Abstract
In contrast to the previous belief that autoreactive B cells are eliminated from the normal repertoire of B cells, many autoreactive B cells actually escape clonal deletion and develop into mature B cells. These autoreactive B cells in healthy individuals perform some beneficial functions in the host and are homeostatically regulated by regulatory T and B cells or other mechanisms to prevent autoimmune diseases. Autoreactive B-1 cells constitutively produce polyreactive natural antibodies for tissue homeostasis. Recently, autoreactive follicular B cells were reported to participate actively in the germinal center reaction. Furthermore, the selection and usefulness of autoreactive marginal zone (MZ) B cells found in autoimmune diseases are not well understood, although the repertoire of MZ B-cell receptors (BCRs) is presumed to be biased to detect bacterial antigens. In this review, we discuss the autoreactive B-cell populations among all three major B-cell subsets and their regulation in immune responses and diseases.
Keywords: B-cell autoreactivity, polyreactivity, autoreactive antibody, B-cell receptor, B-1 lymphocyte, autoreactive germinal center, autoreactive marginal zone B cell
Subject terms: Autoimmunity, B cells
Introduction
The presence of autoreactive B-cell receptors (BCRs) is potentially dangerous to the host since the activation of B cells with autoreactive BCRs can trigger the secretion of autoreactive antibodies (Abs) that cause autoimmune diseases.1 However, paradoxically, the proportion of autoreactive B cells is considerably high in both humans and mice, and nevertheless, they do not cause diseases in the majority of individuals.2,3 Furthermore, autoreactive B cells are currently thought to provide advantages in immune responses against pathogens, as exemplified by the beneficial functions of natural autoAbs and the participation of autoreactive B cells in germinal center (GC) reactions.4,5
The positive selection of autoreactive B cells is well appreciated for B-1 cells, which are derived from fetal progenitor cells and produce natural Ab-producing B cells.6,7 Some level of autoreactivity also exists to positively select follicular (FO) B cells.8–11 The autoreactivity of some marginal zone (MZ) B cells is enigmatic since MZ B cells are thought to be responsible for a rapid response against blood pathogens and biased to react against microbial antigens, not self-antigens.12,13 However, autoreactive MZ B cells are also commonly found in patients and mouse models of autoimmune diseases.14–18 In this review, we will discuss autoreactive B-cell subpopulations among three major B-cell subsets and their functions in healthy individuals and autoimmune diseases. We will also discuss how autoreactive B cells are regulated to not induce autoimmune diseases.
Autoreactivity of BCRs in health and disease
The repertoire of BCRs, membrane-bound immunoglobulins (Igs) associated with the signaling chains Igα and Igβ, should cover a massive range of antigens, whereas the T-cell receptor (TCR) repertoire is biased toward recognizing major histocompatibility complex (MHC) classes I and II.19,20 The antigenic specificities of BCRs encompass proteins, lipids, sugars, small chemicals, and other molecules and are not restricted by antigen-presenting molecules such as MHC.21 Therefore, the BCR repertoire should be adapted to react against all these kinds of antigens. The antigenic specificities of BCRs are determined by the complementary-determining regions (CDRs) of Ig heavy and light chains. Among the six CDRs (CDR-H1, CDR-H2, CDR-H3, CDR-L1, CDR-L2, and CDR-L3), CDR-H3 (CDR3 of the Ig heavy chain) is more diverse than the other CDRs and acts as a highly antigen-specific loop.21,22 Similar to the CDRs of the BCR, CDR-β3 (CDR3 of the TCR β chain) is more structurally diverse than the other CDRs of the TCR α and β chains.23
One of the unique characteristics of adaptive immunity is a high level of specificity, but curiously, degeneracy of the immune responses is also found in both T and B cells. In other words, many BCRs and TCRs specific to given antigens can bind distinctly different antigens as well,24,25 and their binding degeneracy is also referred to as polyreactivity. Polyreactive Abs can be found in the serum of healthy humans and mice,26,27 suggesting effector cell differentiation of polyreactive B cells. The polyreactivity of a given Ab can be measured by binding assays using several different antigens, such as insulin, single-stranded DNA, thyroglobulin, and others. Interestingly, these antigens compete with each other for the antigen-binding site on polyreactive Abs but not on monoreactive Abs.26 The polyreactive BCRs show a substantial level of cross-reactivity to foreign and self-antigens, and therefore, most of the polyreactive B cells in the naive B-cell pool are described as autoreactive B cells.28
It was argued that germline BCRs, not somatically mutated BCRs, have conformational flexibility and a capability of binding to multiple kinds of antigens.29,30 These polyreactive and thus autoreactive B cells are predominantly selected in the early B-cell developmental stage,3 and then a substantial fraction of autoreactive B cells are eliminated by receptor editing, clonal deletion, or follicular exclusion.31,32 However, polyreactive/autoreactive B cells are still observed in the human and mouse mature B-cell pool, and they have various physiological roles.33,34 Although polyreactivity is a well-known and representative characteristic of B-1 cells, polyreactive B cells seem to not be confined to B-1 cells since they constitute ~20% of adult blood B cells and are widely distributed in lymph nodes.34,35 Consistently, previous studies have reported that polyreactive B cells are composed of both mouse B-1+ and B-1− phenotypes.36,37 The presence of polyreactive Abs and BCRs is beneficial to immune responses and homeostasis. Specifically, polyreactive Abs act as the first line of immune defense since they can bind various kinds of viral and bacterial antigens, which can induce effective neutralization and opsonization of pathogens, and their autoreactivity also plays important roles in the clearance of dead cells.26,27,38,39 In sum, polyreactive/autoreactive BCRs are actually selected during B-cell development, even for FO B cells, and B cells with polyreactive BCRs and Abs are advantageous for homeostasis and reactions against many different kinds of antigens.
BCR-mediated signaling is essential not only for the humoral immune response but also for B-cell development and homeostasis.40,41 When B cells suddenly meet their specific antigens on the solid phase of antigen-presenting cells, B cells spread over the antigen-containing surface, contract, and extract antigens via their BCRs, forming immune synapses.42 There is another type of BCR-mediated signaling, which is low-level constitutive signaling in the basal state and is required for B-cell survival.43–45 The ablation of this tonic BCR signaling induces apoptosis of B cells, as shown by the conditional ablation of BCRs in mature B cells.40 Autoreactive B cells induce a higher level of tonic BCR signaling, which can lead to the positive selection and some expansion of autoreactive B cells and the skewing of the BCR repertoire.46 Since the majority of autoreactive B cells observed in the bone marrow (BM) are removed by negative selection in BM or follicular exclusion in the spleen,3,47,48 tonic BCR-mediated signaling less than the threshold for negative selection is critical for B-cell survival, providing survival, and selection advantages for moderately autoreactive B cells.40 Interestingly, tonic BCR signaling is absent in highly autoreactive B cells, which downregulate cell surface IgM upon engagement with self-antigens.49 The contribution of moderately autoreactive B cells to the mature B-cell pool can be confirmed by investigating the expression of Nur77, which is rapidly upregulated in naive B and T cells by BCR and TCR signaling, respectively, upon antigenic encounter.50 Interestingly, a large proportion of mature B cells in all human and mouse B-cell subsets express Nur77,51,52 suggesting that these B cells are actually stimulated strongly enough for the induction of Nur77 via their moderately autoreactive BCRs. Collectively, tonic BCR-mediated signaling is essential for B-cell survival, and moderately autoreactive B cells may have some advantage in survival and expansion.
Although polyreactive/autoreactive B cells are beneficial for the host in many situations, these B cells can be detrimental to the host if dysregulated, leading to the secretion of autoAbs and pathogenic interactions between autoreactive B and T cells.53 Under homeostatic conditions, the strength and quality of BCR-mediated signaling are differentially regulated in B-cell subsets by B-cell costimulatory and coinhibitory receptors.54 For example, CD19, a component of the BCR complex as well as a signaling chain of CD21, contributes differently to BCR-mediated signaling in mouse B-1 cells and FO B cells.55–57 Cytokines and pathogen-associated molecular patterns and their receptors, such as B-cell activating factor receptor (BAFF-R) and toll-like receptors (TLRs), are also important in the activation and survival of human and mouse autoreactive B cells.10,31,58–65 T-cell signals derived from CD40 or SLAM family proteins also greatly influence B-cell activation and selection.66–69 On the other hand, coinhibitory receptors such as CD22, Siglecs (sialic acid binding Ig-like lectins), and FcγRIIb are important in limiting the hyperactivation of B cells.70,71 The expression of costimulatory and coinhibitor receptors is critical for the proper and regulated activation of B cells. Furthermore, their differential expression in B-cell subsets is closely linked to their different levels of autoreactivity.
