Abstract
T cells react to foreign or self-antigens through T cell receptor (TCR) signaling. Several decades of research have delineated the mechanism of TCR signal transduction and its impact on T cell performance. This knowledge provides the foundation for chimeric antigen receptor T cell (CAR-T cell) technology, by which T cells are redirected in a major histocompatibility complex-unrestricted manner. TCR and CAR signaling plays a critical role in determining the T cell state, including exhaustion and memory. Given its artificial nature, CARs might affect or rewire signaling differently than TCRs. A better understanding of CAR signal transduction would greatly facilitate improvements to CAR-T cell technology and advance its usefulness in clinical practice. Herein, we systematically review the knowns and unknowns of TCR and CAR signaling, from the contact of receptors and antigens, proximal signaling, immunological synapse formation, and late signaling outcomes. Signaling through different T cell subtypes and how signaling is translated into practice are also discussed.
Keywords: CAR-T, TCR, Signaling, T cell, Immunological synapse
Subject terms: Signal transduction, Immunotherapy, Tumour immunology, Adaptive immunity, Cell signalling
Introduction
T cells play critical roles in the defense against cancer.1 Among T cell subsets, cytotoxic CD8+ T cells can directly kill tumor cells by the exocytosis of perforin and granzyme B.2 In addition, CD4+ helper T cells are involved in indirect antitumor responses through the secretion of proinflammatory cytokines, including interleukin (IL-2), tumor necrosis factor (TNF), and interferon (IFN)γ, which enhance T cell activation, cytotoxic T lymphocyte (CTL) cytotoxicity and innate immune responses.3–5 Cancer cells expressing highly immunogenic antigens can be recognized and eliminated by T cells during the early stages of cancer development,6 but less immunogenic mutants may escape immune surveillance and acquire an immune-resistant phenotype.7 Recently, various methods have been developed to enhance antitumor T cell responses, including chimeric antigen receptors (CARs).
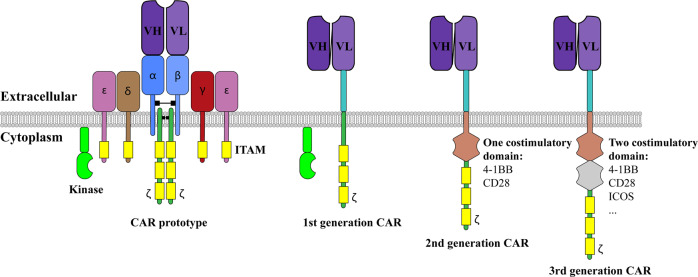
T cell activation relies on T cell receptor (TCR) recognition of antigenic peptides presented on major histocompatibility complex (MHC) molecules, and cancer cells may express very few mutant potentially antigenic peptides. The MHC locus shows extreme polymorphism, and as a result, it is very difficult to generate a universal TCR for immunotherapy. To overcome these problems, CARs were developed to take advantage of antibody recognition specificities. In the first CARs, the variable domains in the TCR-α and/or -β chains were replaced with those of immunoglobulin heavy or light chains from hapten-specific antibodies.8–10 These CARs were expressed on T cells as cell surface proteins and associated with the other endogenous TCR chains and CD3 (Fig. 1).10 The studies of these CARs followed work on chimeric antibodies expressing TCR variable regions11 or other recognition or functional domains,12–14 work that in turn was informed by experiments on switching variable and constant domains of immunoglobulins.15
Fig. 1.
Generation of the chimeric antigen receptor (CAR) and various designs. The prototype of CAR used the variable region (V) of the heavy (H) and light (L) chains from an antibody. The whole complex was assembled as a TCR complex with other CD3 subunits. The 1st generation CAR was streamlined to became the single-chain version. To date, the 2nd generation and 3rd generation CARs have been most commonly used. These proteins contain additional costimulatory signaling domains from CD28 and 4-1BB. All of the designs are based on CD3ζ being the T cell-signaling initiator, which requires phosphorylation by a Src family kinase
T cells expressing these prototype CARs showed effects, such as IL-2 production and cytotoxicity induction, when they met a non-MHC antigen.8–10 However, these CARs were difficult to design and modify (Fig. 1). The discoveries that a single chain variable fragment (scFv), in which the variable regions of the antibody were linked with a flexible linker, had the same recognition ability as the full-length antibody16,17 and that a single CD3ζ subunit was sufficient to activate T cells18 provided fundamental ways to streamline the CAR design. This modification was consolidated into a single chain version of the CAR design, the basis of CARs used today.19
The early success in redirecting T cells in a non-MHC-restricted manner did not necessarily lead to augmented in vivo efficacy, especially in the clinic. The 1st generation CARs did not show any significant in vivo efficacy (reviewed in ref. 20) Efficient T cell responses in vivo require not only the activation signal but also the costimulatory signal. Therefore, the 2nd generation CARs were generated to incorporate intracellular signaling motifs based on costimulatory receptors such as CD28 and 4-1BB (also called CD137 and TNFRSF9) to allow delivery of costimulatory signals upon CAR-T cell stimulation. Although 1st generation CAR-T cells and 2nd generation CAR-T cells show similar cytotoxicity in vitro,21 the 2nd generation CARs outperform the 1st generation CARs in the clinic.
The first clinical advance in CAR-T cell technology was observed in the treatment of patients with lymphoma and chronic lymphocytic leukemia (CLL) by using 2nd generation CAR-T cells with CD28 and 4-1BB costimulatory domains, respectively.22,23 Some patients showed a complete response and the elimination of cancer. A larger-scale clinical trial based on CD19-scFv-4-1BBζ CAR-T cells showed unprecedented results, with an overall survival rate among 75 patients of 90% at 6 months and 76% at 12 months.24 Several other immediate clinical trials also showed dramatic improvements in patients with non-Hodgkin’s lymphoma, CLL, and adult and pediatric acute lymphoblastic lymphoma (ALL).25 In 2017, two CAR-T cell products, tisagenlecleucel (Kymria™) and axicabtagene ciloleucel (Yescarta™), became commercially available for ALL and diffuse large B-cell lymphoma patients. However, CAR-T cell technology faces various challenges, including cytokine release syndrome (CRS) and low efficacy against solid tumors. The emergence of these challenges is related, at least in part, to our inadequate understanding of CAR signaling in T cells. In this review, we focus on the current research progress on the effects and significance of TCR and CAR signaling in T cells.
T cell receptors, CARs and their ligands
TCR and its ligands
The TCR is a heterodimer of two highly variable chains, either α and β chains or γ and δ chains. The structure of the TCR molecule is remarkably similar to that of the Fab region of immunoglobulin (Ig).26,27 Each TCR α or β chain consists of a variable (V) and a constant (C) domain. Each V domain of the TCR α and β chains contains three highly variable complementary determining region loops at the most distal ends that interact directly with the peptide-MHC complex (pMHC).27 The γδ TCR structure is similar to the αβ TCR structure, but the γδ TCR binds to antigens presented by unconventional MHCs or not presented by an MHC.28 Because of the abundance of T cells bearing the αβ TCR structure (95%), this paper focuses on the αβ TCR, henceforth referred to as TCR.
Conventional T cells expressing αβ TCR recognize the complex consisting of a peptide presented by major histocompatibility complex class I (MHC-I) or class II (MHC-II) (certain glycolipids and metabolites are presented by nonclassical MHCs to nonconventional T cells, but they are not the focus of this review).27 TCR recognition of its cognate pMHC for interaction is enhanced by the binding of CD4 or CD8 coreceptors to this pMHC.29,30 During TCR stimulation with a low concentration of antigenic pMHC, CD8 binding to a nonantigenic pMHC can also enhance TCR signaling.31,32 The coreceptors enhance T cell activation not only through binding with pMHC but also through their association with lymphocyte-specific protein tyrosine kinase (Lck), the key Src family kinase involved in the initiation of TCR signaling.33
CARs and their ligands
Currently, a CAR is designed by adopting a recognition domain, typically an scFv encompassing variable regions from heavy and light chains, followed by a hinge region. This extracellular domain is followed by a transmembrane region and intracellular signaling sequences from one (for 2nd generation CARs) or two (for 3rd generation CARs) costimulatory receptors, followed by the intracellular region of CD3ζ. This modular design allows large variations in CAR sequences, differing in antigen recognition domains and costimulatory motifs.
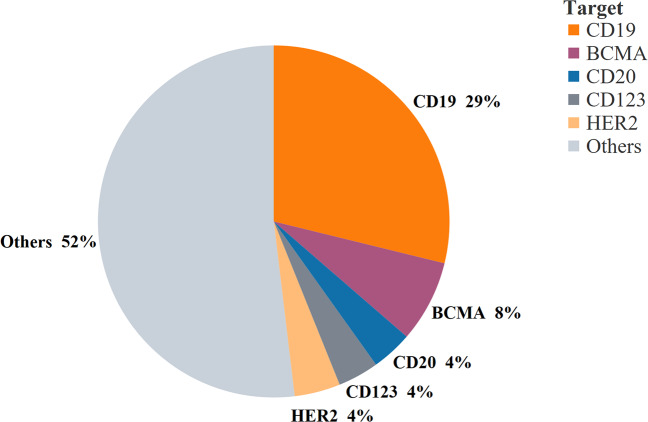
In contrast to the MHC-restricted conventional T cells, CARs are typically designed to recognize non-MHC cell surface proteins. Among the 147 targets in the global CAR-T cell pipeline in 2019, CD19 was the most common target for CAR-T cell therapy (~29%),34 followed by B cell mature antigen (~8%) and CD20 (~4%) (Fig. 2).
Fig. 2.
