limiting the therapeutic outcome of ACT approaches is the low trafficking efficacy and penetrance into the TME.28 Engineering strategies to improve the tumor homing of the transferred T cells to the malignant tissue are under investigation. The principal strategy relies on providing T cells with an exogenous chemokine receptor that recognizes tumor-associated chemokines (Fig. 4c).
Tumor cells abundantly secrete the chemokines CXCL1 and CXCL8, which are known to mediate CXCR1/2-dependent migration of monocytes and neutrophils. Importantly, T cells do not express CXCR1 or 2. Gene insertion of the CXCR2 receptor into tumor-specific T-cells before cell transfer enhanced T cell tumor homing and improved antitumor immune responses in mouse models following intravenous delivery.152 Similar approaches targeting other tumor-associated cytokines, such as CCL2, by overexpressing CCR2 in T cells have also shown improved T-cell homing and subsequent enhanced antitumor responses.153
An alternative way to increase T-cell infiltration has been to overexpress the T-cell recruiting chemokine CCL19 in CAR-T cells.154 The goal of this approach is to increase endogenous T-cell infiltration, and CCL19 may also serve as a calling signal for those transferred cells still on their way to the TME.
Concluding remarks
Adoptive T-cell therapy for cancer is still in its infancy. The potential of cell and gene therapy together in this regard is magnificent. Several areas will be improved by biotechnology and customized cell therapy. Single-cell sequencing and microfluidics, improved T cell culture and reinvigoration techniques, and off-the-shelf edited cultures of T cells will spearhead this progress. Intratumoral and repeated administration are clinically feasible,155 and dealing with cytokine release syndromes and other inflammatory complications are also workable in the clinic. To make the most ACT, we will probably need to combine several tools in our quest to build the best-performing bionic T cells.
Acknowledgements
We acknowledge Dr. Fernando Pastor for his critical reading and suggestions for this review. We are also thankful to all members of Melero´s laboratory for their helpful notes. Managing assistance from Esther Guirado, Cibeles Pinto, and Dr. Belen Palencia are greatly appreciated. English editing by Dr. Paul W. Miller is acknowledged.
Competing interests
I. Melero is a paid consultant for Bristol-Myers Squibb, Roche, AstraZeneca, Pharmamar, Alligator, Numab, F-star, Servier, and MSD and reports receiving commercial research grants from Alligator, Bristol-Myers Squibb, Roche, and Pharmamar. No potential conflict of interest were disclosed by the other authors.
Contributor Information
Iñaki Etxeberria, Email: ietxeberria@alumni.unav.es.
Ignacio Melero, Email: imelero@unav.es.
References
- 1.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimasaki Noriko, Jain Amit, Campana Dario. NK cells for cancer immunotherapy. Nature Reviews Drug Discovery. 2020;19(3):200–218. doi: 10.1038/s41573-019-0052-1. [DOI] [PubMed] [Google Scholar]
- 4.Liu E, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. New Engl. J. Med. 2020;382:545–553. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg SA, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen R, et al. Long-Lasting complete responses in patients with metastatic melanoma after adoptive cell therapy with tumor-infiltrating lymphocytes and an attenuated il2 regimen. Clin. Cancer Res. 2016;22:3734–3745. doi: 10.1158/1078-0432.CCR-15-1879. [DOI] [PubMed] [Google Scholar]
- 7.June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MCCAR. T cell immunotherapy for human cancer. Science. 2018;359:1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 8.Pardoll DM. Building the bionic T cell. Nat. Med. 2007;13:1411–1413. doi: 10.1038/nm1207-1411. [DOI] [PubMed] [Google Scholar]
- 9.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Roberts EW, et al. Critical role for CD103(+)/CD141(+) dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell. 2016;30:324–336. doi: 10.1016/j.ccell.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jansen CS, et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature. 2019;576:465–470. doi: 10.1038/s41586-019-1836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simoni Y, et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018;557:575–579. doi: 10.1038/s41586-018-0130-2. [DOI] [PubMed] [Google Scholar]