The deletion of polyreactive/autoreactive B cells requires proper BCR and TLR signaling. When these signaling pathways are defective in patients with primary immunodeficiency (PID), the proportions of polyreactive/autoreactive B cells increase significantly compared with those in healthy individuals.72 This may predispose PID patients to autoimmune manifestations such as autoimmune hemolytic anemia or rheumatoid arthritis.73 Interestingly, defects in the peripheral selection of B cells lead to the expansion of autoreactive naive B cells recognizing soluble molecules and cytokines in patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy.74
Regulation of autoreactive Ab production
Since there are a substantial number of autoreactive B cells in all B-cell subsets, it is important to understand how these autoreactive B cells are appropriately activated only upon infectious or danger conditions and prevented from activation via self-antigens in homeostatic conditions. We would like to mention some known and possible mechanisms to limit autoreactive B cells in homeostatic conditions or after the control of infectious or danger events. There are also multiple levels of regulation to prevent or reduce the development of autoreactive B cells that have been reviewed elsewhere,75,76 and here, we discuss the regulation of autoreactive B cells after their generation.
First, the activation of regulatory T and B cells can inhibit the hyperactivation of autoreactive B cells through the secretion of IL-10 and TGFβ and contact-dependent mechanisms.77 It is interesting to note that IL-10 production by activated B-1 cells may regulate their own proliferation.78 It is generally accepted that regulatory B cells develop from mouse CD19+CD21highCD23highCD24high precursor MZ B cells,79 but other types of regulatory B cells such as CD5+CD1dhigh B cells or LAG-3+CD138high plasma cells are also reported.77,80 The regulatory B cells themselves are suggested to be autoreactive/polyreactive B cells that produce IL-10 quickly and facilitate the clearance of eliciting antigens by polyreactive Abs.81 Mouse B-1 cell-derived regulatory B cells that produce natural autoAbs are shown to be dependent on the expression of SHIP-1.82
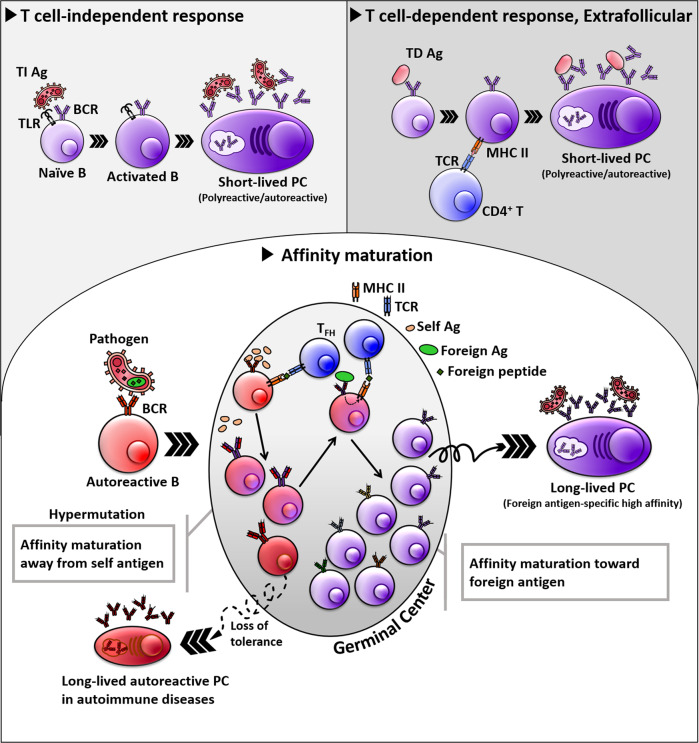
Second, the longevity of plasma cells, effector B cells, is tightly regulated so that polyreactive/autoreactive Abs are secreted during inflammatory processes following infection or other events. The polyreactive/autoreactive Ab-secreting plasma cells generated upon infection are short-lived in most cases, whereas foreign antigen-specific high-affinity plasma cells generated by the GC reaction are long-lived (Fig. 1).83 When polyreactive B cells are costimulated by molecular patterns from microbes, such as TLR4 ligand, and pathogen-derived antigens that can bind to polyreactive BCRs, the stimulated polyreactive B cells can rapidly differentiate into plasma cells, but as the plasma cells are short-lived, the levels of autoreactive/polyreactive Abs will decrease over time after the peak, and the autoimmune Ab responses are eventually terminated. This regulation of the survival of plasma cells ensures that inflammatory reactions by inflammatory mediators and polyreactive/autoreactive Abs are terminated after the elimination of pathogenic agents.84 Therefore, the dysregulation of plasma cell survival can be one of the causes of autoimmune diseases. In this regard, the continuous production of inflammatory cytokines such as type I interferon (IFN) is an important mechanism to generate and maintain autoAb-producing plasma cells in autoimmune diseases such as systemic lupus erythematosus (SLE).85
Fig. 1.
Regulation of the longevity of plasma cells. Polyreactive/autoreactive B cells rapidly react against pathogens in either T cell-independent or T cell-dependent fashions. Without the GC reaction, most of the resulting Ab-secreting cells are short-lived, and therefore, the polyreactive Ab response is self-limiting. Long-lived plasma cells are primarily generated through GC reactions. Polyreactive/autoreactive B cells may be selected to enter into or form GCs that contain foreign antigens. GC B cells with the best-fit Abs that efficiently discriminate self and foreign antigens and have the highest affinity to foreign antigens are selected to become long-lived plasma cells. The negative selection process of autoreactive GC B cells is essential to avoid autoimmune diseases. However, the defective tolerance in GCs caused by several factors, including excessive type I IFN and TLR7 signaling, may give rise to long-lived autoreactive plasma cells and cause certain autoimmune diseases, such as SLE
Third, it is worthwhile to note that the affinity of germline polyreactive/autoreactive Abs to self-antigens is not high since, in most cases, they do not undergo affinity maturation to generate high-affinity autoAbs due to negative selection processes deleting highly autoreactive B cells in the GC.5 It is necessary to address whether persistently elevated levels of low-affinity autoreactive Abs themselves can cause autoimmune diseases without affinity maturation.33
Natural autoantibody-producing B-1a cells and their regulation
Among mouse serosal cavity B cells, including CD5+ B-1a, CD5− B-1b, and B-2 cells, B-1a cells are the most autoreactive B cells producing natural autoAbs.86–88 B-1a cells play a crucial role in maintaining a constant steady-state level of IgM and responding quickly to infection by secreting natural IgM and cytokines (Fig. 2).7 The autoreactivity of B-1a BCRs is established by a germline-encoded canonical repertoire without N-nucleotide addition and the positive selection of autoreactive B cells.6,89 Natural autoAbs secreted by B-1a cells bind various self-antigens, such as dsDNA, phosphatidylcholine (PtC), and oxidized LDL.90–92 Natural autoAbs contribute to homeostatic maintenance by removing dead cells or debris from the body. Human B-1 cells are characterized as CD20+CD27+CD43+CD70− B cells in the umbilical cord or adult blood, not in the serosal cavities, but their autoreactivity and polyspecificity need to be confirmed.93,94 Although the affinities of natural autoAbs to antigens are low and macrophages do not have an Fc receptor for IgM,95,96 pentameric IgM with a high avidity binds well to small particles from apoptotic cells or bacteria and efficiently fixes complement for phagocytic clearance.97,98
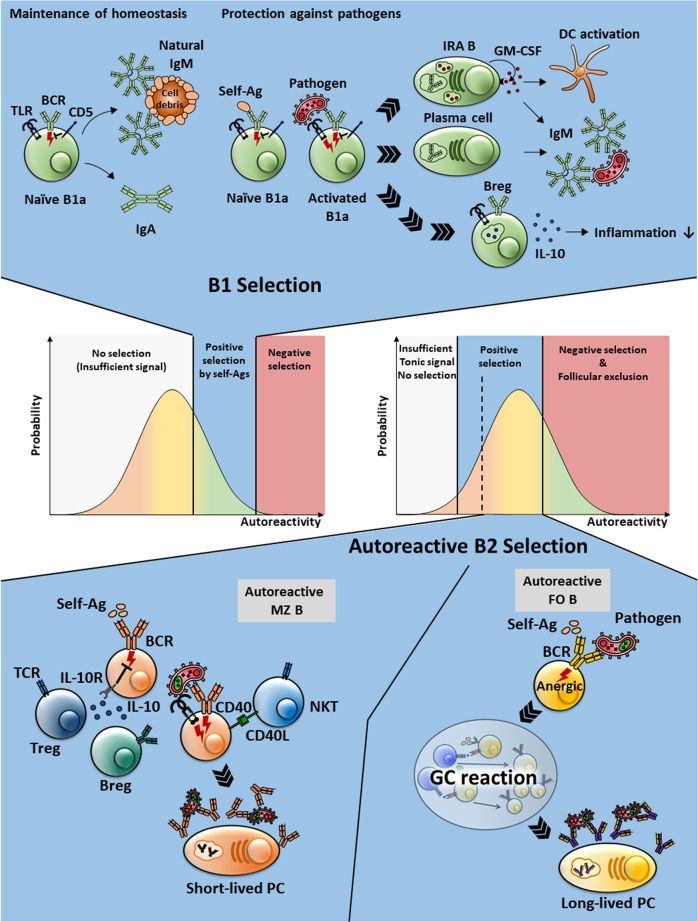
Fig. 2.