Percentage of targets in the 2019 global CAR-T cell pipeline. The top 5 CAR-T cell targets were selected, and a pie chart was plotted based on data from the Cancer Research Institute
TCR and CAR signaling pathways
TCR and CAR signal triggering
The TCR/CD3 complex and CAR share the CD3ζ intracellular region but differ dramatically in their extracellular antigen recognition domains and in their overall architecture, with a single CAR protein playing the role of TCRαβ, CD3ζζ, CD3εδ, and CD3εγ. Given these differences, do TCR/CD3 and CARs use the same mechanism to convey information about ligand binding across the cell membrane to imitate intracellular signaling events? The mechanism of TCR triggering is still incompletely understood, with three main, not necessarily mutually exclusive, mechanisms proposed: receptor clustering, mechanosensing that causes conformational changes within TCR/CD3, and size-dependent protein segregation that leads to changes in the local kinase/phosphatase balance. As activation of conventional T cells does not require pMHC multimers,35 TCR clustering is unlikely to be the main mechanism of TCR triggering; however, it is more likely to be the mechanism for CAR-T cells, as CAR oligomerization is an important step in the initiation of CAR signaling.36,37 There is increasing evidence that TCR acts as a mechanosensor, with the TCR/CD3 complex undergoing force-dependent structural transitions upon ligand binding.38 Whether this mechanism is applicable to CARs has not been determined. However, it is plausible that CAR molecular architecture allows force-dependent intramolecular structural transitions, as CARs have been reported to have a proofreading mechanism similar to that of TCRs, whereby TCRs and CARs respond only when they bind to a ligand with sufficient strength to ensure that the time in contact with an antigen is sufficient.39 This possibility has important implications for optimizing CAR design for sensitivity and specificity. A TCR and its pMHC ligand are relatively small molecules, and TCR ligation leads to size-dependent protein segregation at the T cell–antigen presenting cell (APC) interface, with the formation of “close contact zones” that exclude larger proteins, such as phosphatase CD45.40 This size-dependent exclusion alters the kinase:phosphatase balance in the proximity of TCR/CD3, leading to CD3 phosphorylation. There is evidence supporting the importance of molecular size in conventional T cell signaling, as experimental elongation of pMHC ligands41,42 or a reduction in CD45 ectodomain size43 severely suppressed T cell activation. As with the TCR–APC interface, CD45 is excluded from the interface of CAR-expressing cells and target cells, thereby facilitating signal initiation; hence, size-dependent protein segregation mechanisms might also be important for CAR signaling.44
Proximal TCR and CAR signaling
After TCR-pMHC recognition, TCR signaling is initiated by the Src family kinases Lck and Fyn. Populations of preactivated Lck and Fyn exist prior to TCR-pMHC recognition45 and phosphorylate CD3, zeta-chain-associated protein kinase 70 (Zap-70) and Tec kinases upon TCR ligation. The CD3 chains contain immunoreceptor tyrosine-based activation motifs (ITAM), with CD3γ, δ, and ε having one ITAM each and the ζ chains each having three ITAMs.46 Each ITAM can be phosphorylated by Lck and/or Fyn at two tyrosine residues, and a biphosphorylated ITAM recruits and binds Zap-70.33,47,48
Among the Lck molecules, both coreceptor-bound and coreceptor-unbound Lck can initiate CD3 phosphorylation upon TCR activation, but the coreceptor-unbound Lck can be recruited to trigger TCR signaling earlier than can the coreceptor-bound Lck.49 Therefore, TCR activation is thought to have two distinct stages: active Lck that is not bound to coreceptors is first recruited to a TCR and phosphorylates the CD3 ITAMs; then, a secondary group of coreceptor-bound Lck proteins is recruited, enhancing TCR-pMHC binding at the coreceptor, with the stronger signaling modulated by Lck.49–51 Then, the ITAM-attached Zap-70 molecules are phosphorylated and activated by Lck, resulting in the recruitment of additional Zap-70 molecules and the activation of IL- 2-induced tyrosine kinase (Itk).46
After the initial activation of these tyrosine kinases, two linker molecules, the linker of activated T cells (LAT) and the SH2 domain-containing leukocyte phosphoprotein of 76 kDa (SLP-76), are recruited to modulate downstream signaling.52 LAT phosphorylation can be induced by Zap-70, and the LAT-Zap-70 association is established by through the Lck SH2 domain binding to LAT.53 Furthermore, phosphorylated LAT recruits phospholipase C-gamma (PLCγ), growth factor receptor-bound protein 2 (GRB2) and the GRB2-related adaptor downstream of Shc (Gads).54 GRB2 binds to thymus-expressed molecules involved in selection (Themis),55–57 and Themis interacts with and regulates the activity of Src homology region 2 domain-containing phosphatase-1 (SHP1).57–59 After TCR activation, both SHP1 and Themis are associated with the LAT signalosome.57,60 After LAT phosphorylation, SLP-76 is indirectly associated with LAT through Gads.61,62 Itk molecules are phosphorylated by Lck, and activated Itk is recruited to the LAT signalosome through its association with SLP-76;63 it then activates LAT-associated PLCγ.54
As CARs are designed to utilize CD3ζ to activate T cell effector functions, CARs and TCR are expected to share a similar signaling transduction mechanism. The CAR and TCR use similar molecular machinery to transduce signaling, including PLCγ, Zap-70, LAT, and Src family kinases.64 Nevertheless, further studies on 2nd- and 3rd-generation CARs imply that the CAR has a signal transduction mechanism that differs from that of the TCR. One signal transduction model of TCR and CAR indicates that there might be an unknown mechanism of proximal signaling that makes their activation patterns significantly different.65 In addition, the sensitivity of the CAR and TCR is different, where one TCR can react to as little as one pMHC, each CAR protein requires at least hundreds of antigens, even though CARs have significantly higher affinity for antigens than do TCRs.66 This disparate sensitivity may imply a different intrinsic signal transduction mechanism, which is demonstrated to be associated with sensitivity.67 For example, the recognition of weak (positive selecting) versus strong (negative selecting) ligands by developing thymocytes results in the activation of different mitogen-activated protein kinase (MAPK) pathways in disparate subcellular locations.68
Several studies have investigated phosphorylation events downstream of CAR to decipher its signal transduction mechanism.64,69,70 The results indicate differences in the magnitude and kinetics of the phosphorylation events of CAR-T and TCR-T cells, but not the emergence of new pathways in CAR-T cells. Signalosome and interactome studies identified several novel protein interactions, such as capsule synthesis 1 domain containing 1 (CASD1) with CD28-4-1BB-CAR, butyrophilin-like 3 (BTNL3) with CD28-CAR, and an additional form of CD3ζ, p21, specifically interacting with a 2nd-generation CAR. However, the impact of these molecules on signal transduction is not yet understood.70
Altered signal transduction by 2nd- and 3rd-generation CARs may be caused by the signaling motifs in costimulatory proteins. For example, the CD28 costimulatory domain directly increases the phosphorylation rate of CD3ζ.71 The efficacy of CAR can be fine-tuned by manipulating the CD28-binding domain, similar to the binding motif for Lck.72 Because of the distinct biochemical properties of costimulatory domains, their relative position within the intracellular region in a CAR can affect CAR-T cell activity. The membrane-proximal domain has a stronger influence on cytokine expression than does a more distal domain.73 An inducible T-cell costimulator (ICOS) expressed at a membrane-proximal position in the ICOS‐4-1BB‐CD3ζ construct produced a cytokine profile comparable to that of the ICOS‐CD3ζ construct, whereas the 4-1BB‐ICOS‐CD3ζ construct resulted in a cytokine expression profile similar to that of 4-1BB‐CD3ζ. Tonic signaling from the 4-1BB domain was ameliorated by positioning the 4-1BB domain downstream of CD28.74 This change may have been caused by the 4-1BB domain becoming less accessible to its downstream signal partners, tumor necrosis TNF receptor-associated factors (TRAFs), which are localized at the membrane.
Second-generation CARs with either CD28 or 4-1BB domains recruit coreceptors and bind Lck to amplify the signaling at both the resting state and upon activation. Moreover, 4-1BB-CAR, but not CD28-CAR, has been shown to bind the Themis-SHP1 complex, and this interaction reduces downstream phosphorylation events.75 This ability of 4-1BB-CAR to recruit negative regulators of signal transduction can help to explain why 4-1BB-CAR has reduced in vitro efficacy and lower sensitivity than CD28-CAR.76 Moreover, the presence of different costimulatory motifs in CARs can also alter CAR-T cell sensitivity to negative signaling through inhibitory receptors. As the PD-1-SHP2 complex preferentially targets CD28,77 PD-1 inhibitory signaling is predicted to have a greater effect on CD28 CAR T cells than on 4-1BB CAR T cells. Therefore, anti-PD-1 therapy is predicted to work better with CD28-CAR-T cells in the clinic.78,79 Importantly, even a slight change in the CAR structure can cause dysregulated downstream signaling pathways. For example, the introduction of the ICOS transmembrane domain into the CAR construct upregulated the downstream signaling of 4-1BB-CAR through an enhanced association with Lck.73,80
TCRs and CARs and the immune synapse
T cell activation requires cell–cell contact with the accumulation of receptor–ligand pairs between the T cell and the APC. These cell–cell contacts include the “immunological kinapse”—transient contacts during the migration and search for antigenic pMHC, and the “immunological synapse” (IS)—stable contacts formed upon TCR recognition of its agonist pMHC.81 The IS comprises three different classes of receptors, including TCRs, adhesion receptors and costimulatory or coinhibitory receptors, as well as their associated signal-transduction molecules. During TCR and pMHC recognition, an ~15 nm gap forms between the T cell and the APC,82 which provides space for the receptors and ligands to form an IS.83 Adhesion molecules support the IS by joining cell membranes together tightly and are essential for sustained antigen recognition.82 Finally, the costimulatory or coinhibitory receptors recruited to the IS can substantially tune T cell activation.84,85
Cytotoxic T cell IS formation follows three steps: IS initiation, including the recognition of the TCR-pMHC; the effector stage, including cumulative F-actin, polarization of the microtubule-organizing center and the release of cytolytic granules into the target cell; and the termination stage, when apoptosis is initiated in the target cell.86 The “bull’s eye” structure of the mature IS was named the “supramolecular activation cluster” (SMAC), which has three compartments: the central SMAC (cSMAC), peripheral SMAC (pSMAC), and distal SMAC (dSMAC).81,87 The cSMAC mainly contains TCR-pMHC complexes that formed initially as microclusters (MC), assembling first in the dSMAC and moving through the pSMAC to the cSMAC.88,89 The TCR MC with coreceptors recruits the Src family kinase Lck to initiate TCR signaling, and downstream TCR signaling molecules are then concentrated at the IS. The costimulatory receptor CD28 and coinhibitory receptor PD-1 are also located in the cSMAC.90,91
cSMAC can be split into the TCR-low, CD28-high endo-cSMAC region and the TCR-high, CD28-low, and PD-1-enriched exo-cSMAC region.83 TCR signaling continues in the endo-cSMAC region but is terminated in the exo-cSMAC region. The pSMAC contains adhesion molecules such as lymphocyte function-associated antigen 1 (LFA-1), integrins, and talin. F-actin and filament decorations change dramatically in pSMACs.92The activation and spatial distribution of the actin-binding protein l-plastin was shown to regulate the maturation and stability of the IS.93 The dSMAC is defined by the enrichment of the transmembrane tyrosine phosphatase CD45.94 Although the IS structure can be maintained for hours, the pSMAC and dSMAC structures are renewed rapidly (>1 cycle per minute) to form and bring new TCR MCs to the cSMAC.95
Similar to TCR-T cell IS formation, CAR-T cell IS formation also follows three steps: IS initiation, effector stage, and termination.86 However, the IS pattern formed by CAR-T cells was found to be significantly different from that of TCR-T cells.44,96 The membrane at the interface of the CAR-T cell and target cell was found to be extensively convoluted at first, and the CAR-T cell IS was disorganized and did not form a classical “bull’s eye” structure. Instead, multiple Lck MCs were observed in the contact region, in contrast to the TCR-T cell IS, which is characterized by extensive Lck clustering at the cSMAC region. F-actin was found to be clustered at the CAR-T cell IS, but it did not form a classical ring structure surrounding the cSMAC.96,97 LFA-1, which is located at the pSMAC and stabilizes the IS,98 and an LFA-1-blocking antibody greatly suppressed TCR-T cell and target cell conjugation. However, LFA-1 was disorganized at the CAR-T cell IS. Although the LFA-1 blocking antibody reduced effector CAR-T cell and target cell conjugation, this effect was not as strong as that on the TCR-T cell and target cell conjugation.96 In addition, the lytic granules of the CAR-T cells were observed to be translocated or were recruited faster to the IS than were those of the TCR-T cells. The disorganized CAR-T cell IS and faster granule recruitment potentially explain the rapid killing activity of CAR-T cells compared to that of TCR-T cells.99,100
IS formation in CAR-T cells is still incompletely understood, with several key questions remaining to be answered. For instance, what molecular factors contribute to the disorganized IS structure in CAR-T cells? Although CARs and TCRs differ in many ways, the disorganized IS structure may not be attributed to the high affinity of CAR for antigens or because of its non-MHC restricted nature, since constructs with high-affinity recognition domains fused or bound to a natural TCR showed a classical and organized synapse.101–103 Therefore, it is important to know how the CAR structure relates to synapse formation and quality. It is believed that proper IS formation is essential for enhanced CAR-T cell activity. An imaging system based on a glass-supported planar lipid bilayer system has been used to predict and demonstrate the association of the quality of ISs with CAR-T cell functions.97 Additionally, overexpressing the IS molecule TG2, which enhances the function of LFA-1, enhanced CAR-T cell activity and led to the attenuation of the immune inhibition of CAR-T cell effects on tumors.104 Immunomodulatory drugs such as lenalidomide can also improve CAR-T cell efficacy by increasing actin accumulation in CAR synapses.105
Downstream TCR signaling pathways
The molecular rearrangements and phosphorylation events at the TCR-T cell IS initiate three main signaling pathways: calcium flux, NF-κB (nuclear factor kappa-light chain enhancer of activated B cells) and Ras-MAPK activation. The calcium concentration is approximately 1 mM in the blood but is maintained at only 50–100 nM in resting T cells.106 After T cell activation, the intracellular calcium concentration increases to approximately 1 µM.107 This calcium flux is induced by the phosphorylation of PLCγ. After its activation, PLCγ breaks down phosphatidylinositol 4,5-bisphosphate (PIP2) into diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3).106,107 DAG actuates PKCθ and Ras-MAPK signaling, leading to the expression of transcription factor AP-1 (activator protein 1), which synergizes with nuclear factor of activated T-cells (NFAT) for cytokine gene transcription.108,109 IP3 binds to IP3 receptors on the endoplasmic reticulum (ER) membrane to release calcium stores from the ER into the cytosol.106 The release of ER calcium stores then triggers the opening of calcium release-activated calcium channels (CRAC) in the plasma membrane, allowing an abrupt influx of calcium across the plasma membrane.107 Calcium is critical for NFAT and NF-κB transcription pathways, which both control the expression of cytokine IL-2.108,109 DAG activates PKCζ, which then phosphorylates caspase recruitment domain-containing membrane-associated guanylate kinase protein-1 (CARMA1).110 The phosphorylated CARMA1 then recruits the proteins B-cell lymphoma 10 (BCL10), mucosa-associated lymphoid tissue lymphoma translocation gene 1 (MALT1), and tumor necrosis TNF receptor-associated factor 6 (TRAF6) to form a protein complex that activates the IκB kinase (IKK) complex. The activated IKK complex then triggers the phosphorylation and ubiquitination of IκB, leading to the translocation of NF-κB to the nucleus.111 NF-κB translocation is critical for the regulation of T cell survival, proliferation, differentiation, and cytokine production.112 Ras is activated by two Ras guanine nucleotide exchange factors: Ras guanyl nucleotide-releasing protein (RasGRP) and son of sevenless (SOS). RasGRP can be recruited to the cell membrane by associating with TCR-induced DAG. SOS is bound to GRB2 constitutively and is also recruited to the membrane upon TCR-induced GRB2 and LAT association.54 RasGRP and SOS then activate Ras.113 Activated Ras phosphorylates the serine-threonine kinase Raf-1 to trigger the activation of the MAP kinase (MAPK) chain, including extracellular signal-regulated kinase 1 (Erk1) and Erk2.54 Activated Erk1 and Erk2 then translocate into the cell nucleus and trigger the synthesis of transcription factors that are involved in the cell cycle, cytokine production and cell apoptosis (e.g., AP1 and Bcl-2).114
Downstream CAR signaling pathways
TCRs and CARs share many similar downstream signaling events upon activation. They both secrete perforin and granzyme B to initiate apoptosis of the target cells, upregulate inhibition molecules on the surface or the Fas ligand (FasL) to eliminate bystander tumor cells, and express cytokines and chemokines to boost the immune function or direct proinflammatory responses.115 However, studies have reported significantly different outcomes as discovered in clinical settings, such as CRS, which can be induced by heightened CAR T cell function. CAR-T cells with high affinity for a target and costimulatory signaling enhance killing the effects through increased granzyme B and perforin secretion on target cells, resulting in GASDERMIN-E-induced pyroptosis of the target cells. This cell death leads to macrophage activation by damage-associated molecular patterns secreted from tumor cells and finally causes aberrant cytokine release, i.e., CRS.116 Although not fully understood, these disparate outcomes are believed to be associated with unique CAR designs with different costimulatory domains resulting in distinct downstream signaling.