- 13.Zajac AJ, et al. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zarour HM. Reversing T-cell dysfunction and exhaustion in cancer. Clin. Cancer Res. 2016;22:1856–1864. doi: 10.1158/1078-0432.CCR-15-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chauvin J-M, et al. TIGIT and PD-1 impair tumor antigen-specific CD8+ T cells in melanoma patients. J. Clin. Invest. 2015;125:2046–2058. doi: 10.1172/JCI80445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X-Y, et al. CD155 loss enhances tumor suppression via combined host and tumor-intrinsic mechanisms. J. Clin. Invest. 2018;128:2613–2625. doi: 10.1172/JCI98769. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Wherry EJ. T cell exhaustion. Nat. Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 18.Joshi NS, Kaech SM. Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. J. Immunol. 2008;180:1309–1315. doi: 10.4049/jimmunol.180.3.1309. [DOI] [PubMed] [Google Scholar]
- 19.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev. Immunol. 2013;31:137–161. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Diez A, et al. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood. 2007;109:5346–5354. doi: 10.1182/blood-2006-10-051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alspach E, et al. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature. 2019;574:696–701. doi: 10.1038/s41586-019-1671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett SR, et al. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 23.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 24.Ahrends T, et al. CD4+ T cell help confers a cytotoxic T cell effector program including coinhibitory receptor downregulation and increased tissue invasiveness. Immunity. 2017;47:848–861.e5. doi: 10.1016/j.immuni.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Hunder NN, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl. J. Med. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purwar R, et al. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat. Med. 2012;18:1248–1253. doi: 10.1038/nm.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu Y, et al. Th9 cells represent a unique subset of CD4(+) T cells endowed with the ability to eradicate advanced tumors. Cancer Cell. 2018;33:1048–1060.e7. doi: 10.1016/j.ccell.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melero I, Rouzaut A, Motz GT, Coukos G. T-cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy. Cancer Disco. 2014;4:522–526. doi: 10.1158/2159-8290.CD-13-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalluri R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer. 2016;16:582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 30.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura Kyohei, Smyth Mark J. Myeloid immunosuppression and immune checkpoints in the tumor microenvironment. Cellular & Molecular Immunology. 2019;17(1):1–12. doi: 10.1038/s41423-019-0306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Busse D, et al. Competing feedback loops shape IL-2 signaling between helper and regulatory T lymphocytes in cellular microenvironments. Proc. Natl Acad. Sci. USA. 2010;107:3058–3063. doi: 10.1073/pnas.0812851107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paluskievicz CM, et al. T regulatory cells and priming the suppressive tumor microenvironment. Front Immunol. 2019;10:1–15. doi: 10.3389/fimmu.2019.02453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dudley ME, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenberg SA, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N. Engl. J. Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 36.Lee S, Margolin K. Tumor-infiltrating lymphocytes in melanoma. Curr. Oncol. Rep. 2012;14:468–474. doi: 10.1007/s11912-012-0257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rohaan MW, van den Berg JH, Kvistborg P, Haanen JBAG. Adoptive transfer of tumor-infiltrating lymphocytes in melanoma: a viable treatment option. J. Immunother. Cancer. 2018;6:102. doi: 10.1186/s40425-018-0391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yee C. The use of endogenous T cells for adoptive transfer. Immunol. Rev. 2014;257:250–263. doi: 10.1111/imr.12134. [DOI] [PubMed] [Google Scholar]