The homeostatic and induced functions of polyreactive/autoreactive B cells. In the middle, the self-affinity windows for B-cell subsets are cartooned. Highly autoreactive B cells are selected to develop into B-1a cells, whereas moderately autoreactive B cells become FO B cells. Whereas B-1a cells perform homeostatic and induced functions without somatic hypermutation, FO B cells undergo the GC reaction to change their autoreactive BCRs. The autoreactive MZ B cells are depicted in the bottom left. Although they are presumed to have a regulatory function in homeostatic conditions, they become activated in a T cell-dependent or T cell-independent manner to become short-lived plasma cells
Since B-1a cells are basically autoreactive, their inappropriate activation may trigger the elevation of serum autoAbs and their antigen presentation, leading to CD4+ T-cell activation.99 Therefore, their activity needs to be delicately regulated to prevent the hyperactivation of the B-cell and T-cell compartments. At the same time, B-1a cells act as first-line defenders so that they can be rapidly activated and promote Ab secretion early in infection. Since B-1a cells cannot discriminate between BCR recognition of self-antigens or foreign antigens due to their polyreactivity/autoreactivity,100 activation via pattern recognition receptors, including TLRs, is critical for their rapid functional response.7 Innate-like CD49dhighCD4+ T cells in the serosal cavity were also shown to provide help for B-1a cells, suggesting that B-1a cells can function in collaboration with CD4+ T cells.101,102 Upon infection, B-1a cells residing in serosal cavities instantly migrate into the spleen or draining lymph nodes of infection sites, differentiate into plasma cells, and become the main source of increased serum and local IgM.103–105 B-1a cells also play a pivotal role in protection against sepsis, as they amplify host responses by secreting granulocyte macrophage colony-stimulating factor and IL-3.106 Collectively, B-1a cells have beneficial functions for the host, which are the homeostatic maintenance of the body and the rapid response to life-threatening infection or tissue damage.
Direct pathogenic evidence for B-1a cells in autoimmune diseases has not been definitively provided, although an increased number of B-1a cells was observed in (NZB × NZW) F1 murine lupus mice, one of the autoimmune disease mouse models.107 B-1a cells are currently thought to be heterogeneous and can be further categorized by their degree of autoreactivity.91 The expression of programmed death-ligand 2 (PD-L2) is utilized as the marker of such subdivisions, and PD-L2+ B-1a cells are enriched for more autoreactive specificities, as their BCRs are skewed toward VH11 and VH12 chains specific for PtC. PD-L2+ B-1a cells were more related to the development of lupus in a mouse model than PD-L2− B-1a cells.108 Therefore, it is presumed that hyperactivation of B-1a cells or a subpopulation of B-1a cells can be a pathogenic mechanism for certain autoimmune diseases.
To inhibit the hyperactivation of B-1a cells, B-1a cells possess several inhibitory mechanisms, which put them into a kind of anergic state and limit their expansion in the absence of infection.100,109 CD5 is one of the most representative inhibitory molecules in B-1a cells.110 Whereas wild-type B-1a cells undergo apoptosis upon anti-IgM engagement, CD5-deficient B-1a cells proliferate upon anti-IgM stimulation. Tonic BCR-mediated signaling in B-1a cells leads to the continuous activation of Lyn kinase,111 which in turn phosphorylates the tyrosine residues in the immune receptor tyrosine-based inhibitory motif of CD5. Phosphorylated CD5 constantly recruits SHP-1 (Src homology region 2 domain-containing phosphatase) to the BCR complex that is associated with CD5.112 Another well-known inhibitory molecule of B-1a cells is Siglec G, a member of the family of sialic acid binding proteins.113 Siglec G has been reported to specifically inhibit Ca2+ signaling in B-1a cells and seems to act differently from CD22 in FO B cells.70 In addition, Siglec G is involved in the suppression of survival of B-1a cells since Siglec G-deficient mice have a higher proportion of B-1a cells, and B-1a cells from these mice have a longer lifespan in in vitro culture than those from WT mice.113 The expression of the transcription factor NFATc1, which is essential for the development of B-1a cells,114 is increased in Siglec G-deficient B-1a cells, indicating that Siglec G also regulates the development of B-1a cells. Therefore, B-1a cells are constantly restrained from overactivation via inhibitory molecules such as CD5 and Siglec G.