CD28 family signaling motifs
B7-CD28 signaling is the best-defined costimulatory mechanism. CD28 is constitutively expressed on the T cell membrane, and its ligands, B7-1 and B7-2 (or CD80 and CD86), are expressed on professional APCs (DCs, macrophages, and B cells).117 Upon CD28 cross-linking, the CD28 cytoplasmic domain binds to phosphatidylinositol 3-kinase (PI3K) after it has been phosphorylated on its tyrosine by Lck.117 PI3K can transform phosphatidylinositol4,5 trisphosphate (PIP2) into phosphatidylinositol3–5 trisphosphate (PIP3) at the cell membrane. PIP3 recruits 3-phosphoinositide-dependent protein kinase 1 (PDK1) and Akt to the cell membrane.54 Akt is then activated by PDK1 and phosphorylates multiple targets. Activated Akt enhances signaling pathways, including NF-κB, NFAT, and AP1, that mediate T cell survival, proliferation, cytokine production, cell apoptosis, and differentiation.54,108 Another CD28 family member, the inducible T-cell costimulator (ICOS or CD278), binds exclusively to ICOSL118 and causes increased PI3K recruitment, enhanced PIP3 production, and higher AKT phosphorylation than does CD28 after its activation.119,120
Two costimulatory molecules from the CD28 family have been adopted to generate CAR-T cells, the widely used CD28 and ICOS. CD28 plays an essential role in initiating T cell activity and is present in resting T cells, in contrast to other costimulatory molecules, which are expressed upon activation.121 The integration of CD28 into CARs confers a higher response than do other costimulatory domains; for example, 4-1BB induces Th1 cytokine secretion (IL-2, TNF, and IFNγ) via the upregulation of the PI3K/AKT signaling pathway.122 This elevated Akt activity prevents CAR-T cells from triggering activation-induced cell death and attenuates the inhibition induced by Treg cells, although enhanced IL-2 production was also shown to increase Treg cell inhibition in some models.123–125 Nevertheless, many reports have indicated that the increased activation of the PI3K/AKT pathway by CD28 results in a more differentiated memory phenotype and a reduction in mitochondrial biogenesis.126,127 The constant suboptimal activation stimulated by CD28—that is, tonic signaling—induces CAR-T cell exhaustion by upregulation of exhaustion-associated transcription factors, such as T-bet, eomesodermin (EOMES), Blimp-1, and Helios.128 Both the differentiated memory phenotype and increased exhaustion lead to a reduced in vivo efficacy. Transient costimulatory signals from CD28 primed mitochondrial biogenesis and led to memory T cell differentiation.129 Moreover, CD28-CAR-T cells became persistent central memory T cells when the CAR expression level was lowered by targeting a CD28-CAR construct to the TCR α-chain C-region (TRAC) locus.130 Therefore, the timing, degree, and duration of CD28 signaling may be crucial for driving CD28 CAR T cell metabolism and differentiation.
ICOS is upregulated upon T cell activation and boosts activity via PI3K signaling in a manner similar to CD28. However, its transmembrane domain is critical and sufficient to recruit Lck to augment CAR-T cell signaling.80 In addition, the ICOS intracellular domain has a different structure than the CD28 domain, leading to reduced IL-2 production, possibly due to the inability to recruit Grb2.131 In contrast to CD28, the ICOS costimulatory domain induces CAR-T cells toward Th1/Th17 polarization, with increased expression of IL-17A, IL-17F, IL-22, IFNγ, and T-bet. Because of this Th1/Th17 polarization, ICOS-based CAR-T cells had a significantly higher subpopulation of persistent CD4+ CAR-T cells in vivo than either CD28- or 4-1BB-based CAR-T cells.132
TNF receptor superfamily signaling motifs
The TNF receptor superfamily (TNFRSF) on T cells provides costimulatory or coinhibitory signals in T cell responses. For example, the TNF receptor TNFR2 on activated T cells induces a costimulatory signal for T cell proliferation and differentiation.133 In addition, 4-1BB (CD137, TNFRSF9) is transiently expressed on T cells upon TCR stimulation and provides a costimulatory signal for T cell proliferation,134 survival,135 effector function, and differentiation into memory T cells.136 Another member in this family, Herpes virus entry mediator (HVEM), is both a costimulatory receptor for TNF-like molecule LIGHT137 and a ligand for the inhibitory receptor BTLA.133
As the most commonly used TNF-receptor-related costimulatory domain in CARs, 4-1BB exploits TNFR-associated factor family members (TRAFs) to form the signalosome and promotes the nuclear translocation and transcriptional activity of NF-κB via the activation of the IκB kinase complex (IKKα/β). Although 4-1BB-CAR-T cells increase IL-2 and IFNγ secretion, not to the same extent as CD28 CARs.138 In addition, 4-1BB-CARs induce the upregulation of anti-apoptotic protein Bcl-xL, and most importantly, 4-1BB-CAR induces proliferation, Tcm cell differentiation, and increased fatty acid oxidation through increased mitochondrial biogenesis to a greater extent than is induced by CD28-CARs.127 Because of these characteristics, 4-1BB-CAR-T cells showed great potency in clinical settings.24 Nevertheless, long-term exposure to resistant cancer cells induces dysfunction-associated gene expression in 4-1BB-CAR-T cells. In fact, 4-1BB-CAR-T cells show enhanced promoter accessibility to several key immunosuppressive transcription factors (TOX2, IRF8, and PRDM1).139 In addition, the overexpression of 4-1BB-CAR in T cells can lead to a negative effect through the induction of apoptosis via the upregulation of ICAM-1 and Fas/FasL.140,141 This fratricide was observed during the ex vivo expansion of 4-1BB-CAR-T cells when CD5 was targeted.
Other TNFRSF molecules have also been tested as costimulatory domains, although their impact and downstream signaling after integration into CARs have not yet been systematically studied. HVEM belongs to the TNFRSF family. It has been shown to increase T cell functions such as IL-2 production, oxidative phosphorylation, and glycolysis, although it is also involved in the coinhibitory network described above. HVEM, as a costimulatory domain in CAR, attenuated the exhaustion of CAR-T cells and induced a balanced central memory (Tcm) and effector memory (Tem) cell population.142 OX40 is another TNFRSF protein used as a costimulatory domain in constructed CARs. OX40 activates both the PI3K/AKT and NF-κB signaling pathways. Interestingly, the addition of OX40 after the CD28 and CD3ζ domains diminished the production of IL-10 in the CD28-CAR-T cells, although the detailed mechanism is not well understood.143
Cytokine receptor protein domains in CARs
The purpose of costimulatory domain integration is to provide signal 2 for T cell activation. However, full T cell activation can be achieved when signal 3 from the cytokine receptor is also triggered. The importance of the cytokine signaling pathway in CAR-T cells is indicated by a biomarker study in which CAR-T cells from patients with complete or partial responses showed upregulated activation of the STAT3 pathway.144 Retrospectively, this outcome is perhaps unsurprising, since signaling through STAT3 is involved in the generation and maintenance of memory T cells, which is beneficial for expansion and long-term survival in vivo.145 Therefore, activation of cytokine signaling pathways by adding cytokine receptor signaling motifs to 2nd generation CARs has considerable therapeutic potential. Currently, only one such CAR design has been reported, with IL-2Rβ and a STAT3-binding motif inserted into CD28-CAR to activate STAT5 and STAT3 signaling, respectively. The proliferation and survival of these CAR-T cells were augmented, and CAR-T cells with cytokine receptor signaling motifs had a less differentiated phenotype. All these factors resulted in greatly improved in vivo efficacy for both liquid and solid tumors.146
These examples illustrate that a better understanding of how CAR T cells initiate and regulate signaling pathways can lead to improved CAR design and therapeutic strategies. The remaining part of the review therefore focuses on the role of CAR ligands and recognition domains, CAR signaling in different T cell subsets, and how CAR signaling can be modulated to increase CAR T cell functionality.
Impact of CAR ligand and ligand recognition domains on CAR-T cell activation
CAR ligands and CAR-T cell activation thresholds
The diversity and unique characteristics of each target affect CAR-T cell signaling significantly in several ways (Table 1). In contrast to the intrinsic fixed size of pMHC-TCR, the size of ligand-CAR can vary. Early research on the hinge/spacer region of CAR and the response of CAR to antigens of different sizes indicated that the receptor and ligand dimensions are critical for CAR-T cell activation.147,148 It has been shown that membrane-proximal CAR epitopes can induce higher levels of CAR-T cell activation.149,150 The results of several studies suggest that the more proximal the target epitope to the membrane (e.g., CD20), the longer the length of the hinge/spacer required to optimize the receptor–ligand size. The converse is true for longer target epitopes to achieve correct immunological synapse formation and optimal activation.149–152
Table 1.