- 39.Gros A, et al. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J. Clin. Invest. 2014;124:2246–2259. doi: 10.1172/JCI73639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ye Q, et al. CD137 accurately identifies and enriches for naturally occurring tumor-reactive T cells in tumor. Clin. Cancer Res. 2014;20:44–55. doi: 10.1158/1078-0432.CCR-13-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gros A, et al. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat. Med. 2016;22:433–438. doi: 10.1038/nm.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandez-Poma, S. M. et al. Expansion of tumor-infiltrating CD8+ T cells expressing PD-1 improves the efficacy of adoptive T cell therapy. Cancer Res.http://cancerres.aacrjournals.org/lookup/doi/10.1158/0008-5472.CAN-17-0236 (2017) [DOI] [PubMed]
- 43.Kelderman S, et al. Antigen-specific TIL therapy for melanoma: a flexible platform for personalized cancer immunotherapy. Eur. J. Immunol. 2016;46:1351–1360. doi: 10.1002/eji.201545849. [DOI] [PubMed] [Google Scholar]
- 44.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 45.Yang S, et al. Modulating the differentiation status of ex vivo-cultured anti-tumor T cells using cytokine cocktails. Cancer Immunol. Immunother. 2013;62:727–736. doi: 10.1007/s00262-012-1378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chacon JA, et al. Co-stimulation through 4-1BB/CD137 improves the expansion and function of CD8(+) melanoma tumor-infiltrating lymphocytes for adoptive T-cell therapy. PLoS ONE. 2013;8:e60031–e60031. doi: 10.1371/journal.pone.0060031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hervas-Stubbs S, et al. CD8 T cell priming in the presence of IFN-α renders CTLs with improved responsiveness to homeostatic cytokines and recall antigens: important traits for adoptive T cell therapy. J. Immunol. 2012;189:3299–3310. doi: 10.4049/jimmunol.1102495. [DOI] [PubMed] [Google Scholar]
- 48.Gattinoni L, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arenas-Ramirez N, Woytschak J, Boyman O. Interleukin-2: biology, design and application. Trends Immunol. 2015;36:763–777. doi: 10.1016/j.it.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Romano E, et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc. Natl Acad. Sci. USA. 2015;112:6140–6145. doi: 10.1073/pnas.1417320112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alexandrov LB, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jazaeri AA, et al. Safety and efficacy of adoptive cell transfer using autologous tumor infiltrating lymphocytes (LN-145) for treatment of recurrent, metastatic, or persistent cervical carcinoma. J. Clin. Oncol. 2019;37:2538. [Google Scholar]
- 53.Radvanyi LG. Tumor-infiltrating lymphocyte therapy: addressing prevailing questions. Cancer J. 2015;21:450–464. doi: 10.1097/PPO.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 54.Chandran SS, et al. Treatment of metastatic uveal melanoma with adoptive transfer of tumour-infiltrating lymphocytes: a single-centre, two-stage, single-arm, phase 2 study. Lancet Oncol. 2017;18:792–802. doi: 10.1016/S1470-2045(17)30251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Solinas C, Carbognin L, De Silva P, Criscitiello C, Lambertini M. Tumor-infiltrating lymphocytes in breast cancer according to tumor subtype: current state of the art. Breast. 2017;35:142–150. doi: 10.1016/j.breast.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 56.Pedersen M, et al. Adoptive cell therapy with tumor-infiltrating lymphocytes in patients with metastatic ovarian cancer: a pilot study. Oncoimmunology. 2018;7:e1502905–e1502905. doi: 10.1080/2162402X.2018.1502905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chandran SS, Klebanoff CA. T cell receptor-based cancer immunotherapy: Emerging efficacy and pathways of resistance. Immunol. Rev. 2019;290:127–147. doi: 10.1111/imr.12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morgan RA, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parkhurst MR, et al. T cells targeting carcinoembryonic antigen can eradiate regression of metastatic colorectal cancer but induce severe transient colitis. Mol. Ther. 2011;19:620–626. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simpson AJG, Caballero OL, Jungbluth A, Chen Y-T, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat. Rev. Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 61.Robbins PF, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu Y-C, et al. Treatment of patients with metastatic cancer using a major histocompatibility complex class II-restricted T-cell receptor targeting the cancer germline antigen MAGE-A3. J. Clin. Oncol. 2017;35:3322–3329. doi: 10.1200/JCO.2017.74.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cameron BJ, et al. Identification of a titin-derived HLA-A1–presented peptide as a cross-reactive target for engineered MAGE A3–directed T cells. Sci. Transl. Med. 2013;5:197ra103. doi: 10.1126/scitranslmed.3006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morgan RA, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother. 2013;36:133–151. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jin BY, et al. Engineered T cells targeting E7 mediate regression of human papillomavirus cancers in a murine model. JCI insight. 2018;3:e99488. doi: 10.1172/jci.insight.99488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang QJ, et al. Identification of T-cell receptors targeting KRAS-mutated human tumors. Cancer Immunol. Res. 2016;4:204–214. doi: 10.1158/2326-6066.CIR-15-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malekzadeh Parisa, Yossef Rami, Cafri Gal, Paria Biman C., Lowery Frank J., Jafferji Mohammad, Good Meghan L., Sachs Abraham, Copeland Amy R., Kim Sanghyun P., Kivitz Scott, Parkhurst Maria R., Robbins Paul F., Ray Satyajit, Xi Liqiang, Raffeld Mark, Yu Zhiya, Restifo Nicholas P., Somerville Robert P.T., Rosenberg Steven A., Deniger Drew C. Antigen Experienced T Cells from Peripheral Blood Recognize p53 Neoantigens. Clinical Cancer Research. 2020;26(6):1267–1276. doi: 10.1158/1078-0432.CCR-19-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Veatch JR, et al. Tumor-infiltrating BRAFV600E-specific CD4+ T cells correlated with complete clinical response in melanoma. J. Clin. Invest. 2018;128:1563–1568. doi: 10.1172/JCI98689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tran E, et al. T-cell transfer therapy targeting mutant KRAS in cancer. N. Engl. J. Med. 2016;375:2255–2262. doi: 10.1056/NEJMoa1609279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Olivera Irene, Etxeberria Iñaki, Bolaños Elixabet, Gato-Cañas María, Melero Ignacio. Exploiting TCR Recognition of Shared Hotspot Oncogene-encoded Neoantigens. Clinical Cancer Research. 2020;26(6):1203–1204. doi: 10.1158/1078-0432.CCR-19-3813. [DOI] [PubMed] [Google Scholar]
- 71.Guedan S, Calderon H, Posey AD, Maus MV. Engineering and design of chimeric antigen receptors. Mol. Ther. Methods Clin. Dev. 2019;12:145–156. doi: 10.1016/j.omtm.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cohen AD, et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J. Clin. Invest. 2019;129:2210–2221. doi: 10.1172/JCI126397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fry TJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 2018;1:20–28. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lim WA, June CH. The principles of engineering immune cells to treat cancer. Cell. 2017;168:724–740. doi: 10.1016/j.cell.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schmidts A, Maus MV, Making CAR. T cells a solid option for solid tumors. Front Immunol. 2018;9:2593. doi: 10.3389/fimmu.2018.02593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spear TT, Nagato K, Nishimura MI. Strategies to genetically engineer T cells for cancer immunotherapy. Cancer Immunol. Immunother. 2016;65:631–649. doi: 10.1007/s00262-016-1842-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ren Y-B, Sun S-J, Han S-Y. Safety strategies of genetically engineered T cells in cancer immunotherapy. Curr. Pharm. Des. 2018;24:78–83. doi: 10.2174/1381612824666171227222624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jackson SR, Yuan J, Teague RM. Targeting CD8+ T-cell tolerance for cancer immunotherapy. Immunother. 