Moderately autoreactive follicular B cells in the T cell-dependent Ab response
FO B cells are the major population of mature B cells, recirculating through blood and secondary lymphoid tissues. They undergo clonal expansion, isotype switching, and differentiation into Ab-secreting cells when they are exposed to specific antigens and obtain T cell help. In the traditional view, nonautoreactive FO B cells are predominant, and rare antigen-specific FO B cells are selected according to the clonal selection theory, become activated upon antigenic challenge, and undergo the GC reaction to generate high-affinity Ab-producing plasma cells or memory B cells. However, it has now been shown that moderately autoreactive FO B cells are predominant in secondary lymphoid tissues and are ready to respond to foreign antigens. The combination of BCR- and BAFF-mediated signaling is crucial for the selection of moderately autoreactive FO B cells.10 For example, human B cells with IGHV4–34 heavy chains that bind an autoantigen, carbohydrate I/i antigens, constitute 7% of naive B cells.115 The development of autoreactive FO B cells can be confirmed in mouse hen-egg lysozyme (HEL) transgenic (Tg) and anti-HEL Tg mouse models. In these models, the large population of anti-HEL B cells was not deleted in the presence of HEL in the body but was maintained in the secondary lymphoid tissues.116,117 We also previously reported a subpopulation of mouse splenic FO B cells, CD138intIgMlowIgDhigh FO B cells, which are presumed to be a polyclonal population of anergic B cells.118 They have features of anergic B cells such as a high expression of IgD and blunted Ca2+ response upon BCR engagement.117,119 Previously, these autoreactive FO B cells were expected to be eliminated eventually and not to participate in the immune responses. It is interesting to address how these autoreactive B cells survive in the secondary lymphoid tissues, and Bruton’s tyrosine kinase (BTK) may be one mechanism promoting their survival, as the expression of BTK increased with the maturation of FO B cells.120,121 Unexpectedly, these autoreactive/anergic B cells were not silent but participated in the immune reaction to foreign antigens, undergoing the GC reaction upon stimulation with multivalent foreign Ags, and modified their BCRs to have a higher affinity for foreign Ags and a lower affinity for self-Ags through somatic hypermutation and selection in the GC.122 These findings suggest that anergic B cells are apparently not truly anergic and have some capability for generating active immune responses.123
The fact that the majority of FO B cells are moderately autoreactive raises the question of how FO B cells can selectively respond to foreign antigens while ignoring self-antigens under homeostatic conditions. The high expression of IgD and the low expression of IgM are suggested to account for the tolerance against common self-antigens since IgD is less sensitive than IgM to endogenous antigen in mice.124 Another BCR signaling feature of FO B cells is their capability for antigen extraction from particulate pathogens when they meet high-affinity antigens on the solid phase.125 This priming signaling of FO B cells also involves coordination between BCR signaling and actin cytoskeletal rearrangement.126 Mouse Fc receptor-like 1 (FcRL1) is reported to be one of the BCR signaling molecules responsible for this type of priming signaling but is not responsible for tonic BCR signaling.127
Taken together, these findings show that autoreactive B cells are favored to develop into FO B cells through relatively strong tonic BCR signaling during development and are maintained through the weakening of tonic BCR signaling by the high expression of IgD. Therefore, despite strong tonic BCR signaling, autoreactive FO B cells appear not to fall into the anergic state, maintaining their immune response capability. The redemption hypothesis argues that these autoreactive FO B cells modify their BCRs to lose self-reactivity and enhance their affinity to foreign antigens.123 To prevent the selection of high-affinity autoreactive B cells, some delicate selection mechanisms are required to discriminate the signaling induced by infrequent foreign antigens and commonly encountered self-antigens.128
Autoreactive MZ B cells in immunity and autoimmune diseases
MZ B cells are innate-like B cells that rapidly differentiate into Ab-secreting cells upon stimulation with blood–borne pathogens.129 Human MZ B cells are different from mouse MZ B cells in that human MZ B cells have the capability of recirculating in the blood and some somatic mutations in their BCRs.130 MZ B cells are identified as CD23lowIgDlowCD21highIgMhighCD1dhigh B cells in mice and IgD+IgM+CD27+CD1c+ B cells in humans. MZ B cells are also excellent antigen-presenting cells for CD4+ T cells.131 Since MZ B cells have a low threshold for activation and hyperactivation of MZ B cells is implicated in the pathogenesis of certain autoimmune diseases,132 a strict repertoire selection of MZ B cells may be required for rapid immune response as well as the prevention of dysregulated Ab responses. BCR repertoire analysis of FO B and MZ B cells shows distinctive repertoires in the two B-cell subsets.133
BCR-mediated signaling is also critical for the development of MZ B cells, as it is for other B-cell subsets, but unlike in FO B and B-1 cells, weak BCR signaling is thought to be sufficient for intracytoplasmic signaling of Notch2, as the developmental choice of transitional B cells between FO B and MZ B cells is determined by Notch2 signaling.12,134,135 Whereas mutations that attenuate BCR signaling, such as mutations in Aiolos or CD22, result in a decrease in the number of MZ B cells,136,137 weakening of the BTK signaling pathway preserves MZ B-cell development despite a marked decrease in FO B cells.138 In view of the BCR signal strength model, strong BCR signaling is supposed to block Notch2 signaling and inhibit MZ B-cell development.12 However, as the BCR signaling strength is not measured by only one parameter, such as BTK activation, but can be assessed by multiple parameters, such as MAP kinase activity, actin rearrangement, or the duration of kinase activity, it is not clear which signaling is responsible for MZ B-cell commitment and maintenance. It is interesting to note that the generation of MZ B cells is dependent on CD19 but not on FcRL1,127,139 which may give us a hint of the BCR signaling characteristics for MZ B-cell development.
On the other hand, it is difficult to find common ground between the BCR signaling hypothesis of MZ B-cell development and previous observations of the presence of low-affinity autoreactive MZ B cells in experimental autoimmune disease models.14 As MZ B cells were previously argued to be composed of heterogeneous populations,129 alternative pathways leading to the MZ B-cell phenotype may be possible. There is a previous report showing a Notch-independent pathway of MZ B-cell development.140 More importantly, MZ B cells have self-renewal capacity, as the MZ B-cell population could be sustained in the absence of new B-cell influx.141 When RAG-2 was inducibly deleted at the age of 8–10 weeks, splenic FO B cells were gradually lost over a year, but the pools of MZ B cells in the spleen and of B-1 cells in the peritoneal cavity were kept at normal levels.129 It is not known whether the sustained MZ B-cell population in the absence of new B-cell entry contains all heterogeneous populations of MZ B cells. Since ~2 years or a few weeks are needed to establish the MZ in the spleen and generate MZ B cells, MZ B cells are thought to be derived from adult BM hematopoietic stem cells. However, fetal-type MZ B cells were also found in terminal deoxynucleotidyl transferase-deficient mice.142 Other studies showed the development of polyreactive anti-DNA MZ B cells in quasimonoclonal mice or the anti-DNA B6.56R mouse model.143,144 In K/BxN mice, which are rheumatoid arthritis (RA) models that develop spontaneous inflammatory joint disease, glucose-6-phosphate-isomerase-reactive B cells display a splenic MZ phenotype.14 We also identified a polyclonal autoreactive MZ B-cell population that expresses a high level of CD80 and includes type II collagen-reactive B cells.15
Collectively, the MZ B-cell population also harbors autoreactive B cells as well as microbe-biased B cells. Therefore, the regulation of autoreactive MZ B cells is important to prevent the harmful effects of their rapid Ab responses. It will be interesting to address whether MZ precursor B cells are linked to the counteraction of autoreactive MZ B cells and whether T cells are responsible for the regulation of MZ B cells.
Clinical implications of B-cell autoreactivity
The presence of autoreactive B cells in healthy individuals suggests that selective ablation of the autoreactive B cells for the treatment of autoimmune diseases may be very difficult to achieve.145 Although strong BCR signaling leads to negative selection or follicular exclusion of B cells in most cases, the moderately autoreactive B cells present in normal individuals are thought to be adapted to signaling through the BCR and other receptors for their survival. Strong BCR-mediated signaling sometimes contributes to the survival of autoreactive B cells, as exemplified in B-cell lymphoma cells with oncogenic mutations.146,147 Therefore, BCR signaling inhibitors such as ibrutinib are used for the treatment of B-cell malignancies. However, it is not clear whether BCR signaling blockade can also preferentially inhibit normal autoreactive B cells. The beneficial effects of BCR signaling inhibitors on autoimmune diseases may be anticipated based on their general inhibition of Ab production and the antigen-presenting capability of B cells, and the results of the current clinal trials using BCR signaling inhibitors in autoimmune diseases are awaited.148 We previously showed that a low dose of ibrutinib, a BTK inhibitor, selectively removed the autoreactive population of MZ B cells in DBA/1 mice,15 suggesting the possibility of using BCR signaling inhibitors to kill the autoreactive B-cell population. An understanding of the differential BCR signaling in different subsets and stages of B cells is important for selecting appropriate signaling inhibitors for the treatment of autoimmune diseases.149
B-cell depletion therapy is now widely used for the treatment of autoimmune diseases, and its beneficial results have been confirmed in many autoimmune diseases, such as RA, multiple sclerosis, and type 1 diabetes.150–152 However, the effectiveness of B-cell depletion therapy varies among different autoimmune diseases and different individuals with the same disease. Among RA patients, the presence of anti-cyclic citrullinated peptide Abs or rheumatoid factor predicted a better response to B-cell depletion therapy using rituximab.153 Rituximab does not decrease the total serum IgG levels since the expression of CD20, the target of rituximab, is lost in long-lived plasma cells. Fortunately, most autoreactive plasma cells are short-lived and, therefore, susceptible to B-cell depletion therapy, whereas antimicrobial plasma cells are not depleted by the therapy.154 However, once autoreactive B cells differentiate into long-lived plasma cells, they are resistant to B-cell depletion therapy.155 Other possible mechanisms for the failure or ineffectiveness of B-cell depletion therapy include the predominant depletion of regulatory B cells by therapy or phagocytic defects of macrophages due to the excess amounts of immune complexes.156–158 Collectively, an immunotherapeutic approach for the inhibition or depletion of autoreactive B cells is partially successful and needs to be improved in the future.
Conclusion
To react against all kinds of antigens, BCRs should be adapted to fit these extremely diverse epitopes. The development of B cells with polyreactive/autoreactive BCRs is one of the solutions for this requirement. Polyreactive B cells are normally found in humans and mice and, interestingly, in all kinds of B-cell subsets that have different compartmentalized functions (Fig. 2). Although this polyreactivity is beneficial to host survival, it may lead to the development of autoimmune diseases in some individuals. To overcome B cell-mediated autoimmune diseases, it is necessary to understand how polyreactive/autoreactive B cells are regulated in different B-cell subsets.