Summary of the ways the CAR-T cell targets influence CAR signaling
| Approaches | Influence |
|---|---|
| Length of antigen protrusion from target membrane | Long targets reduce the efficacy of immunological synapse formation and other functions when CAR has a longer spacer/hinge, e.g., proper degranulation. In contrast, short target molecules require a longer spacer/hinge region in order to reach the antigen-binding site. |
| Antigen density | The antigen density determines the activation level of the CAR-T cells. CAR-T cells respond to target cells when the antigen density expressed exceeds a threshold level. CAR-T cells can be designed to recognize cancer cells expressing higher amount of antigens instead of the relatively low amount of antigen expressed by normal cells. |
| Original function | Different antigens distinctly affect CAR-T signaling due to their original properties. For example, pMHC as the target antigen may affect CAR-T cell signaling by recruiting coreceptors. An affinity threshold for CAR also exists for pMHC, where high affinity CARs lose the ability to recognize a target pMHC with specificity. |
TCR-T cells can respond to very low amounts of antigenic pMHC, with reports of T cell activation by a single-antigenic pMHC.153 In contrast, the density of the target antigen plays an important role in the modulation of CAR-T cell signaling. In the clinic, this criterion may be of critical importance during responses to cancer escape variants, where recurrent cancer cells bear reduced antigen density, leading to reduced CAR-T cell activation in vitro and in vivo.154,155 A threshold of antigen density exists such that CAR-T cells are activated only after it reaches a threshold amount. It is also noteworthy that the threshold required for cytokine secretion is significantly higher than that for cytolytic activity; therefore, the killing of target cells can be triggered without cytokine secretion.34,156,157
CAR-T cell targets have distinct molecular properties that can influence CAR signaling. For example, glypican-3 (GPC3) is a surface proteoglycan expressed on the majority of hepatocellular carcinoma (HCC) cells. GPC3-specific CAR-T cells face signal inhibition by soluble GPC3 secreted by HCC cells.158 TCR-like CAR-T cells with an scFv recognizing a specific pMHC complex have an affinity threshold such that CAR-T cells lose some peptide specificity when the scFv affinity is too high.159 Additionally, coreceptors may be involved in CAR signaling when pMHC is targeted.
CAR ligand recognition domains and CAR-T cell activation thresholds
The extracellular recognition domains of CARs have been demonstrated to critically affect downstream signals. Theoretically, any protein that has the capacity to bind with its specific partner can be utilized as a recognition domain in CAR. To date, various forms of extracellular domains have been tested, including scFv, single domain antibodies, natural ligands, synthetic peptide ligands, and designed ankyrin repeat proteins.160 However, much of our knowledge of how the recognition domain affects CAR signaling was obtained from scFv, easily the most commonly used module. One of the most important questions is the influence of the affinity of the recognition domain for a target on downstream CAR signals.
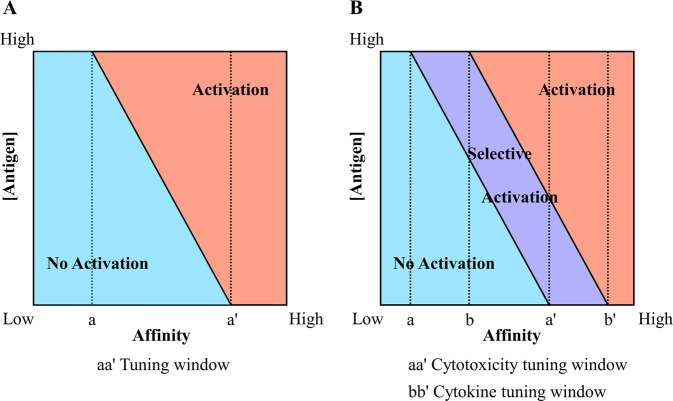
The binding affinity determines the T cell activation threshold, where CAR-T cells start to respond when the amount of a targeted antigen reaches a threshold level, and the magnitude and type of the response is determined by the costimulatory signaling domain(s).161 This action suggests that there is an optimal affinity window for CAR-T cells (Fig. 3a). Within this window, CAR-T cells have selective effects on tumor cells expressing high amounts of target antigen but spare normal cells expressing the same antigen at a lower level.162–164 Outside this window, CAR-T cells can show either an inability to distinguish between high- and low-antigen expressing cells or an inability to respond effectively.165 In other words, CARs with lower affinity do not respond to even high amounts of antigen, and CARs with higher affinity do not distinguish between target cells bearing low and high antigen loads.165
Fig. 3.
The influence of binding affinity of the CAR recognition domain and the antigen load on CAR-T signal and function. a The activation threshold of CAR-T cells is mediated by their binding affinity. Within the tuning window (a < affinity < a′), the antigen load required to activate CAR-T cells is inversely related to the affinity such that the CAR-T cell can discriminate between tumor and normal cells because of their different antigen loads. The CAR-T cell will not be activated regardless of how high the antigen load is when its affinity < a, whereas the CAR-T cell will be activated by even a very low antigen load when affinity > a′. b The affinity can be tuned to selectively control CAR-T cell responses. The activation threshold of cytokine secretion is several-fold higher than that of cytolytic function; hence, the tuning windows of cytotoxicity and cytokine secretion are distinct. These discrepant windows result in a selective activation zone, colored purple, where the cytotoxic function can be activated without activating the cytokine secretion function
Importantly, different T cell effector functions have distinct activation thresholds. For example, cytotoxicity has a lower activation threshold than cytokine secretion.157,166 Therefore, it is feasible to fine-tune the affinity of the recognition domains to selectively control the CAR-T cell responses such that the cytolytic capacity is retained, but cytokine secretion levels are reduced (Fig. 3b).167
In addition to affinity, the molecular characteristics of CAR extracellular domains can affect signaling. Screening of a series of scFvs showed that a particular scFv induced significantly higher tonic signaling, thus affecting the in vitro and in vivo efficacy of CAR-T cells.168 This outcome may be due to constitutive CAR clustering related to the increased probability of scFv oligomerization.169 In addition, a tandem CAR (TanCAR), in which two recognition domains are tethered through a linker and integrated into a single CAR structure, had dramatically enhanced signaling and CAR-T cell function because of this bivalent recognition compared with that of CAR-T cells simultaneously expressing two CARs with the same specificities.170
TCR and CAR signaling in different T cell subtypes
Because of the heterogeneity of T cell subsets, the distinct signaling networks and functional properties of these subsets of T cells can modulate CAR-T cell functions.
CD4+ and CD8+ αβ T cells
The major subsets of αβ T cells, CD4+ and CD8+, exhibit dramatic differences in signaling and function as CAR-T cells. CD8-CAR-T cells have a higher intracellular content of granzyme B and perforin and show faster killing kinetics than do CD4-CAR-T cells when both undertake sequential killing.171 However, CD4-CAR-T cells have significantly higher capacity to secrete effector cytokines such as IFNγ, TNF, and IL-2 and higher persistence in vivo than do CD8-CAR-T cells.172
The effects of endogenous TCR of CD4 and CD8 T cells also make a difference in CAR-T cell activity. Endogenous TCR activation of CD8-CAR-T cells makes them more prone to exhaustion and apoptosis, thus reducing the efficacy of TCRs and CARs concomitantly activated.173 However, longer exposure to activated CD4-CAR-T cells increases the risk of toxicity by inducing the constant release of Th2-type cytokines, such as IL-4, IL-6, IL-10, and IL-13.174 In addition, the differential influence of the costimulatory signaling by CAR on these two types of T cells can be inferred from early studies showing that CD28 costimulation preferentially expanded CD4+ T cells but resulted in CD8+ T cell exhaustion or anergy in the long term.175 Although 4‐1BB costimulation enhances the proliferation of both CD8+ and CD4+ T cells, it also supports memory CD8+ T cell expansion, in contrast to CD28 costimulation.176 Therefore, the costimulatory domains, i.e., CD28 or 4-1BB, in CARs might play different roles in CD4+ and CD8+ CAR-T cells.
Numerous studies have implied that the induction of memory CAR-T cells, particularly those with a central memory phenotype, may have greater in vivo efficacy than effector CAR-T cells.127,130 Hence, preselecting memory CD45RA−-CAR-T cells before injection significantly increased the in vivo antitumor effects compared to those of CD45RA+-CAR-T cells.177 The secretion of the cytokines IFNγ, TNF, and IL-2 from both CD4+ and CD8+ CAR-T cells in different memory subsets followed the trend of Tnaive (n) > Tcentral memory (cm) > Teffector memory (em) cells.172 The in vivo persistence and efficacy of CD4+ CAR-T cells followed this trend, whereas CD8+ CAR-T cells followed a trend of Tcm > Tn > Tem cells. Importantly, CD8+ CAR-Tcm cells administered with CD4+ CAR-Tn or -Tcm cells had a synergistic effect in vivo, as the antitumor efficacy was dramatically enhanced compared with that of individual injections of CD4+ or CD8+ cells.
Treg cells
CARs are not only powerful tools to boost immune function but are also great potential treatments for autoimmune conditions, such as CAR-Treg cells. The impacts of CARs on Treg function are largely dictated by the costimulatory signaling of CAR. CD28-CAR seems to confer Treg cells with a specific proinflammatory property in addition to their regulatory function, as CD28-CAR-Treg cells secrete cytokines such as IFNγ and TNF, which were not observed in the 4-1BB-CAR-Treg cells.178,179 However, 4-1BB-CAR increased the secretion of the anti-inflammatory cytokine IL-10. Nevertheless, CD28-CAR, but not 4-1BB-CAR, boosted the anti-effector T cell function of CAR-Treg cells both in vivo and in vitro.180 Hence, CD28-CAR might play a more important role than 4-1BB-CAR in controlling Treg cell function.
γδ T cells
In contrast to αβ T cells, γδ T cells recognize not only classical pMHC181 but also diverse molecules, such as phosphoantigens, lipopeptides, microorganism-derived proteins, stress-associated proteins, and nonclassical MHC.181,182 Because of their antitumor and proinflammatory functions, γδ T cells have drawn high levels of attention as CAR-T cells. Additionally, some types of γδ TCRs, e.g., Vγ9Vδ2 T cells, naturally recognize stressed cells, such as tumor cells.183 By taking advantage of a tumor cell-recognizing TCR, an AND-gate CAR-T cell was designed using a combination of a Vγ9Vδ2 TCR and a CAR with a DAP10 intracellular domain, Therefore, on-target, off-tumor effects were minimized since the CAR-T cells were activated only when the CAR-T cells meet two antigens, i.e., antigens for Vγ9Vδ2 TCR and CAR, respectively, expressed on tumor cells.184 γδ CAR-T cells with a less differentiated cell phenotype retained cytotoxicity to a greater extent than did αβ CAR-T cells, and they also serve as APCs when directed to cancer cells, presenting neoantigens for αβ T cells in the tumor environment.185 Moreover, γδ CAR-T cells potentially have a lower risk of mediating unwanted side effects, including CRS.186
Applications: modulating CAR signaling to control CAR-T cell functions
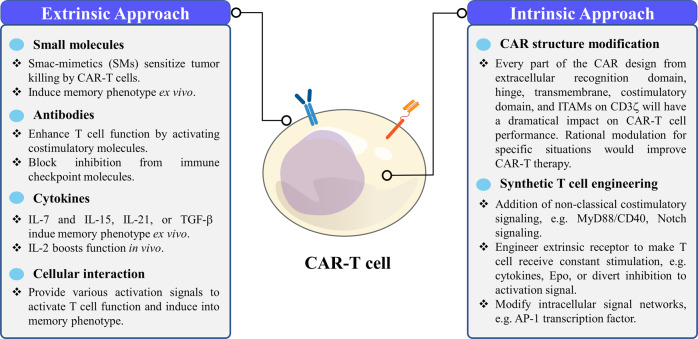
CAR-T cell signaling and functions can be tuned through extrinsic and intrinsic methods (Fig. 4). Extrinsic methods use external molecules, such as chemicals, cytokines, or antibodies, to boost CAR-T cell functions during ex vivo culture and in vivo therapy. Intrinsic methods include engineering CAR-T cells and bestowing them with additional signaling capacity.
Fig. 4.