2014;6:833–852. doi: 10.2217/imt.14.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aleksic M, et al. Different affinity windows for virus and cancer-specific T-cell receptors: implications for therapeutic strategies. Eur. J. Immunol. 2012;42:3174–3179. doi: 10.1002/eji.201242606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Parkhurst MR, et al. Characterization of genetically modified T-cell receptors that recognize the CEA:691-699 peptide in the context of HLA-A2.1 on human colorectal cancer cells. Clin. Cancer Res. 2009;15:169–180. doi: 10.1158/1078-0432.CCR-08-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li Y, et al. Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nat. Biotechnol. 2005;23:349–354. doi: 10.1038/nbt1070. [DOI] [PubMed] [Google Scholar]
- 82.Linette GP, et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122:863–871. doi: 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Palmer DC, et al. Cish actively silences TCR signaling in CD8+ T cells to maintain tumor tolerance. J. Exp. Med. 2015;212:1–19. doi: 10.1084/jem.20150304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stromnes IM, et al. Abrogation of SRC homology region 2 domain-containing phosphatase 1 in tumor-specific T cells improves efficacy of adoptive immunotherapy by enhancing the effector function and accumulation of short-lived effector T cells in vivo. J. Immunol. 2012;189:1812–1825. doi: 10.4049/jimmunol.1200552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Riese MJ, et al. Enhanced effector responses in activated CD8+ T cells deficient in diacylglycerol kinases. Cancer Res. 2013;73:3566–3577. doi: 10.1158/0008-5472.CAN-12-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brownlie, R. J., Wright, D., Zamoyska, R. & Salmond, R. J. Deletion of PTPN22 improves effector and memory CD8+ T cell responses to tumors. JCI Insight4, e127847 (2019). [DOI] [PMC free article] [PubMed]
- 87.Thaker YR, Raab M, Strebhardt K, Rudd CE. GTPase-activating protein Rasal1 associates with ZAP-70 of the TCR and negatively regulates T-cell tumor immunity. Nat. Commun. 2019;10:1–13. doi: 10.1038/s41467-019-12544-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.LaFleur MW, et al. A CRISPR-Cas9 delivery system for in vivo screening of genes in the immune system. Nat. Commun. 2019;10:1–10. doi: 10.1038/s41467-019-09656-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shifrut E, et al. Genome-wide CRISPR screens in primary human T cells reveal key regulators of immune function. Cell. 2018;175:1958–1971.e15. doi: 10.1016/j.cell.2018.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Manguso RT, et al. In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature. 2017;547:413–418. doi: 10.1038/nature23270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Patel SJ, et al. Identification of essential genes for cancer immunotherapy. Nature. 2017;548:537–542. doi: 10.1038/nature23477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ramishetti S. & Peer D. Engineering lymphocytes with RNAi. Adv. Drug Deliv. Rev.141, 55–66 (2019). [DOI] [PubMed]
- 93.Tang R, Langdon WY, Zhang J. Regulation of immune responses by E3 ubiquitin ligase Cbl-b. Cell Immunol. 2019;340:103878. doi: 10.1016/j.cellimm.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hinterleitner R, et al. Adoptive transfer of siRNA Cblb-silenced CD8+ T lymphocytes augments tumor vaccine efficacy in a B16 melanoma model. PLoS ONE. 2012;7:1–9. doi: 10.1371/journal.pone.0044295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ramakrishna S, et al. Modulation of target antigen density improves CAR T-cell functionality and persistence. Clin. Cancer Res. 2019;25:5329–5341. doi: 10.1158/1078-0432.CCR-18-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ghorashian S, et al. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat. Med. 2019;25:1408–1414. doi: 10.1038/s41591-019-0549-5. [DOI] [PubMed] [Google Scholar]