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. NRF-2019R1A2C2006717).
Competing interests
The authors declare no competing interests.
References
- 1.Martin F, Chan AC. Pathogenic roles of B cells in human autoimmunity; insights from the clinic. Immunity. 2004;20:517–527. doi: 10.1016/s1074-7613(04)00112-8. [DOI] [PubMed] [Google Scholar]
- 2.Koelsch K, et al. Mature B cells class switched to IgD are autoreactive in healthy individuals. J. Clin. Investig. 2007;117:1558–1565. doi: 10.1172/JCI27628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wardemann H, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 4.Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nat. Rev. Immunol. 2010;10:778–786. doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- 5.Burnett DL, et al. Germinal center antibody mutation trajectories are determined by rapid self/foreign discrimination. Science. 2018;360:223–226. doi: 10.1126/science.aao3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardy RR, Hayakawa K. Perspectives on fetal derived CD5+ B1 B cells. Eur. J. Immunol. 2015;45:2978–2984. doi: 10.1002/eji.201445146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 8.Herzog S, Jumaa H. Self-recognition and clonal selection: autoreactivity drives the generation of B cells. Curr. Opin. Immunol. 2012;24:166–172. doi: 10.1016/j.coi.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Cancro MP. Signalling crosstalk in B cells: managing worth and need. Nat. Rev. Immunol. 2009;9:657–661. doi: 10.1038/nri2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Z, Davidson A. BAFF and selection of autoreactive B cells. Trends Immunol. 2011;32:388–394. doi: 10.1016/j.it.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cancro MP, Kearney JF. B cell positive selection: road map to the primary repertoire. J. Immunol. 2004;173:15–19. doi: 10.4049/jimmunol.173.1.15. [DOI] [PubMed] [Google Scholar]
- 12.Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat. Rev. Immunol. 2009;9:767–777. doi: 10.1038/nri2656. [DOI] [PubMed] [Google Scholar]
- 13.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 14.Mandik-Nayak L, Racz J, Sleckman BP, Allen PM. Autoreactive marginal zone B cells are spontaneously activated but lymph node B cells require T cell help. J. Exp. Med. 2006;203:1985–1998. doi: 10.1084/jem.20060701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park C, et al. Positive selection of type II collagen-reactive CD80(high) marginal zone B cells in DBA/1 mice. Clin. Immunol. 2017;178:64–73. doi: 10.1016/j.clim.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Kishi Y, et al. Apoptotic marginal zone deletion of anti-Sm/ribonucleoprotein B cells. Proc. Natl Acad. Sci. USA. 2012;109:7811–7816. doi: 10.1073/pnas.1204509109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palm AK, et al. Function and regulation of self-reactive marginal zone B cells in autoimmune arthritis. Cell Mol. Immunol. 2015;12:493–504. doi: 10.1038/cmi.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyagawa-Hayashino A, et al. Increase of MZB1 in B cells in systemic lupus erythematosus: proteomic analysis of biopsied lymph nodes. Arthritis Res Ther. 2018;20:13. doi: 10.1186/s13075-018-1511-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong, W. K., Leem J., Deane C. M. Comparative analysis of the CDR loops of antigen receptors. BioRxiv.0, 2019. 10.1101/709840. [DOI] [PMC free article] [PubMed]
- 20.Van Laethem F, Tikhonova AN, Singer A. MHC restriction is imposed on a diverse T cell receptor repertoire by CD4 and CD8 co-receptors during thymic selection. Trends Immunol. 2012;33:437–441. doi: 10.1016/j.it.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis MM. The evolutionary and structural ‘logic’ of antigen receptor diversity. Semin Immunol. 2004;16:239–243. doi: 10.1016/j.smim.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Weitzner BD, Dunbrack RL, Gray JJ. The origin of CDR H3 structural diversity. Structure. 2015;23:302–311. doi: 10.1016/j.str.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong WK, Leem J, Deane CM. Comparative analysis of the CDR loops of antigen receptors. Front Immunol. 2019;10:2454. doi: 10.3389/fimmu.2019.02454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisen HN, Chakraborty AK. Evolving concepts of specificity in immune reactions. Proc. Natl Acad. Sci. USA. 2010;107:22373–22380. doi: 10.1073/pnas.1012051108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birnbaum ME, et al. Deconstructing the peptide-MHC specificity of T cell recognition. Cell. 2014;157:1073–1087. doi: 10.1016/j.cell.2014.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Notkins AL. Polyreactivity of antibody molecules. Trends Immunol. 2004;25:174–179. doi: 10.1016/j.it.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Zhou ZH, et al. The broad antibacterial activity of the natural antibody repertoire is due to polyreactive antibodies. Cell Host Microbe. 2007;1:51–61. doi: 10.1016/j.chom.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Köhler F, et al. Autoreactive B cell receptors mimic autonomous pre-B cell receptor signaling and induce proliferation of early B cells. Immunity. 2008;29:912–921. doi: 10.1016/j.immuni.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Sethi DK, Agarwal A, Manivel V, Rao KV, Salunke DM. Differential epitope positioning within the germline antibody paratope enhances promiscuity in the primary immune response. Immunity. 2006;24:429–438. doi: 10.1016/j.immuni.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Manivel V, Sahoo NC, Salunke DM, Rao KV. Maturation of an antibody response is governed by modulations in flexibility of the antigen-combining site. Immunity. 2000;13:611–620. doi: 10.1016/s1074-7613(00)00061-3. [DOI] [PubMed] [Google Scholar]
- 31.Lesley R, et al. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–453. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 32.Melamed D, Benschop RJ, Cambier JC, Nemazee D. Developmental regulation of B lymphocyte immune tolerance compartmentalizes clonal selection from receptor selection. Cell. 1998;92:173–182. doi: 10.1016/s0092-8674(00)80912-5. [DOI] [PubMed] [Google Scholar]
- 33.Dimitrov JD, et al. Antibody polyreactivity in health and disease: statu variabilis. J. Immunol. 2013;191:993–999. doi: 10.4049/jimmunol.1300880. [DOI] [PubMed] [Google Scholar]
- 34.Chen ZJ, et al. Polyreactive antigen-binding B cells are the predominant cell type in the newborn B cell repertoire. Eur. J. Immunol. 1998;28:989–994. doi: 10.1002/(SICI)1521-4141(199803)28:03<989::AID-IMMU989>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 35.Gunti S, et al. Stimulation of toll-like receptors profoundly influences the titer of polyreactive antibodies in the circulation. Sci. Rep. 2015;5:15066. doi: 10.1038/srep15066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ZHOU ZHAO-HUA, NOTKINS AL. Polyreactive antigen-binding B (PAB+) cells are widely distributed and the PAB+ population consists of both B-1+ and B-1- phenotypes. Clin. Exp. Immunol. 2004;137:88–100. doi: 10.1111/j.1365-2249.2004.02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian Q, et al. B cells expressing a natural polyreactive autoantibody have a distinct phenotype and are overrepresented in immunoglobulin heavy chain transgenic mice. J. Immunol. 2006;177:2412–2422. doi: 10.4049/jimmunol.177.4.2412. [DOI] [PubMed] [Google Scholar]
- 38.Jones DD, DeIulio GA, Winslow GM. Antigen-driven induction of polyreactive IgM during intracellular bacterial infection. J. Immunol. 2012;189:1440–1447. doi: 10.4049/jimmunol.1200878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casali P, Schettino EW. Structure and function of natural antibodies. Curr. Top. Microbiol Immunol. 1996;210:167–179. doi: 10.1007/978-3-642-85226-8_17. [DOI] [PubMed] [Google Scholar]
- 40.Lam KP, Kühn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 41.Cyster JG, et al. Regulation of B-lymphocyte negative and positive selection by tyrosine phosphatase CD45. Nature. 1996;381:325–328. doi: 10.1038/381325a0. [DOI] [PubMed] [Google Scholar]