Summary of the extrinsic and intrinsic approaches to regulate CAR-T cells through signaling
Extrinsic regulation of CAR-T cell function
Various chemicals have been developed to suppress cancer progression. It is thus intriguing to combine CAR-T cell therapy with traditional chemotherapy to synergistically enhance anticancer effects. A screen of approximately 500 drugs revealed that the second mitochondria-derived activator of caspase (smac)-mimetics (SMs) are leading candidates to sensitize CAR-T cell killing in B cell malignancies.187 SMs antagonize inhibitor of apoptosis protein (IAP), thereby inducing cancer cell death, but they also have a distinct effect on T cells, increasing NF-κB signaling and thus leading to enhanced T cell function.188 Therefore, synergistic therapeutic effects of SMs were observed when they were administered in combination with CAR-T cell therapy.189 An inhibitor of phosphatase 2A (PP2A), LB-100, enhanced CAR-T cell activity by enhancing mTORC1 signaling, T cell activation, and proliferation.190 Chemicals can also be used to alter the CAR-T cell differentiation state by modulating signaling in ex vivo culture. Treatment with an AKT inhibitor during ex vivo expansion significantly increased the memory T cell percentage in the population, thus significantly increasing the efficacy of CAR-T cells in vivo.191 Beyond the efficacy enhancement, chemicals can also be applied to regulate CAR-T cell activity in vivo. For example, the use of Src family kinase inhibitors reduced side effects associated with CAR-T cell therapy by preventing excessive activation of CAR-T cells.192,193
Cytokines provide CAR-T cells with signal 3, which is critical to modulate T cell functions and its differentiation state. Culturing CAR-T cells with IL-7 and IL-15, IL-21, or TGFβ induced a less differentiated memory state, such as stem cell-like memory T (Tscm) cells or central memory T cells (Tcm).194–196 Memory CAR-T cells exhibited prolonged survival and expansion in vivo and enhanced antitumor efficacy. On the other hand, CAR-T cell antitumor efficacy can also be boosted with additional cytokine injections, such as engineered orthogonal IL-2, after CAR-T cell transfusion.197
Antibodies can induce specific costimulatory signaling to promote CAR-T cell function in the absence of ligands for costimulatory receptors in the tumor environment. For example, antibodies were used to coactivate 4-1BB expressed in CD28-CAR-T cells, leading to enhanced CAR-T cell antitumor function and survival in vivo.198 Moreover, CAR-T cell function can also be enhanced by releasing inhibitory factors via the immune checkpoint blockade.199,200
CAR-T cell signaling can be modulated through cellular interactions. CAR-T cells cocultured with a Notch ligand-expressing cell line, OP9-hDLL1, were reprogrammed into Tscm, promoting anticancer efficacy by increasing the survival of CAR-T cells in vivo.201 DCs provide a variety of costimulatory signals for T cells, and the use of modified DCs or vaccinations can induce interactions between CAR-T cells and DCs, resulting in better CAR-T cell responses.202,203
Intrinsic regulation of CAR-T cell functions
The most common intrinsic method used to modify the CAR construct involves adjusting its signaling. Each part of the CAR design, from the recognition domain to the ITAMs of CD3ζ can be optimized to enhance downstream signaling. For example, increasing the affinity of scFv within the therapeutic optimal affinity window (Fig. 3) improves the activity of CAR-T cells.66 Blocking the second and third ITAM in CD3ζ was found to make CAR-T cells more memory-like compared to those generated by CD3ζ with the full complement of ITAMs.204 The optimal solution is to create a rational design or screening of CAR costimulatory domains to ensure that they can both highly discriminate between low and high antigen expression and boost CAR-T cell activity.
With the advent of synthetic biology and cell engineering, researchers were equipped with versatile tools to manipulate CAR-T cells to optimize their anticancer efficacy. Numerous studies have been performed to intrinsically modify CAR-T cell signaling. These studies can be summarized as pursuits of activity enhancement and inhibition reversal. To enhance the activity of CAR-T cells by boosting signaling, it is critical to increase CAR-T cell proliferation and survival in vivo, which are usually accompanied by a sustained T cell memory phenotype. This outcome can be achieved by integrating other costimulatory signaling domains in CAR-T cells along with 4-1BB or CD28, for example, with MyD88, CD40, and a Notch signaling sequence to promote proliferation and survival and induce a Tscm phenotype, respectively.201,205 CAR-T cells can also be engineered to constantly receive stimulation required for optimal functioning, such as stimulation by cytokines such as IL-7,206 IL-12,207 IL-15,208 IL-18,209 IL-23,37 or Epo.210 These modifications prevent the risk caused by systematically injecting stimulants and promote the survival and persistence of the CAR-T cells. Another approach to engineering CAR-T cells is to reverse inhibitory signaling received from the tumor microenvironment (TME) or intracellularly upon activation. The TME is notorious for presenting multiple immune inhibition molecules, such as PD-L1 on cancer cells or suppressive cytokines IL-4 and TGFβ .211 These inhibitions can be reversed by constructing chimeric cytokine receptors to divert IL-4 and TGFβ signaling to be IL-7 or 4-1BB signaling, respectively, or by conferring CAR-T cells with the ability to secrete anti-PD-1 scFv.212–214 Upregulation of basic leucine zipper (bZIP) and interferon regulatory factor (IRF) family transcription factors led to an exhausted gene signature in CAR-T cells.215 Overexpression of the AP-1 transcription factor c-JUN blocked these proteins and reversed the state of CAR-T cell exhaustion, resulting in the expansion and survival of the CAR-T cells and their increased in vivo antitumor efficacy.
Concluding remarks
In this review, we analyzed TCR and CAR signaling starting from the engagement of the antigen and receptor to downstream signaling. We also reviewed the interaction between different cell subtypes and CAR signaling and ways to modulate TCR-T and CAR-T cells through extrinsic or intrinsic signaling. Because of the artificial nature of CARs, it is meaningful to dissect the fundamental differences between TCR and CAR signaling to apply these receptors in different situations. The cumulative understanding of CAR signaling conferred by each part of the CAR and its role in different T cell subtypes will help researchers rationally design proper CAR-T cell therapies to address diverse challenges posed by various diseases. It is important that different CAR designs show disparate efficacy and patient responses toward different kinds of cancers. It has been observed that CD28-CAR-T cell therapy might be better for patients with a heavy cancer burden, whereas 4-1BB-CAR-T cell therapy may be more effective for patients with chronic cancer. Given the range of tools and approaches now available, there is great promise that CAR-T cell functions can be tuned by all kinds of methods, including costimulatory selection, scFv modulation, and cytokine induction during ex vivo expansion, to ultimately produce optimal CAR-based therapy for a particular disease.
Competing interests
The authors declare no competing interests.
References
- 1.Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32:1267–1284. doi: 10.1101/gad.314617.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanson HL, et al. Eradication of established tumors by CD8+ T cell adoptive immunotherapy. Immunity. 2000;13:265–276. doi: 10.1016/s1074-7613(00)00026-1. [DOI] [PubMed] [Google Scholar]
- 3.Kalams SA, Walker BD. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J. Exp. Med. 1998;188:2199–2204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr. Opin. Immunol. 1998;10:588–594. doi: 10.1016/s0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- 5.Shankaran V, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 6.Matsushita H, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482:400–404. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teng MW, Galon J, Fridman WH, Smyth MJ. From mice to humans: developments in cancer immunoediting. J. Clin. Invest. 2015;125:3338–3346. doi: 10.1172/JCI80004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker ML, et al. Expression of a hybrid immunoglobulin-T cell receptor protein in transgenic mice. Cell. 1989;58:911–921. doi: 10.1016/0092-8674(89)90943-4. [DOI] [PubMed] [Google Scholar]
- 9.Goverman J, et al. Chimeric immunoglobulin-T cell receptor proteins form functional receptors: implications for T cell receptor complex formation and activation. Cell. 1990;60:929–939. doi: 10.1016/0092-8674(90)90341-b. [DOI] [PubMed] [Google Scholar]
- 10.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl. Acad. Sci. USA. 1989;86:10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gascoigne NR, Goodnow CC, Dudzik KI, Oi VT, Davis MM. Secretion of a chimeric T-cell receptor-immunoglobulin protein. Proc. Natl. Acad. Sci. USA. 1987;84:2936–2940. doi: 10.1073/pnas.84.9.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neuberger MS, Williams GT, Fox RO. Recombinant antibodies possessing novel effector functions. Nature. 1984;312:604–608. doi: 10.1038/312604a0. [DOI] [PubMed] [Google Scholar]
- 13.Traunecker A, Luke W, Karjalainen K. Soluble CD4 molecules neutralize human immunodeficiency virus type 1. Nature. 1988;331:84–86. doi: 10.1038/331084a0. [DOI] [PubMed] [Google Scholar]
- 14.Smith DH, et al. Blocking of HIV-1 infectivity by a soluble, secreted form of the CD4 antigen. Science. 1987;238:1704–1707. doi: 10.1126/science.3500514. [DOI] [PubMed] [Google Scholar]
- 15.Morrison SL, Johnson MJ, Herzenberg LA, Oi VT. Chimeric human antibody molecules: mouse antigen-binding domains with human constant region domains. Proc. Natl. Acad. Sci. USA. 1984;81:6851–6855. doi: 10.1073/pnas.81.21.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bird RE, et al. Single-chain antigen-binding proteins. Science. 1988;242:423–426. doi: 10.1126/science.3140379. [DOI] [PubMed] [Google Scholar]
- 17.Huston JS, et al. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc. Natl. Acad. Sci. USA. 1988;85:5879–5883. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irving BA, Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991;64:891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- 19.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl. Acad. Sci. USA. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat. Rev. Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 21.Hombach A, et al. Tumor-specific T cell activation by recombinant immunoreceptors: CD3 signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3 signaling receptor molecule. J. Immunol. 2001;167:6123–6131. doi: 10.4049/jimmunol.167.11.6123. [DOI] [PubMed] [Google Scholar]
- 22.Kochenderfer JN, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maude SL, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.June CH, Sadelain M. Chimeric antigen receptor therapy. N. Engl. J. Med. 2018;379:64–73. doi: 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis MM, Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334:395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- 27.Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu. Rev. Immunol. 2006;24:419–466. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- 28.Kabelitz D, Marischen L, Oberg H-H, Holtmeier W, Wesch D. Epithelial defence by γδ T cells. Int. Arch. Allergy Immunol. 2005;137:73–81. doi: 10.1159/000085107. [DOI] [PubMed] [Google Scholar]
- 29.Gao GF, Jakobsen BK. Molecular interactions of coreceptor CD8 and MHC class I: the molecular basis for functional coordination with the T-cell receptor. Immunol. Today. 2000;21:630–636. doi: 10.1016/s0167-5699(00)01750-3. [DOI] [PubMed] [Google Scholar]
- 30.Gao GF, Rao Z, Bell JI. Molecular coordination of αβ T-cell receptors and coreceptors CD8 and CD4 in their recognition of peptide-MHC ligands. Trends Immunol. 2002;23:408–413. doi: 10.1016/s1471-4906(02)02282-2. [DOI] [PubMed] [Google Scholar]
- 31.Yachi PP, Ampudia J, Gascoigne NR, Zal T. Nonstimulatory peptides contribute to antigen-induced CD8-T cell receptor interaction at the immunological synapse. Nat. Immunol. 2005;6:785–792. doi: 10.1038/ni1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao X, et al. Nonstimulatory peptide-MHC enhances human T-cell antigen-specific responses by amplifying proximal TCR signaling. Nat. Commun. 2018;9:2716. doi: 10.1038/s41467-018-05288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palacios EH, Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. 2004;23:7990–8000. doi: 10.1038/sj.onc.1208074. [DOI] [PubMed] [Google Scholar]