- 97.Weinkove R, George P, Dasyam N, McLellan AD. Selecting costimulatory domains for chimeric antigen receptors: functional and clinical considerations. Clin. Transl. Immunol. 2019;8:e1049–e1049. doi: 10.1002/cti2.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen P-L, et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Disco. 2016;6:827–837. doi: 10.1158/2159-8290.CD-15-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peng W, et al. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-γ inducible chemokines. Cancer Res. 2012;72:5209–5218. doi: 10.1158/0008-5472.CAN-12-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mahvi DA, et al. Ctla-4 blockade plus adoptive T-cell transfer promotes optimal melanoma immunity in mice. J. Immunother. 2015;38:54–61. doi: 10.1097/CJI.0000000000000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shi LZ, et al. Blockade of CTLA-4 and PD-1 enhances adoptive t-cell therapy efficacy in an ICOS-mediated manner. Cancer Immunol. Res. 2019;7:1803–1812. doi: 10.1158/2326-6066.CIR-18-0873. [DOI] [PubMed] [Google Scholar]
- 102.Weigelin B, et al. Focusing and sustaining the antitumor CTL effector killer response by agonist anti-CD137 mAb. Proc. Natl Acad. Sci. USA. 2015;112:7551–7556. doi: 10.1073/pnas.1506357112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kjaergaard J, et al. Augmentation versus inhibition: effects of conjunctional OX-40 receptor monoclonal antibody and IL-2 treatment on adoptive immunotherapy of advanced tumor. J. Immunol. 2001;167:6669–6677. doi: 10.4049/jimmunol.167.11.6669. [DOI] [PubMed] [Google Scholar]
- 104.Imai N, et al. Glucocorticoid-induced tumor necrosis factor receptor stimulation enhances the multifunctionality of adoptively transferred tumor antigen-specific CD8+ T cells with tumor regression. Cancer Sci. 2009;100:1317–1325. doi: 10.1111/j.1349-7006.2009.01179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu C, et al. Agonistic antibody to CD40 boosts the antitumor activity of adoptively transferred T cells in vivo. J. Immunother. 2012;35:276–282. doi: 10.1097/CJI.0b013e31824e7f43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cherkassky L, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J. Clin. Invest. 2016;126:3130–3144. doi: 10.1172/JCI83092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Choi BD, et al. CRISPR-Cas9 disruption of PD-1 enhances activity of universal EGFRvIII CAR T cells in a preclinical model of human glioblastoma. J. Immunother. Cancer. 2019;7:304. doi: 10.1186/s40425-019-0806-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stadtmauer, E. A., et al. CRISPR-engineered T cells in patients with refractory cancer. Science367, eaba7365 (2020). [DOI] [PMC free article] [PubMed]
- 109.Stephan MT, et al. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nat. Med. 2007;13:1440–1449. doi: 10.1038/nm1676. [DOI] [PubMed] [Google Scholar]
- 110.Abbas Abul K., Trotta Eleonora, R. Simeonov Dimitre, Marson Alexander, Bluestone Jeffrey A. Revisiting IL-2: Biology and therapeutic prospects. Science Immunology. 2018;3(25):eaat1482. doi: 10.1126/sciimmunol.aat1482. [DOI] [PubMed] [Google Scholar]
- 111.Berraondo, P.,et al. Cytokines in clinical cancer immunotherapy. Br. J. Cancerhttps://www.nature.com/articles/s41416-018-0328-y (2018). [DOI] [PMC free article] [PubMed]
- 112.Sockolosky JT, et al. Selective targeting of engineered T cells using orthogonal IL-2 cytokine-receptor complexes. Science. 2018;359:1037–1042. doi: 10.1126/science.aar3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shum T, et al. Constitutive signaling from an engineered IL7 receptor promotes durable tumor elimination by tumor-redirected T Cells. Cancer Disco. 2017;7:1238–1247. doi: 10.1158/2159-8290.CD-17-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Leonard JP, et al. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood. 1997;90:2541–2548. [PubMed] [Google Scholar]
- 115.Kerkar SP, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J. Clin. Invest. 2011;121:4746–4757. doi: 10.1172/JCI58814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang L, et al. Tumor-infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clin. Cancer Res. 2015;21:2278–2288. doi: 10.1158/1078-0432.CCR-14-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang L, et al. Enhanced efficacy and limited systemic cytokine exposure with membrane-anchored interleukin-12 T-cell therapy in murine tumor models. J. Immunother. 2020;8:1–12. doi: 10.1136/jitc-2019-000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kunert A, et al. Intra-tumoral production of IL18, but not IL12, by TCR-engineered T cells is non-toxic and counteracts immune evasion of solid tumors. Oncoimmunology. 2017;7:e1378842–e1378842. doi: 10.1080/2162402X.2017.1378842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu Y, et al. Armored inducible expression of IL-12 enhances antitumor activity of glypican-3-targeted chimeric antigen receptor-engineered T cells in hepatocellular carcinoma. J. Immunol. 2019;203:198–207. doi: 10.4049/jimmunol.1800033. [DOI] [PubMed] [Google Scholar]