- 42.Kwak K, Akkaya M, Pierce SK. B cell signaling in context. Nat. Immunol. 2019;20:963–969. doi: 10.1038/s41590-019-0427-9. [DOI] [PubMed] [Google Scholar]
- 43.Myers DR, Zikherman J, Roose JP. Tonic signals: why do lymphocytes bother. Trends Immunol. 2017;38:844–857. doi: 10.1016/j.it.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srinivasan L, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139:573–586. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaudin E, et al. Positive selection of B cells expressing low densities of self-reactive BCRs. J. Exp. Med. 2004;199:843–853. doi: 10.1084/jem.20030955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Übelhart R, Jumaa H. Autoreactivity and the positive selection of B cells. Eur. J. Immunol. 2015;45:2971–2977. doi: 10.1002/eji.201444622. [DOI] [PubMed] [Google Scholar]
- 47.Rawlings DJ, Metzler G, Wray-Dutra M, Jackson SW. Altered B cell signalling in autoimmunity. Nat. Rev. Immunol. 2017;17:421–436. doi: 10.1038/nri.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mandik-Nayak L, Bui A, Noorchashm H, Eaton A, Erikson J. Regulation of anti-double-stranded DNA B cells in nonautoimmune mice: localization to the T-B interface of the splenic follicle. J. Exp. Med. 1997;186:1257–1267. doi: 10.1084/jem.186.8.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schram BR, et al. B cell receptor basal signaling regulates antigen-induced Ig light chain rearrangements. J. Immunol. 2008;180:4728–4741. doi: 10.4049/jimmunol.180.7.4728. [DOI] [PubMed] [Google Scholar]
- 50.Zikherman J, Parameswaran R, Weiss A. Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature. 2012;489:160–164. doi: 10.1038/nature11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ashouri JF, Weiss A. Endogenous Nur77 is a specific indicator of antigen receptor signaling in human T and B cells. J. Immunol. 2017;198:657–668. doi: 10.4049/jimmunol.1601301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steach HR, et al. Cross-reactivity with self-antigen tunes the functional potential of naive B cells specific for foreign antigens. J. Immunol. 2020;204:498–509. doi: 10.4049/jimmunol.1900799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4(+) T cell immunity. Nat. Rev. Immunol. 2010;10:236–247. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Irish JM, Czerwinski DK, Nolan GP, Levy R. Kinetics of B cell receptor signaling in human B cell subsets mapped by phosphospecific flow cytometry. J. Immunol. 2006;177:1581–1589. doi: 10.4049/jimmunol.177.3.1581. [DOI] [PubMed] [Google Scholar]
- 55.Depoil D, et al. CD19 is essential for B cell activation by promoting B cell receptor-antigen microcluster formation in response to membrane-bound ligand. Nat. Immunol. 2008;9:63–72. doi: 10.1038/ni1547. [DOI] [PubMed] [Google Scholar]
- 56.Dasu T, Sindhava V, Clarke SH, Bondada S. CD19 signaling is impaired in murine peritoneal and splenic B-1 B lymphocytes. Mol. Immunol. 2009;46:2655–2665. doi: 10.1016/j.molimm.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 58.Mackay F, Schneider P. Cracking the BAFF code. Nat. Rev. Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 59.Fairfax KA, et al. BAFF-driven autoimmunity requires CD19 expression. J. Autoimmun. 2015;62:1–10. doi: 10.1016/j.jaut.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 60.Schiemann B, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 61.Thien M, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 62.Rawlings DJ, Schwartz MA, Jackson SW, Meyer-Bahlburg A. Integration of B cell responses through Toll-like receptors and antigen receptors. Nat. Rev. Immunol. 2012;12:282–294. doi: 10.1038/nri3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hua Z, Hou B. TLR signaling in B-cell development and activation. Cell Mol. Immunol. 2013;10:103–106. doi: 10.1038/cmi.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lobo PI, Schlegel KH, Bajwa A, Huang L, Okusa MD. Natural IgM and TLR agonists switch murine splenic Pan-B to “regulatory” cells that suppress ischemia-induced innate inflammation via regulating NKT-1 cells. Front Immunol. 2017;8:974. doi: 10.3389/fimmu.2017.00974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kolhatkar NS, et al. Altered BCR and TLR signals promote enhanced positive selection of autoreactive transitional B cells in Wiskott-Aldrich syndrome. J. Exp. Med. 2015;212:1663–1677. doi: 10.1084/jem.20150585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sater RA, Sandel PC, Monroe JG. B cell receptor-induced apoptosis in primary transitional murine B cells: signaling requirements and modulation by T cell help. Int Immunol. 1998;10:1673–1682. doi: 10.1093/intimm/10.11.1673. [DOI] [PubMed] [Google Scholar]
- 67.Lesley R, Kelly LM, Xu Y, Cyster JG. Naive CD4 T cells constitutively express CD40L and augment autoreactive B cell survival. Proc. Natl Acad. Sci. USA. 2006;103:10717–10722. doi: 10.1073/pnas.0601539103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schwartz MA, Kolhatkar NS, Thouvenel C, Khim S, Rawlings DJ. CD4+ T cells and CD40 participate in selection and homeostasis of peripheral B cells. J. Immunol. 2014;193:3492–3502. doi: 10.4049/jimmunol.1400798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Menard L, et al. Signaling lymphocytic activation molecule (SLAM)/SLAM-associated protein pathway regulates human B-cell tolerance. J. Allergy Clin. Immunol. 2014;133:1149–1161. doi: 10.1016/j.jaci.2013.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nitschke L. CD22 and Siglec-G: B-cell inhibitory receptors with distinct functions. Immunol. Rev. 2009;230:128–143. doi: 10.1111/j.1600-065X.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- 71.Avalos AM, Uccellini MB, Lenert P, Viglianti GA, Marshak-Rothstein A. FcγRIIB regulation of BCR/TLR-dependent autoreactive B-cell responses. Eur. J. Immunol. 2010;40:2692–2698. doi: 10.1002/eji.200940184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meffre E. The establishment of early B cell tolerance in humans: lessons from primary immunodeficiency diseases. Ann. N. Y. Acad. Sci. 2011;1246:1–10. doi: 10.1111/j.1749-6632.2011.06347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Todoric K, Koontz JB, Mattox D, Tarrant TK. Autoimmunity in Immunodeficiency. Curr. Allergy Asthma Rep. 2013;13:361–370. doi: 10.1007/s11882-013-0350-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sng J, et al. AIRE expression controls the peripheral selection of autoreactive B cells. Sci. Immunol. 2019;4:eaav6778. doi: 10.1126/sciimmunol.aav6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nemazee D. Receptor editing in lymphocyte development and central tolerance. Nat. Rev. Immunol. 2006;6:728–740. doi: 10.1038/nri1939. [DOI] [PubMed] [Google Scholar]
- 76.Goodnow CC, Sprent J, Fazekas de St Groth B, Vinuesa CG. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature. 2005;435:590–597. doi: 10.1038/nature03724. [DOI] [PubMed] [Google Scholar]
- 77.Horikawa M, et al. Regulatory B cell (B10 Cell) expansion during Listeria infection governs innate and cellular immune responses in mice. J. Immunol. 2013;190:1158–1168. doi: 10.4049/jimmunol.1201427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sindhava V, Woodman ME, Stevenson B, Bondada S. Interleukin-10 mediated autoregulation of murine B-1 B-cells and its role in Borrelia hermsii infection. PLoS ONE. 2010;5:e11445. doi: 10.1371/journal.pone.0011445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42:607–612. doi: 10.1016/j.immuni.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 80.Lino AC, et al. LAG-3 inhibitory receptor expression identifies immunosuppressive natural regulatory plasma cells. Immunity. 2018;49:120–133.e9. doi: 10.1016/j.immuni.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maseda D, et al. Regulatory B10 cells differentiate into antibody-secreting cells after transient IL-10 production in vivo. J. Immunol. 2012;188:1036–1048. doi: 10.4049/jimmunol.1102500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen Y, et al. SHIP-1 deficiency in AID(+) B cells leads to the impaired function of B10 cells with spontaneous autoimmunity. J. Immunol. 2017;199:3063–3073. doi: 10.4049/jimmunol.1700138. [DOI] [PubMed] [Google Scholar]