- 34.Nerreter T, et al. Super-resolution microscopy reveals ultra-low CD19 expression on myeloma cells that triggers elimination by CD19 CAR-T. Nat. Commun. 2019;10:3137. doi: 10.1038/s41467-019-10948-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brameshuber M, et al. Monomeric TCRs drive T cell antigen recognition. Nat. Immunol. 2018;19:487–496. doi: 10.1038/s41590-018-0092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang ZL, et al. Rewiring T-cell responses to soluble factors with chimeric antigen receptors. Nat. Chem. Biol. 2018;14:317–324. doi: 10.1038/nchembio.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma X, et al. Interleukin-23 engineering improves CAR T cell function in solid tumors. Nat. Biotechnol. 2020;38:448–459. doi: 10.1038/s41587-019-0398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng Y, Reinherz EL, Lang MJ. alphabeta T cell receptor mechanosensing forces out serial engagement. Trends Immunol. 2018;39:596–609. doi: 10.1016/j.it.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tischer DK, Weiner OD. Light-based tuning of ligand half-life supports kinetic proofreading model of T cell signaling. eLife. 2019;8:e42498. doi: 10.7554/eLife.42498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis SJ, van der Merwe PA. The kinetic-segregation model: TCR triggering and beyond. Nat. Immunol. 2006;7:803–809. doi: 10.1038/ni1369. [DOI] [PubMed] [Google Scholar]
- 41.Choudhuri K, Wiseman D, Brown MH, Gould K, van der Merwe PA. T-cell receptor triggering is critically dependent on the dimensions of its peptide-MHC ligand. Nature. 2005;436:578–582. doi: 10.1038/nature03843. [DOI] [PubMed] [Google Scholar]
- 42.Choudhuri K, et al. Peptide-major histocompatibility complex dimensions control proximal kinase-phosphatase balance during T cell activation. J. Biol. Chem. 2009;284:26096–26105. doi: 10.1074/jbc.M109.039966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cordoba SP, et al. The large ectodomains of CD45 and CD148 regulate their segregation from and inhibition of ligated T-cell receptor. Blood. 2013;121:4295–4302. doi: 10.1182/blood-2012-07-442251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.James JR, Vale RD. Biophysical mechanism of T-cell receptor triggering in a reconstituted system. Nature. 2012;487:64–69. doi: 10.1038/nature11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nika K, et al. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity. 2010;32:766–777. doi: 10.1016/j.immuni.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nel AE. T-cell activation through the antigen receptor. Part 1: signaling components, signaling pathways, and signal integration at the T-cell antigen receptor synapse. J. Allergy Clin. Immunol. 2002;109:758–770. doi: 10.1067/mai.2002.124259. [DOI] [PubMed] [Google Scholar]
- 47.Denny MF, Patai B, Straus DB. Differential T-cell antigen receptor signaling mediated by the Src family kinases Lck and Fyn. Mol. Cell. Biol. 2000;20:1426–1435. doi: 10.1128/mcb.20.4.1426-1435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 49.Casas J, et al. Ligand-engaged TCR is triggered by Lck not associated with CD8 coreceptor. Nat. Commun. 2014;5:5624. doi: 10.1038/ncomms6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gascoigne NR, Casas J, Brzostek J, Rybakin V. Initiation of TCR phosphorylation and signal transduction. Front. Immunol. 2011;2:72. doi: 10.3389/fimmu.2011.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang N, et al. Two-stage cooperative T cell receptor-peptide major histocompatibility complex-CD8 trimolecular interactions amplify antigen discrimination. Immunity. 2011;34:13–23. doi: 10.1016/j.immuni.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samelson LE. Signal transduction edited by the T cell antigen receptor: the role of adapter proteins. Annu. Rev. Immunol. 2002;20:371–394. doi: 10.1146/annurev.immunol.20.092601.111357. [DOI] [PubMed] [Google Scholar]
- 53.Lo WL, et al. Lck promotes Zap70-dependent LAT phosphorylation by bridging Zap70 to LAT. Nat. Immunol. 2018;19:733–741. doi: 10.1038/s41590-018-0131-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu. Rev. Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lesourne R, et al. Themis, a T cell-specific protein important for late thymocyte development. Nat. Immunol. 2009;10:840–847. doi: 10.1038/ni.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson AL, et al. Themis is a member of a new metazoan gene family and is required for the completion of thymocyte positive selection. Nat. Immunol. 2009;10:831–839. doi: 10.1038/ni.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fu G, et al. Themis sets the signal threshold for positive and negative selection in T-cell development. Nature. 2013;504:441–445. doi: 10.1038/nature12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choi S, et al. THEMIS enhances TCR signaling and enables positive selection by selective inhibition of the phosphatase SHP-1. Nat. Immunol. 2017;18:433–441. doi: 10.1038/ni.3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paster W, et al. A THEMIS:SHP1 complex promotes T-cell survival. EMBO J. 2015;34:393–409. doi: 10.15252/embj.201387725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fu G, et al. Themis controls thymocyte selection through regulation of T cell antigen receptor-mediated signaling. Nat. Immunol. 2009;10:848–856. doi: 10.1038/ni.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu SK, Fang N, Koretzky GA, McGlade CJ. The hematopoietic-specific adaptor protein gads functions in T-cell signaling via interactions with the SLP-76 and LAT adaptors. Curr. Biol. 1999;9:67–75. doi: 10.1016/s0960-9822(99)80017-7. [DOI] [PubMed] [Google Scholar]
- 62.Shim EK, Jung SH, Lee JR. Role of two adaptor molecules SLP-76 and LAT in the PI3K signaling pathway in activated T cells. J. Immunol. 2011;186:2926–2935. doi: 10.4049/jimmunol.1001785. [DOI] [PubMed] [Google Scholar]
- 63.Bogin Y, Ainey C, Beach D, Yablonski D. SLP-76 mediates and maintains activation of the Tec family kinase ITK via the T cell antigen receptor-induced association between SLP-76 and ITK. Proc. Natl. Acad. Sci. USA. 2007;104:6638–6643. doi: 10.1073/pnas.0609771104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salter AI, et al. Phosphoproteomic analysis of chimeric antigen receptor signaling reveals kinetic and quantitative differences that affect cell function. Sci. Signal. 2018;11:eaat6753. doi: 10.1126/scisignal.aat6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harris DT, et al. Comparison of T cell activities mediated by human TCRs and CARs that use the same recognition domains. J. Immunol. 2018;200:1088–1100. doi: 10.4049/jimmunol.1700236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watanabe K, Kuramitsu S, Posey AD, Jr., June CH. Expanding the therapeutic window for CAR T cell therapy in solid tumors: the knowns and unknowns of CAR T cell biology. Front. Immunol. 2018;9:2486. doi: 10.3389/fimmu.2018.02486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gascoigne NR, Rybakin V, Acuto O, Brzostek J. TCR signal strength and T cell development. Annu. Rev. Cell Dev. Biol. 2016;32:327–348. doi: 10.1146/annurev-cellbio-111315-125324. [DOI] [PubMed] [Google Scholar]
- 68.Daniels MA, et al. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 69.Karlsson H, et al. Evaluation of intracellular signaling downstream chimeric antigen receptors. PLoS ONE. 2015;10:e0144787. doi: 10.1371/journal.pone.0144787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ramello MC, et al. An immunoproteomic approach to characterize the CAR interactome and signalosome. Sci. Signal. 2019;12:eaap9777. doi: 10.1126/scisignal.aap9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rohrs JA, Zheng D, Graham NA, Wang P, Finley SD. Computational model of chimeric antigen receptors explains site-specific phosphorylation kinetics. Biophys. J. 2018;115:1116–1129. doi: 10.1016/j.bpj.2018.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gulati P, et al. Aberrant Lck signal via CD28 costimulation augments antigen-specific functionality and tumor control by redirected T cells with PD-1 blockade in humanized mice. Clin. Cancer Res. 2018;24:3981–3993. doi: 10.1158/1078-0432.CCR-17-1788. [DOI] [PubMed] [Google Scholar]
- 73.Guedan S, et al. Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation. JCI Insight. 2018;3:96976. doi: 10.1172/jci.insight.96976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gomes da Silva D, et al. Direct comparison of in vivo fate of second and third-generation CD19-specific chimeric antigen receptor (CAR)-T cells in patients with B-cell lymphoma: reversal of toxicity from tonic signaling. Blood. 2016;128:1851. [Google Scholar]
- 75.Sun C, et al. THEMIS-SHP1 recruitment by 4-1BB tunes LCK-mediated priming of chimeric antigen receptor-redirected T cells. Cancer Cell. 2020;37:216–225. doi: 10.1016/j.ccell.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hamieh M, et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature. 2019;568:112–116. doi: 10.1038/s41586-019-1054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hui E, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. 2017;355:1428–1433. doi: 10.1126/science.aaf1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zolov SN, Rietberg SP, Bonifant CL. Programmed cell death protein 1 activation preferentially inhibits CD28.CAR-T cells. Cytotherapy. 2018;20:1259–1266. doi: 10.1016/j.jcyt.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 79.Kamphorst AO, et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science. 2017;355:1423–1427. doi: 10.1126/science.aaf0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wan Z, et al. Transmembrane domain-mediated Lck association underlies bystander and costimulatory ICOS signaling. Cell. Mol. Immunol. 2020;17:143–152. doi: 10.1038/s41423-018-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fooksman DR, et al. Functional anatomy of T cell activation and synapse formation. Annu. Rev. Immunol. 2010;28:79–105. doi: 10.1146/annurev-immunol-030409-101308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 83.Dustin ML. The immunological synapse. Cancer Immunol. Res. 2014;2:1023–1033. doi: 10.1158/2326-6066.CIR-14-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Korman, A. J., Peggs, K. S. & Allison J. P. Checkpoint Blockade in Cancer Immunotherapy, Vol. 90 297–339 (Elsevier; 2006). [DOI] [PMC free article] [PubMed]
- 85.Yokosuka T, et al. Spatiotemporal regulation of T cell costimulation by TCR-CD28 microclusters and protein kinase Cθ translocation. Immunity. 2008;29:589–601. doi: 10.1016/j.immuni.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mukherjee M, Mace EM, Carisey AF, Ahmed N, Orange JS. Quantitative imaging approaches to study the CAR immunological synapse. Mol. Ther. 2017;25:1757–1768. doi: 10.1016/j.ymthe.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Monks CRF, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 88.Campi G, Varma R, Dustin ML. Actin and agonist MHC–peptide complex–dependent T cell receptor microclusters as scaffolds for signaling. J. Exp. Med. 2005;202:1031–1036. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Varma R, Campi G, Yokosuka T, Saito T, Dustin MLT. Cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saito T, Yokosuka T, Hashimoto-Tane A. Dynamic regulation of T cell activation and co-stimulation through TCR-microclusters. FEBS Lett. 2010;584:4865–4871. doi: 10.1016/j.febslet.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 91.Zinselmeyer BH, et al. PD-1 promotes immune exhaustion by inducing antiviral T cell motility paralysis. J. Exp. Med. 2013;210:757–774. doi: 10.1084/jem.20121416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yi J, Wu XS, Crites T, Hammer JA., III Actin retrograde flow and actomyosin II arc contraction drive receptor cluster dynamics at the immunological synapse in Jurkat T cells. Mol. Biol. Cell. 2012;23:834–852. doi: 10.1091/mbc.E11-08-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wabnitz G, Balta E, Samstag Y. l-Plastin regulates the stability of the immune synapse of naive and effector T-cells. Adv. Biol. Regul. 2017;63:107–114. doi: 10.1016/j.jbior.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 94.Johnson KG, Bromley SK, Dustin ML, Thomas ML. A supramolecular basis for CD45 tyrosine phosphatase regulation in sustained T cell activation. Proc. Natl. Acad. Sci. USA. 2000;97:10138–10143. doi: 10.1073/pnas.97.18.10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sims TN, et al. Opposing effects of PKCtheta and WASp on symmetry breaking and relocation of the immunological synapse. Cell. 2007;129:773–785. doi: 10.1016/j.cell.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 96.Davenport AJ, et al. Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity. Proc. Natl. Acad. Sci. USA. 2018;115:E2068–E2076. doi: 10.1073/pnas.1716266115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xiong W, et al. Immunological synapse predicts effectiveness of chimeric antigen receptor cells. Mol. Ther. 2018;26:963–975. doi: 10.1016/j.ymthe.2018.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Davenport AJ, et al. CAR-T cells inflict sequential killing of multiple tumor target cells. Cancer Immunol. Res. 2015;3:483–494. doi: 10.1158/2326-6066.CIR-15-0048. [DOI] [PubMed] [Google Scholar]