- 120.Hurton LV, et al. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc. Natl Acad. Sci. USA. 2016;113:E7788–E7797. doi: 10.1073/pnas.1610544113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hu B, et al. CAR T cells secreting IL18 augment antitumor immunity and increase T cell proliferation and costimulation 2. Cell Rep. 2017;20:3025–3033. doi: 10.1016/j.celrep.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ma Xingcong, Shou Peishun, Smith Christof, Chen Yuhui, Du Hongwei, Sun Chuang, Porterfield Kren Nancy, Michaud Daniel, Ahn Sarah, Vincent Benjamin, Savoldo Barbara, Pylayeva-Gupta Yuliya, Zhang Shuqun, Dotti Gianpietro, Xu Yang. Interleukin-23 engineering improves CAR T cell function in solid tumors. Nature Biotechnology. 2020;38(4):448–459. doi: 10.1038/s41587-019-0398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Etxeberria I, et al. Intratumor adoptive transfer of IL-12 mRNA transiently engineered antitumor CD8+ T Cells. Cancer Cell. 2019;36:613–629.e7. doi: 10.1016/j.ccell.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 124.Leen AM, et al. Reversal of tumor immune inhibition using a chimeric cytokine receptor. Mol. Ther. 2014;22:1211–1220. doi: 10.1038/mt.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sukumaran S, et al. Enhancing the potency and specificity of engineered T cells for cancer treatment. Cancer Disco. 2018;8:972–987. doi: 10.1158/2159-8290.CD-17-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kloss CC, et al. Dominant-negative TGF-β receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol. Ther. 2018;26:1855–1866. doi: 10.1016/j.ymthe.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tang, N. et al. TGFβ inhibition via CRISPR promotes the long-term efficacy of CAR-T cells against solid tumors. JCI insight5, e133977 (2020). [DOI] [PMC free article] [PubMed]
- 128.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat. Immunol. 2005;6:600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 129.Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Crompton JG, Sukumar M, Restifo NP. Uncoupling T-cell expansion from effector differentiation in cell-based immunotherapy. Immunol. Rev. 2014;257:264–276. doi: 10.1111/imr.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gautam S, et al. The transcription factor c-Myb regulates CD8 + T cell stemness and antitumor immunity. Nat. Immunol. 2019;20:337–349. doi: 10.1038/s41590-018-0311-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen J, et al. NR4A transcription factors limit CAR T cell function in solid tumours. Nature. 2019;567:530–534. doi: 10.1038/s41586-019-0985-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Seo H, et al. TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8+ T cell exhaustion. Proc. Natl Acad. Sci. USA. 2019;116:12410–12415. doi: 10.1073/pnas.1905675116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fraietta JA, et al. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature. 2018;558:307–312. doi: 10.1038/s41586-018-0178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wei J, et al. Targeting REGNASE-1 programs long-lived effector T cells for cancer therapy. Nature. 2019;576:471–476. doi: 10.1038/s41586-019-1821-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.LaFleur MW, et al. PTPN2 regulates the generation of exhausted CD8+ T cell subpopulations and restrains tumor immunity. Nat. Immunol. 2019;20:1335–1347. doi: 10.1038/s41590-019-0480-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lynn RC, et al. c-Jun overexpression in CAR T cells induces exhaustion resistance. Nature. 2019;576:293–300. doi: 10.1038/s41586-019-1805-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhao Z, et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell. 2015;28:415–428. doi: 10.1016/j.ccell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Teijeira A, et al. Metabolic consequences of T-cell costimulation in anticancer immunity. Cancer Immunol. Res. 2019;7:1564–1569. doi: 10.1158/2326-6066.CIR-19-0115. [DOI] [PubMed] [Google Scholar]