- 83.Lam WY, et al. Mitochondrial pyruvate import promotes long-term survival of antibody-secreting plasma cells. Immunity. 2016;45:60–73. doi: 10.1016/j.immuni.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin F-R, et al. ASK1 promotes apoptosis of normal and malignant plasma cells. Blood. 2012;120:1039–1047. doi: 10.1182/blood-2011-12-399808. [DOI] [PubMed] [Google Scholar]
- 85.Crow MK. Type I interferon in the pathogenesis of lupus. J. Immunol. 2014;192:5459–5468. doi: 10.4049/jimmunol.1002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berberich S, Forster R, Pabst O. The peritoneal micromilieu commits B cells to home to body cavities and the small intestine. Blood. 2007;109:4627–4634. doi: 10.1182/blood-2006-12-064345. [DOI] [PubMed] [Google Scholar]
- 87.Berberich S, et al. Differential molecular and anatomical basis for B cell migration into the peritoneal cavity and omental milky spots. J. Immunol. 2008;180:2196–2203. doi: 10.4049/jimmunol.180.4.2196. [DOI] [PubMed] [Google Scholar]
- 88.Baumgarth N. B-1 cell heterogeneity and the regulation of natural and antigen-induced IgM production. Front Immunol. 2016;7:324. doi: 10.3389/fimmu.2016.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hayakawa K, et al. Positive selection of natural autoreactive B cells. Science. 1999;285:113–116. doi: 10.1126/science.285.5424.113. [DOI] [PubMed] [Google Scholar]
- 90.Cruz-Leal Y, et al. Role of B-1 cells in the immune response against an antigen encapsulated into phosphatidylcholine-containing liposomes. Int Immunol. 2014;26:427–437. doi: 10.1093/intimm/dxu042. [DOI] [PubMed] [Google Scholar]
- 91.Zhong X, Tumang JR, Gao W, Bai C, Rothstein TL. PD-L2 expression extends beyond dendritic cells/macrophages to B1 cells enriched for V(H)11/V(H)12 and phosphatidylcholine binding. Eur. J. Immunol. 2007;37:2405–2410. doi: 10.1002/eji.200737461. [DOI] [PubMed] [Google Scholar]
- 92.Karvonen J, Päivänsalo M, Kesäniemi YA, Hörkkö S. Immunoglobulin M type of autoantibodies to oxidized low-density lipoprotein has an inverse relation to carotid artery atherosclerosis. Circulation. 2003;108:2107–2112. doi: 10.1161/01.CIR.0000092891.55157.A7. [DOI] [PubMed] [Google Scholar]
- 93.Rothstein TL, Quach TD. The human counterpart of mouse B-1 cells. Ann. N. Y Acad. Sci. 2015;1362:143–152. doi: 10.1111/nyas.12790. [DOI] [PubMed] [Google Scholar]
- 94.Pashov A, et al. Diagnostic profiling of the human public IgM repertoire with scalable mimotope libraries. Front Immunol. 2019;10:2796. doi: 10.3389/fimmu.2019.02796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hodgkin PD. An antigen valence theory to explain the evolution and organization of the humoral immune response. Immunol. Cell Biol. 1997;75:604–618. doi: 10.1038/icb.1997.95. [DOI] [PubMed] [Google Scholar]
- 96.Shima H, et al. Identification of TOSO/FAIM3 as an Fc receptor for IgM. Int Immunol. 2010;22:149–156. doi: 10.1093/intimm/dxp121. [DOI] [PubMed] [Google Scholar]
- 97.Litvack ML, Post M, Palaniyar N. IgM promotes the clearance of small particles and apoptotic microparticles by macrophages. PLoS ONE. 2011;6:e17223. doi: 10.1371/journal.pone.0017223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kaveri SV, Silverman GJ, Bayry J. Natural IgM in immune equilibrium and harnessing their therapeutic potential. J. Immunol. 2012;188:939–945. doi: 10.4049/jimmunol.1102107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Margry B, Wieland WH, van Kooten PJ, van Eden W, Broere F. Peritoneal cavity B-1a cells promote peripheral CD4+ T-cell activation. Eur. J. Immunol. 2013;43:2317–2326. doi: 10.1002/eji.201343418. [DOI] [PubMed] [Google Scholar]
- 100.Sindhava VJ, Bondada S. Multiple regulatory mechanisms control B-1 B cell activation. Front Immunol. 2012;3:372. doi: 10.3389/fimmu.2012.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moon H, et al. Early development in the peritoneal cavity of CD49dhigh Th1 memory phenotype CD4+ T cells with enhanced B cell helper activity. J. Immunol. 2015;195:564–575. doi: 10.4049/jimmunol.1401661. [DOI] [PubMed] [Google Scholar]
- 102.Lee J-G, et al. Identification of human B-1 helper T cells with a Th1-like memory phenotype and high integrin CD49d expression. Front Immunol. 2018;9:1617. doi: 10.3389/fimmu.2018.01617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ha SA, et al. Regulation of B1 cell migration by signals through toll-like receptors. J. Exp. Med. 2006;203:2541–2550. doi: 10.1084/jem.20061041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Choi YS, Baumgarth N. Dual role for B-1a cells in immunity to influenza virus infection. J. Exp. Med. 2008;205:3053–3064. doi: 10.1084/jem.20080979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moon H, Lee JG, Shin SH, Kim TJ. LPS-induced migration of peritoneal B-1 cells is associated with upregulation of CXCR4 and increased migratory sensitivity to CXCL12. J. Korean Med Sci. 2012;27:27–35. doi: 10.3346/jkms.2012.27.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rauch PJ, et al. Innate response activator B cells protect against microbial sepsis. Science. 2012;335:597–601. doi: 10.1126/science.1215173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Duan B, Morel L. Role of B-1a cells in autoimmunity. Autoimmun. Rev. 2006;5:403–408. doi: 10.1016/j.autrev.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 108.Zhong X, et al. A novel subpopulation of B-1 cells is enriched with autoreactivity in normal and lupus-prone mice. Arthritis Rheum. 2009;60:3734–3743. doi: 10.1002/art.25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cambier JC, Gauld SB, Merrell KT, Vilen BJ. B-cell anergy: from transgenic models to naturally occurring anergic B cells? Nat. Rev. Immunol. 2007;7:633–643. doi: 10.1038/nri2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bikah G, Carey J, Ciallella JR, Tarakhovsky A, Bondada S. CD5-mediated negative regulation of antigen receptor-induced growth signals in B-1 B cells. Science. 1996;274:1906–1909. doi: 10.1126/science.274.5294.1906. [DOI] [PubMed] [Google Scholar]
- 111.DeFranco AL, Chan VW, Lowell CA. Positive and negative roles of the tyrosine kinase Lyn in B cell function. Semin Immunol. 1998;10:299–307. doi: 10.1006/smim.1998.0122. [DOI] [PubMed] [Google Scholar]
- 112.Sen G, Bikah G, Venkataraman C, Bondada S. Negative regulation of antigen receptor-mediated signaling by constitutive association of CD5 with the SHP-1 protein tyrosine phosphatase in B-1 B cells. Eur. J. Immunol. 1999;29:3319–3328. doi: 10.1002/(SICI)1521-4141(199910)29:10<3319::AID-IMMU3319>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 113.Jellusova J, et al. Siglec-G regulates B1 cell survival and selection. J. Immunol. 2010;185:3277–3284. doi: 10.4049/jimmunol.1001792. [DOI] [PubMed] [Google Scholar]
- 114.Berland R, Wortis HH. Normal B-1a cell development requires B cell-intrinsic NFATc1 activity. Proc. Natl Acad. Sci. USA. 2003;100:13459–13464. doi: 10.1073/pnas.2233620100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cappione A, et al. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J. Clin. Investig. 2005;115:3205–3216. doi: 10.1172/JCI24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Goodnow CC, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 117.Cyster JG, Hartley SB, Goodnow CC. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994;371:389–395. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- 118.Lee JG, et al. Reversible expression of CD138 on mature follicular B cells is downregulated by IL-4. Immunol. Lett. 2013;156:38–45. doi: 10.1016/j.imlet.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 119.Sabouri Z, et al. IgD attenuates the IgM-induced anergy response in transitional and mature B cells. Nat. Commun. 2016;7:13381. doi: 10.1038/ncomms13381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bonami RH, et al. Bruton’s tyrosine kinase promotes persistence of mature anti-insulin B cells. J. Immunol. 2014;192:1459–1470. doi: 10.4049/jimmunol.1300125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kil LP, et al. Btk levels set the threshold for B-cell activation and negative selection of autoreactive B cells in mice. Blood. 2012;119:3744–3756. doi: 10.1182/blood-2011-12-397919. [DOI] [PubMed] [Google Scholar]