- 100.Davenport AJ, Jenkins MR. Programming a serial killer: CAR T cells form non-classical immune synapses. Oncoscience. 2018;5:69–70. doi: 10.18632/oncoscience.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baeuerle PA, et al. Synthetic TRuC receptors engaging the complete T cell receptor for potent anti-tumor response. Nat. Commun. 2019;10:2087. doi: 10.1038/s41467-019-10097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Offner S, Hofmeister R, Romaniuk A, Kufer P, Baeuerle PA. Induction of regular cytolytic T cell synapses by bispecific single-chain antibody constructs on MHC class I-negative tumor cells. Mol. Immunol. 2006;43:763–771. doi: 10.1016/j.molimm.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 103.Helsen CW, et al. The chimeric TAC receptor co-opts the T cell receptor yielding robust anti-tumor activity without toxicity. Nat. Commun. 2018;9:3049. doi: 10.1038/s41467-018-05395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jeon BN, et al. Actin stabilizer TAGLN2 potentiates adoptive T cell therapy by boosting the inside-out costimulation via lymphocyte function-associated antigen-1. Oncoimmunology. 2018;7:e1500674. doi: 10.1080/2162402X.2018.1500674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang X, et al. Lenalidomide enhances the function of CS1 chimeric antigen receptor-redirected T cells against multiple myeloma. Clin. Cancer Res. 2018;24:106–119. doi: 10.1158/1078-0432.CCR-17-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Joseph N, Reicher B, Barda-Saad M. The calcium feedback loop and T cell activation: how cytoskeleton networks control intracellular calcium flux. Biochim. Biophys. Acta. 2014;1838:557–568. doi: 10.1016/j.bbamem.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 107.Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu. Rev. Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- 108.Schulze-Luehrmann J, Ghosh S. Antigen-receptor signaling to nuclear factor kappa B. Immunity. 2006;25:701–715. doi: 10.1016/j.immuni.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 109.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat. Rev. Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 110.Roche MI, Ramadas RA, Medoff BD. The role of CARMA1 in T cells. Crit. Rev. Immunol. 2013;33:219–243. doi: 10.1615/critrevimmunol.2013007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sun W, Yang J. Molecular basis of lysophosphatidic acid-induced NF-kappaB activation. Cell. Signal. 2010;22:1799–1803. doi: 10.1016/j.cellsig.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 113.Roose JP, Mollenauer M, Ho M, Kurosaki T, Weiss A. Unusual interplay of two types of Ras activators, RasGRP and SOS, establishes sensitive and robust Ras activation in lymphocytes. Mol. Cell. Biol. 2007;27:2732–2745. doi: 10.1128/MCB.01882-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Roskoski R., Jr. ERK1/2 MAP kinases: structure, function, and regulation. Pharm. Res. 2012;66:105–143. doi: 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 115.Benmebarek MR, et al. Killing mechanisms of chimeric antigen receptor (CAR) T cells. Int. J. Mol. Sci. 2019;20:E1283. doi: 10.3390/ijms20061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu Y, et al. Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Sci. Immunol. 2020;5:eaax7969. doi: 10.1126/sciimmunol.aax7969. [DOI] [PubMed] [Google Scholar]
- 117.Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol. Rev. 2009;229:12–26. doi: 10.1111/j.1600-065X.2009.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yoshinaga SK, et al. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- 119.Fos C, et al. ICOS ligation recruits the p50alpha PI3K regulatory subunit to the immunological synapse. J. Immunol. 2008;181:1969–1977. doi: 10.4049/jimmunol.181.3.1969. [DOI] [PubMed] [Google Scholar]
- 120.Wikenheiser DJ, Stumhofer JS. ICOS co-stimulation: friend or foe? Front. Immunol. 2016;7:304. doi: 10.3389/fimmu.2016.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Esensten JH, Helou YA, Chopra G, Weiss A, Bluestone JA. CD28 costimulation: from mechanism to therapy. Immunity. 2016;44:973–988. doi: 10.1016/j.immuni.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cheng Z, et al. In vivo expansion and antitumor activity of coinfused CD28- and 4-1BB-engineered CAR-T Cells in patients with B cell leukemia. Mol. Ther. 2018;26:976–985. doi: 10.1016/j.ymthe.2018.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kegler A, et al. T cells engrafted with a UniCAR 28/z outperform UniCAR BB/z-transduced T cells in the face of regulatory T cell-mediated immunosuppression. Oncoimmunology. 2019;8:e1621676. doi: 10.1080/2162402X.2019.1621676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Weinkove R, George P, Dasyam N, McLellan AD. Selecting costimulatory domains for chimeric antigen receptors: functional and clinical considerations. Clin. Transl. Immunol. 2019;8:e1049. doi: 10.1002/cti2.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kofler DM, et al. CD28 costimulation Impairs the efficacy of a redirected t-cell antitumor attack in the presence of regulatory t cells which can be overcome by preventing Lck activation. Mol. Ther. 2011;19:760–767. doi: 10.1038/mt.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zheng W, et al. PI3K orchestration of the in vivo persistence of chimeric antigen receptor-modified T cells. Leukemia. 2018;32:1157–1167. doi: 10.1038/s41375-017-0008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kawalekar OU, et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity. 2016;44:380–390. doi: 10.1016/j.immuni.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 128.Ajina A, Maher J. Strategies to address chimeric antigen receptor tonic signaling. Mol. Cancer Ther. 2018;17:1795–1815. doi: 10.1158/1535-7163.MCT-17-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Klein Geltink RI, et al. Mitochondrial priming by CD28. Cell. 2017;171:385–397. doi: 10.1016/j.cell.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Eyquem J, et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543:113–117. doi: 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Harada Y, et al. A single amino acid alteration in cytoplasmic domain determines IL-2 promoter activation by ligation of CD28 but not inducible costimulator (ICOS) J. Exp. Med. 2003;197:257–262. doi: 10.1084/jem.20021305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guedan S, et al. ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood. 2014;124:1070–1080. doi: 10.1182/blood-2013-10-535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ward-Kavanagh LK, Lin WW, Sedy JR, Ware CF. The TNF receptor superfamily in co-stimulating and co-inhibitory responses. Immunity. 2016;44:1005–1019. doi: 10.1016/j.immuni.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Oh HS, et al. 4-1BB signaling enhances primary and secondary population expansion of CD8+ T cells by maximizing autocrine IL-2/IL-2 receptor signaling. PLoS ONE. 2015;10:e0126765. doi: 10.1371/journal.pone.0126765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Snell LM, Lin GH, McPherson AJ, Moraes TJ, Watts TH. T-cell intrinsic effects of GITR and 4-1BB during viral infection and cancer immunotherapy. Immunol. Rev. 2011;244:197–217. doi: 10.1111/j.1600-065X.2011.01063.x. [DOI] [PubMed] [Google Scholar]
- 136.Bartkowiak T, Curran MA. 4-1BB agonists: multi-potent potentiators of tumor immunity. Front. Oncol. 2015;5:117. doi: 10.3389/fonc.2015.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tamada K, et al. LIGHT, a TNF-like molecule, costimulates T cell proliferation and is required for dendritic cell-mediated allogeneic T cell response. J. Immunol. 2000;164:4105–4110. doi: 10.4049/jimmunol.164.8.4105. [DOI] [PubMed] [Google Scholar]
- 138.Milone MC, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol. Ther. 2009;17:1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Singh N, et al. Impaired death receptor signaling in leukemia causes antigen-independent resistance by inducing CAR T cell dysfunction. Cancer Discov. 2020;10:552–567. doi: 10.1158/2159-8290.CD-19-0813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mamonkin M, et al. Reversible transgene expression reduces fratricide and permits 4-1BB costimulation of CAR T cells directed to T-cell malignancies. Cancer Immunol. Res. 2018;6:47–58. doi: 10.1158/2326-6066.CIR-17-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kunkele A, et al. Functional tuning of CARs reveals signaling threshold above which CD8+ CTL antitumor potency is attenuated due to cell Fas-FasL-dependent AICD. Cancer Immunol. Res. 2015;3:368–379. doi: 10.1158/2326-6066.CIR-14-0200. [DOI] [PubMed] [Google Scholar]
- 142.Nunoya JI, Masuda M, Ye C, Su L. Chimeric antigen receptor T cell bearing herpes virus entry mediator co-stimulatory signal domain exhibits high functional potency. Mol. Ther. Oncolytics. 2019;14:27–37. doi: 10.1016/j.omto.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hombach AA, Heiders J, Foppe M, Chmielewski M, Abken H. OX40 costimulation by a chimeric antigen receptor abrogates CD28 and IL-2 induced IL-10 secretion by redirected CD4(+) T cells. Oncoimmunology. 2012;1:458–466. doi: 10.4161/onci.19855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fraietta JA, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat. Med. 2018;24:563–571. doi: 10.1038/s41591-018-0010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Siegel AM, et al. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity. 2011;35:806–818. doi: 10.1016/j.immuni.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kagoya Y, et al. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat. Med. 2018;24:352–359. doi: 10.1038/nm.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Guest RD, et al. The role of extracellular spacer regions in the optimal design of chimeric immune receptors: evaluation of four different scFvs and antigens. J. Immunother. 2005;28:203–211. doi: 10.1097/01.cji.0000161397.96582.59. [DOI] [PubMed] [Google Scholar]
- 148.Hombach A, et al. T cell activation by recombinant FcepsilonRI gamma-chain immune receptors: an extracellular spacer domain impairs antigen-dependent T cell activation but not antigen recognition. Gene Ther. 2000;7:1067–1075. doi: 10.1038/sj.gt.3301195. [DOI] [PubMed] [Google Scholar]
- 149.Hombach AA, et al. T cell activation by antibody-like immunoreceptors: the position of the binding epitope within the target molecule determines the efficiency of activation of redirected T cells. J. Immunol. 2007;178:4650–4657. doi: 10.4049/jimmunol.178.7.4650. [DOI] [PubMed] [Google Scholar]
- 150.James SE, et al. Antigen sensitivity of CD22-specific chimeric TCR is modulated by target epitope distance from the cell membrane. J. Immunol. 2008;180:7028–7038. doi: 10.4049/jimmunol.180.10.7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zah E, Lin MY, Silva-Benedict A, Jensen MC, Chen YY. T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol. Res. 2016;4:498–508. doi: 10.1158/2326-6066.CIR-15-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Qin H, et al. Eradication of B-ALL using chimeric antigen receptor-expressing T cells targeting the TSLPR oncoprotein. Blood. 2015;126:629–639. doi: 10.1182/blood-2014-11-612903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–849. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- 154.Fry TJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 2018;24:20–28. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sun B, et al. Eradication of hepatocellular carcinoma by NKG2D-based CAR-T cells. Cancer Immunol. Res. 2019;7:1813–1823. doi: 10.1158/2326-6066.CIR-19-0026. [DOI] [PubMed] [Google Scholar]
- 156.Stone JD, Aggen DH, Schietinger A, Schreiber H, Kranz DM. A sensitivity scale for targeting T cells with chimeric antigen receptors (CARs) and bispecific T-cell engagers (BiTEs) Oncoimmunology. 2012;1:863–873. doi: 10.4161/onci.20592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Watanabe K, et al. Target antigen density governs the efficacy of anti-CD20-CD28-CD3 zeta chimeric antigen receptor-modified effector CD8+ T cells. J. Immunol. 2015;194:911–920. doi: 10.4049/jimmunol.1402346. [DOI] [PubMed] [Google Scholar]
- 158.Hoseini SS, Cheung NV. Immunotherapy of hepatocellular carcinoma using chimeric antigen receptors and bispecific antibodies. Cancer Lett. 2017;399:44–52. doi: 10.1016/j.canlet.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Oren R, et al. Functional comparison of engineered T cells carrying a native TCR versus TCR-like antibody-based chimeric antigen receptors indicates affinity/avidity thresholds. J. Immunol. 2014;193:5733–5743. doi: 10.4049/jimmunol.1301769. [DOI] [PubMed] [Google Scholar]