- 140.Kishton RJ, Sukumar M, Restifo NP. Metabolic regulation of T cell longevity and function in tumor immunotherapy. Cell Metab. 2017;26:94–109. doi: 10.1016/j.cmet.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ho PC, et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell. 2015;162:1217–1228. doi: 10.1016/j.cell.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Palazon A, et al. An HIF-1α/VEGF-A axis in cytotoxic T cells regulates tumor progression. Cancer Cell. 2017;32:669–683.e5. doi: 10.1016/j.ccell.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang Y, et al. Enhancing CD8(+) T cell fatty acid catabolism within a metabolically challenging tumor microenvironment increases the efficacy of melanoma immunotherapy. Cancer Cell. 2017;32:377–391.e9. doi: 10.1016/j.ccell.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dumauthioz, N. et al. Enforced PGC-1α expression promotes CD8 T cell fitness, memory formation and antitumor immunity. Cell Mol. Immunol.10.1038/s41423-020-0365-3 (2020) [DOI] [PMC free article] [PubMed]
- 145.Scharping NE, et al. The tumor microenvironment represses T cell mitochondrial biogenesis to drive intratumoral T cell metabolic insufficiency and dysfunction. Immunity. 2016;45:374–388. doi: 10.1016/j.immuni.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kawalekar OU, et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity. 2016;44:380–390. doi: 10.1016/j.immuni.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 147.Teijeira A, et al. Mitochondrial morphological and functional reprogramming following CD137 (4-1BB) costimulation. Cancer Immunol. Res. 2018;6:798–811. doi: 10.1158/2326-6066.CIR-17-0767. [DOI] [PubMed] [Google Scholar]
- 148.Menk AV, et al. 4-1BB costimulation induces T cell mitochondrial function and biogenesis enabling cancer immunotherapeutic responses. J. Exp. Med. 2018;215:1091–1100. doi: 10.1084/jem.20171068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zah E, Lin M-Y, Silva-Benedict A, Jensen MC, Chen YYT. Cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol. Res. 2016;4:498–508. doi: 10.1158/2326-6066.CIR-15-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kuhn NF, et al. CD40 ligand-modified chimeric antigen receptor T cells enhance antitumor function by eliciting an endogenous antitumor response. Cancer Cell. 2019;35:473–488.e6. doi: 10.1016/j.ccell.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sánchez-Paulete A.R., Teijeira A., Cueto F.J., Garasa S., Pérez-Gracia J.L., Sánchez-Arráez A., Sancho D., Melero I. Antigen cross-presentation and T-cell cross-priming in cancer immunology and immunotherapy. Annals of Oncology. 2017;28:xii44–xii55. doi: 10.1093/annonc/mdx237. [DOI] [PubMed] [Google Scholar]
- 152.Peng W, et al. Transduction of tumor-specific T cells with CXCR2 chemokine receptor improves migration to tumor and antitumor immune responses. Clin. Cancer Res. 2010;16:5458–5468. doi: 10.1158/1078-0432.CCR-10-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Moon EK, et al. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin. Cancer Res. 2011;17:4719–4730. doi: 10.1158/1078-0432.CCR-11-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Adachi K, et al. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat. Biotechnol. 2018;36:346–351. doi: 10.1038/nbt.4086. [DOI] [PubMed] [Google Scholar]
- 155.Brown Christine E., Alizadeh Darya, Starr Renate, Weng Lihong, Wagner Jamie R., Naranjo Araceli, Ostberg Julie R., Blanchard M. Suzette, Kilpatrick Julie, Simpson Jennifer, Kurien Anita, Priceman Saul J., Wang Xiuli, Harshbarger Todd L., D’Apuzzo Massimo, Ressler Julie A., Jensen Michael C., Barish Michael E., Chen Mike, Portnow Jana, Forman Stephen J., Badie Behnam. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. New England Journal of Medicine. 2016;375(26):2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]