- 122.Kara EE, Nussenzweig MC. Redemption for self-reactive antibodies. Science. 2018;360:152–153. doi: 10.1126/science.aat5758. [DOI] [PubMed] [Google Scholar]
- 123.Reed JH, Jackson J, Christ D, Goodnow CC. Clonal redemption of autoantibodies by somatic hypermutation away from self-reactivity during human immunization. J. Exp. Med. 2016;213:1255–1265. doi: 10.1084/jem.20151978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Noviski M, et al. IgM and IgD B cell receptors differentially respond to endogenous antigens and control B cell fate. Elife. 2018;7:e35074. doi: 10.7554/eLife.35074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yuseff MI, et al. Polarized secretion of lysosomes at the B cell synapse couples antigen extraction to processing and presentation. Immunity. 2011;35:361–374. doi: 10.1016/j.immuni.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 126.Li J, et al. The coordination between B cell receptor signaling and the actin cytoskeleton during B cell activation. Front Immunol. 2018;9:3096. doi: 10.3389/fimmu.2018.03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhao X, et al. Fc receptor–like 1 intrinsically recruits c-Abl to enhance B cell activation and function. Sci. Adv. 2019;5:eaaw0315. doi: 10.1126/sciadv.aaw0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Khalil AM, Cambier JC, Shlomchik MJ. B cell receptor signal transduction in the GC is short-circuited by high phosphatase activity. Science. 2012;336:1178–1181. doi: 10.1126/science.1213368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Martin F, Kearney JF. Marginal-zone B cells. Nat. Rev. Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 130.Weller S, et al. Somatic diversification in the absence of antigen-driven responses is the hallmark of the IgM+ IgD+ CD27+ B cell repertoire in infants. J. Exp. Med. 2008;205:1331–1342. doi: 10.1084/jem.20071555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Attanavanich K, Kearney JF. Marginal zone, but not follicular B cells, are potent activators of naive CD4 T cells. J. Immunol. 2004;172:803–811. doi: 10.4049/jimmunol.172.2.803. [DOI] [PubMed] [Google Scholar]
- 132.Sang A, Zheng YY, Morel L. Contributions of B cells to lupus pathogenesis. Mol. Immunol. 2014;62:329–338. doi: 10.1016/j.molimm.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang Y, et al. Distinct mechanisms define murine B cell lineage immunoglobulin heavy chain (IgH) repertoires. Elife. 2015;4:e09083. doi: 10.7554/eLife.09083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Martin F, Kearney JF. Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19, and btk. Immunity. 2000;12:39–49. doi: 10.1016/s1074-7613(00)80157-0. [DOI] [PubMed] [Google Scholar]
- 135.Saito T, et al. Notch2 is preferentially expressed in mature B cells and indispensable for marginal zone B lineage development. Immunity. 2003;18:675–685. doi: 10.1016/s1074-7613(03)00111-0. [DOI] [PubMed] [Google Scholar]
- 136.Cariappa A, et al. The follicular versus marginal zone B lymphocyte cell fate decision is regulated by Aiolos, Btk, andCD21. Immunity. 2001;14:603–615. doi: 10.1016/s1074-7613(01)00135-2. [DOI] [PubMed] [Google Scholar]
- 137.Samardzic T, et al. Reduction of marginal zone B cells in CD22-deficient mice. Eur. J. Immunol. 2002;32:561–567. doi: 10.1002/1521-4141(200202)32:2<561::AID-IMMU561>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 138.Makowska A, Faizunnessa NN, Anderson P, Midtvedt T, Cardell S. CD1high B cells: a population of mixed origin. Eur. J. Immunol. 1999;29:3285–3294. doi: 10.1002/(SICI)1521-4141(199910)29:10<3285::AID-IMMU3285>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 139.Vaeth M, et al. Follicular regulatory T cells control humoral autoimmunity via NFAT2-regulated CXCR5 expression. J. Exp. Med. 2014;211:545–561. doi: 10.1084/jem.20130604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang Z, et al. Notch-RBP-J-independent marginal zone B cell development in IgH transgenic mice with VH derived from a natural polyreactive antibody. PLoS ONE. 2012;7:e38894. doi: 10.1371/journal.pone.0038894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hao Z, Rajewsky K. Homeostasis of peripheral B cells in the absence of B cell influx from the bone marrow. J. Exp. Med. 2001;194:1151–1164. doi: 10.1084/jem.194.8.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Carey JB, Moffatt-Blue CS, Watson LC, Gavin AL, Feeney AJ. Repertoire-based selection into the marginal zone compartment during B cell development. J. Exp. Med. 2008;205:2043–2052. doi: 10.1084/jem.20080559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kanayama N, Cascalho M, Ohmori H. Analysis of marginal zone B cell development in the mouse with limited B cell diversity: role of the antigen receptor signals in the recruitment of B cells to the marginal zone. J. Immunol. 2005;174:1438–1445. doi: 10.4049/jimmunol.174.3.1438. [DOI] [PubMed] [Google Scholar]
- 144.Gies V, et al. Phenotyping of autoreactive B cells with labeled nucleosomes in 56R transgenic mice. Sci. Rep. 2017;7:13232. doi: 10.1038/s41598-017-13422-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hofmann K, Clauder AK, Manz RA. Targeting B cells and plasma cells in autoimmune diseases. Front Immunol. 2018;9:835. doi: 10.3389/fimmu.2018.00835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rickert RC. New insights into pre-BCR and BCR signalling with relevance to B cell malignancies. Nat. Rev. Immunol. 2013;13:578–591. doi: 10.1038/nri3487. [DOI] [PubMed] [Google Scholar]
- 147.Seda V, Mraz M. B-cell receptor signalling and its crosstalk with other pathways in normal and malignant cells. Eur. J. Haematol. 2015;94:193–205. doi: 10.1111/ejh.12427. [DOI] [PubMed] [Google Scholar]
- 148.Vukelic M, Li Y, Kyttaris VC. Novel treatments in lupus. Front Immunol. 2018;9:2658. doi: 10.3389/fimmu.2018.02658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu W, Tolar P, Song W, Kim TJ. Editorial: BCR signaling and B cell activation. Front Immunol. 2020;11:45. doi: 10.3389/fimmu.2020.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gürcan HM, et al. A review of the current use of rituximab in autoimmune diseases. Int Immunopharmacol. 2009;9:10–25. doi: 10.1016/j.intimp.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 151.Hauser SL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N. Engl. J. Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 152.Yu L, et al. Rituximab selectively suppresses specific islet antibodies. Diabetes. 2011;60:2560–2565. doi: 10.2337/db11-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sellam J, et al. B cell activation biomarkers as predictive factors for the response to rituximab in rheumatoid arthritis: a six-month, national, multicenter, open-label study. Arthritis Rheum. 2011;63:933–938. doi: 10.1002/art.30233. [DOI] [PubMed] [Google Scholar]
- 154.Huang H, Benoist C, Mathis D. Rituximab specifically depletes short-lived autoreactive plasma cells in a mouse model of inflammatory arthritis. Proc. Natl Acad. Sci. USA. 2010;107:4658–4663. doi: 10.1073/pnas.1001074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chang HD, et al. Pathogenic memory plasma cells in autoimmunity. Curr. Opin. Immunol. 2019;61:86–91. doi: 10.1016/j.coi.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Musette P, Bouaziz JD. B cell modulation strategies in autoimmune diseases: new concepts. Front Immunol. 2018;9:622. doi: 10.3389/fimmu.2018.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ahuja A, et al. An acquired defect in IgG-dependent phagocytosis explains the impairment in antibody-mediated cellular depletion in Lupus. J. Immunol. 2011;187:3888–3894. doi: 10.4049/jimmunol.1101629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gomez Mendez LM, et al. Peripheral blood B cell depletion after rituximab and complete response in lupus nephritis. Clin. J. Am. Soc. Nephrol. 2018;13:1502–1509. doi: 10.2215/CJN.01070118. [DOI] [PMC free article] [PubMed] [Google Scholar]