- 160.Roselli E, et al. CAR-T engineering: optimizing signal transduction and effector mechanisms. BioDrugs. 2019;33:647–659. doi: 10.1007/s40259-019-00384-z. [DOI] [PubMed] [Google Scholar]
- 161.Chmielewski M, Hombach A, Heuser C, Adams GP, Abken HT. Cell activation by antibody-like immunoreceptors: increase in affinity of the single-chain fragment domain above threshold does not increase T cell activation against antigen-positive target cells but decreases selectivity. J. Immunol. 2004;173:7647–7653. doi: 10.4049/jimmunol.173.12.7647. [DOI] [PubMed] [Google Scholar]
- 162.Caruso HG, et al. Tuning sensitivity of CAR to EGFR density limits recognition of normal tissue while maintaining potent antitumor activity. Cancer Res. 2015;75:3505–3518. doi: 10.1158/0008-5472.CAN-15-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu X, et al. Affinity-tuned ErbB2 or EGFR chimeric antigen receptor T cells exhibit an increased therapeutic index against tumors in mice. Cancer Res. 2015;75:3596–3607. doi: 10.1158/0008-5472.CAN-15-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Talavera A, et al. Nimotuzumab, an antitumor antibody that targets the epidermal growth factor receptor, blocks ligand binding while permitting the active receptor conformation. Cancer Res. 2009;69:5851–5859. doi: 10.1158/0008-5472.CAN-08-4518. [DOI] [PubMed] [Google Scholar]
- 165.Lynn RC, et al. High-affinity FRbeta-specific CAR T cells eradicate AML and normal myeloid lineage without HSC toxicity. Leukemia. 2016;30:1355–1364. doi: 10.1038/leu.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Walker AJ, et al. Tumor antigen and receptor densities regulate efficacy of a chimeric antigen receptor targeting anaplastic lymphoma kinase. Mol. Ther. 2017;25:2189–2201. doi: 10.1016/j.ymthe.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hudecek M, et al. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin. Cancer Res. 2013;19:3153–3164. doi: 10.1158/1078-0432.CCR-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Smith EL, et al. GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci. Transl. Med. 2019;11:eaau7746. doi: 10.1126/scitranslmed.aau7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Long AH, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 2015;21:581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hegde M, et al. Tandem CAR T cells targeting HER2 and IL13Ralpha2 mitigate tumor antigen escape. J. Clin. Invest. 2016;126:3036–3052. doi: 10.1172/JCI83416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liadi I, et al. Individual motile CD4(+) T cells can participate in efficient multikilling through conjugation to multiple tumor cells. Cancer Immunol. Res. 2015;3:473–482. doi: 10.1158/2326-6066.CIR-14-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sommermeyer D, et al. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia. 2016;30:492–500. doi: 10.1038/leu.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yang Y, et al. TCR engagement negatively affects CD8 but not CD4 CAR T cell expansion and leukemic clearance. Sci. Transl. Med. 2017;9:eaag1209. doi: 10.1126/scitranslmed.aag1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cheadle EJ, et al. Differential role of Th1 and Th2 cytokines in autotoxicity driven by CD19-specific second-generation chimeric antigen receptor T cells in a mouse model. J. Immunol. 2014;192:3654–3665. doi: 10.4049/jimmunol.1302148. [DOI] [PubMed] [Google Scholar]
- 175.Laux I, et al. Response differences between human CD4(+) and CD8(+) T-cells during CD28 costimulation: implications for immune cell-based therapies and studies related to the expansion of double-positive T-cells during aging. Clin. Immunol. 2000;96:187–197. doi: 10.1006/clim.2000.4902. [DOI] [PubMed] [Google Scholar]
- 176.Zhang H, et al. 4-1BB is superior to CD28 costimulation for generating CD8+ cytotoxic lymphocytes for adoptive immunotherapy. J. Immunol. 2007;179:4910–4918. doi: 10.4049/jimmunol.179.7.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chan WK, et al. Chimeric antigen receptor-redirected CD45RA-negative T cells have potent antileukemia and pathogen memory response without graft-versus-host activity. Leukemia. 2015;29:387–395. doi: 10.1038/leu.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Koristka S, et al. Engrafting human regulatory T cells with a flexible modular chimeric antigen receptor technology. J. Autoimmun. 2018;90:116–131. doi: 10.1016/j.jaut.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 179.Hombach AA, Abken H. Most do, but some do not: CD4(+)CD25(−) T cells, but not CD4(+)CD25(+) Treg cells, are cytolytic when redirected by a chimeric antigen receptor (CAR) Cancers. 2017;9:112. doi: 10.3390/cancers9090112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Boroughs AC, et al. Chimeric antigen receptor costimulation domains modulate human regulatory T cell function. JCI Insight. 2019;5:e126194. doi: 10.1172/jci.insight.126194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Benveniste PM, et al. Generation and molecular recognition of melanoma-associated antigen-specific human gammadelta T cells. Sci. Immunol. 2018;3:eaav4036. doi: 10.1126/sciimmunol.aav4036. [DOI] [PubMed] [Google Scholar]
- 182.Vermijlen D, Gatti D, Kouzeli A, Rus T, Eberl M. gammadelta T cell responses: how many ligands will it take till we know? Semin. Cell Dev. Biol. 2018;84:75–86. doi: 10.1016/j.semcdb.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 183.Mirzaei HR, Mirzaei H, Lee SY, Hadjati J, Till BG. Prospects for chimeric antigen receptor (CAR) gammadelta T cells: a potential game changer for adoptive T cell cancer immunotherapy. Cancer Lett. 2016;380:413–423. doi: 10.1016/j.canlet.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Fisher J, et al. Avoidance of on-target off-tumor activation using a co-stimulation-only chimeric antigen receptor. Mol. Ther. 2017;25:1234–1247. doi: 10.1016/j.ymthe.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Capsomidis A, et al. Chimeric antigen receptor-engineered human gamma delta T cells: enhanced cytotoxicity with retention of cross presentation. Mol. Ther. 2018;26:354–365. doi: 10.1016/j.ymthe.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sebestyen Z, Prinz I, Dechanet-Merville J, Silva-Santos B, Kuball J. Translating gammadelta (γδ) T cells and their receptors into cancer cell therapies. Nat. Rev. Drug Discov. 2019;19:169–184. doi: 10.1038/s41573-019-0038-z. [DOI] [PubMed] [Google Scholar]
- 187.Dufva O, et al. Integrated drug profiling and CRISPR screening identify essential pathways for CAR T cell cytotoxicity. Blood. 2019;135:597–609. doi: 10.1182/blood.2019002121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dougan M, et al. IAP inhibitors enhance co-stimulation to promote tumor immunity. J. Exp. Med. 2010;207:2195–2206. doi: 10.1084/jem.20101123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Michie J, et al. Antagonism of IAPs enhances CAR T-cell efficacy. Cancer Immunol. Res. 2019;7:183–192. doi: 10.1158/2326-6066.CIR-18-0428. [DOI] [PubMed] [Google Scholar]
- 190.Cui J, et al. Inhibition of PP2A with LB-100 enhances efficacy of CAR-T cell therapy against glioblastoma. Cancers. 2020;12:139. doi: 10.3390/cancers12010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kim EH, Suresh M. Role of PI3K/Akt signaling in memory CD8 T cell differentiation. Front. Immunol. 2013;4:20. doi: 10.3389/fimmu.2013.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mestermann K, et al. The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Sci. Transl. Med. 2019;11:eaau5907. doi: 10.1126/scitranslmed.aau5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Weber EW, et al. Pharmacologic control of CAR-T cell function using dasatinib. Blood Adv. 2019;3:711–717. doi: 10.1182/bloodadvances.2018028720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dahmani A, et al. TGFbeta programs central memory differentiation in ex vivo-stimulated human T cells. Cancer Immunol. Res. 2019;7:1426–1439. doi: 10.1158/2326-6066.CIR-18-0691. [DOI] [PubMed] [Google Scholar]
- 195.Singh H, et al. Reprogramming CD19-specific T cells with IL-21 signaling can improve adoptive immunotherapy of B-lineage malignancies. Cancer Res. 2011;71:3516–3527. doi: 10.1158/0008-5472.CAN-10-3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Cieri N, et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood. 2013;121:573–584. doi: 10.1182/blood-2012-05-431718. [DOI] [PubMed] [Google Scholar]
- 197.Sockolosky JT, et al. Selective targeting of engineered T cells using orthogonal IL-2 cytokine-receptor complexes. Science. 2018;359:1037–1042. doi: 10.1126/science.aar3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mardiana S, et al. A multifunctional role for adjuvant anti-4-1BB therapy in augmenting antitumor response by chimeric antigen receptor T cells. Cancer Res. 2017;77:1296–1309. doi: 10.1158/0008-5472.CAN-16-1831. [DOI] [PubMed] [Google Scholar]
- 199.Grosser R, Cherkassky L, Chintala N, Adusumilli PS. Combination immunotherapy with CAR T cells and checkpoint blockade for the treatment of solid tumors. Cancer Cell. 2019;36:471–482. doi: 10.1016/j.ccell.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Marshall N, et al. Antitumor T-cell homeostatic activation is uncoupled from homeostatic inhibition by checkpoint blockade. Cancer Discov. 2019;9:1520–1537. doi: 10.1158/2159-8290.CD-19-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kondo T, et al. The NOTCH-FOXM1 axis plays a key role in mitochondrial biogenesis in the induction of human stem cell memory-like CAR-T cells. Cancer Res. 2020;80:471–483. doi: 10.1158/0008-5472.CAN-19-1196. [DOI] [PubMed] [Google Scholar]
- 202.Akahori Y, et al. Antitumor activity of CAR-T cells targeting the intracellular oncoprotein WT1 can be enhanced by vaccination. Blood. 2018;132:1134–1145. doi: 10.1182/blood-2017-08-802926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Slaney CY, et al. Dual-specific chimeric antigen receptor T cells and an indirect vaccine eradicate a variety of large solid tumors in an immunocompetent, self-antigen setting. Clin. Cancer Res. 2017;23:2478–2490. doi: 10.1158/1078-0432.CCR-16-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Feucht J, et al. Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency. Nat. Med. 2019;25:82–88. doi: 10.1038/s41591-018-0290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Collinson-Pautz MR, et al. Constitutively active MyD88/CD40 costimulation enhances expansion and efficacy of chimeric antigen receptor T cells targeting hematological malignancies. Leukemia. 2019;33:2195–2207. doi: 10.1038/s41375-019-0417-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Shum T, et al. Constitutive signaling from an engineered IL7 receptor promotes durable tumor elimination by tumor-redirected T cells. Cancer Discov. 2017;7:1238–1247. doi: 10.1158/2159-8290.CD-17-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yeku OO, Purdon TJ, Koneru M, Spriggs D, Brentjens RJ. Armored CAR T cells enhance antitumor efficacy and overcome the tumor microenvironment. Sci. Rep. 2017;7:10541. doi: 10.1038/s41598-017-10940-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hurton LV, et al. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc. Natl. Acad. Sci. USA. 2016;113:E7788–E7797. doi: 10.1073/pnas.1610544113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hu B, et al. Augmentation of antitumor immunity by human and mouse CAR T cells secreting IL-18. Cell Rep. 2017;20:3025–3033. doi: 10.1016/j.celrep.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Vinanica N, et al. Specific stimulation of T lymphocytes with erythropoietin for adoptive immunotherapy. Blood. 2019;135:668–679. doi: 10.1182/blood.2019001645. [DOI] [PubMed] [Google Scholar]
- 211.Mocellin S, Wang E, Marincola FM. Cytokines and immune response in the tumor microenvironment. J. Immunother. 2001;24:392–407. [PubMed] [Google Scholar]
- 212.Nakajima M, Sakoda Y, Adachi K, Nagano H, Tamada K. Improved survival of chimeric antigen receptor-engineered T (CAR-T) and tumor-specific T cells caused by anti-programmed cell death protein 1 single-chain variable fragment-producing CAR-T cells. Cancer Sci. 2019;110:3079–3088. doi: 10.1111/cas.14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sukumaran S, et al. Enhancing the potency and specificity of engineered T cells for cancer treatment. Cancer Discov. 2018;8:972–987. doi: 10.1158/2159-8290.CD-17-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bajgain P, et al. CAR T cell therapy for breast cancer: harnessing the tumor milieu to drive T cell activation. J. Immunother. Cancer. 2018;6:34. doi: 10.1186/s40425-018-0347-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lynn RC, et al. c-Jun overexpression in CAR T cells induces exhaustion resistance. Nature. 2019;576:293–300. doi: 10.1038/s41586-019-1805-z. [DOI] [PMC free article] [PubMed] [Google Scholar]