Since the discovery of dendritic cells (DCs) by the Nobel laureate Professor Ralph Steinman et al. in 1973, a plethora of literature has accumulated on the functional roles of DCs in humans and animal models.1 DCs are involved in the innate sensing and modulation of adaptive immunity to pathogens. CD103+ DCs constitute a classical nonlymphoid DC subset that has an important role in generating immunity and maintaining tolerance.2 Pulmonary CD103+ DCs (CD103+ PDCs), which reside in close association with the airway epithelium, are particularly critical in controlling T-cell immunity against lung infections.2,3 In this article, we discuss recent evidence that shines light on the role and mechanism of CD103+ PDCs in modulating CD8+ and CD4+ T-cell responses against various pathogens, including bacteria and viruses. A deeper understanding of CD103+ PDC function may provide new translational avenues for the development of vaccines and therapeutics against infectious diseases.
Mouse CD103+ PDCs are phenotypically characterized by the expression of αE(CD103)β7, CD11chi, CD207, MHC-II, TLR3, XCR1, and Clec9a/DNGR1 but not CD64 and CD11b.3 The transcription factors Batf3 and Irf8 are critical for the development of CD103+ PDCs, as shown by the lack of these DCs in Batf3- or Irf8-deficient mice.3 Following pulmonary infection, CD103+ PDCs upregulate costimulatory molecules (CD40, CD80, and CD86), produce large quantities of several cytokines (IL-4, IL-13, IL-12, IL-10, IL-23, and IL-6), migrate to the lung-draining mediastinal lymph nodes, and prime naive CD4+ and CD8+ T cells to induce antigen-specific immune responses.4–6 Recent studies indicate that CD103+ DCs, along with lymphoid CD8α+ DCs, form a new class of DCs referred to as type 1 DCs (DC1s), which express the chemokine receptor XCR1 and perform the unique function of cross-presenting exogenous antigens with MHC-I molecules to CD8+ T cells.7 Owing to similar phenotypic and functional characteristics, human CD141/BDCA-3+ DCs are considered equivalent to murine DC1s.8 Overall, these DCs constitute a unified DC subset in mice and humans that is developmentally and functionally related.
An accumulating wealth of evidence stemming from mouse studies has focused on the function of CD103+ PDCs during viral and bacterial infections.4,6,9 Upon challenge with respiratory influenza A virus or poxvirus infection, mice that lack CD103+ DCs, such as Batf3−/− and Clec9A− diphtheria toxin receptor transgenic mice, failed to induce protective immunity, in contrast to control mice, suggesting a protective role for CD103+ PDCs in viral infections.6,9,10 Following influenza A virus infection, CD103+ PDCs acquired and processed apoptotic cell-associated viral antigens in their endocytic compartment, migrated to the mediastinal lymph nodes, and cross-presented the antigens on MHC-I molecules to naive CD8+ T cells to elicit protective virus-specific cytotoxic responses.6,11 Interestingly, CD103+ PDCs could cross-present antigens from virally infected cells because of their ability to resist infection by influenza virus via a type I interferon-mediated antiviral state.6 On the other hand, ablation of CD103+ PDCs resulted in decreased production of IFN-γ by CD8+ T cells and reduced expression of the activation and transcription markers Ki67, CD25, and T-bet in these cells after respiratory vaccinia virus infection.10 Liang Ng et al.12 have further shown that CD103+ PDCs not only control cross-priming of CD8+ T cells but also regulate their migration, viability, and memory responses during influenza infection. In doing so, CD103+ PDCs induce upregulated levels of sphingosine-1-phosphate receptor, which is important for egress of lymphocytes from the lymph nodes, on activated CD8+ T cells12. Moreover, adoptive transfer of bone marrow-derived CD103+ DCs in CD103+ DC-ablated mice promoted the survival of virus-specific effector CD8+ T cells.12 In addition, CD103+ PDCs purified from the lungs of influenza virus-infected mice expressed an enhanced level of IL-15, which is a critical cytokine for the maintenance of naive and memory T cells.12 Thus, CD103+ PDCs occupy a central position in regulating various aspects of CD8+ T-cell responses against viral infections.
Hemann et al.13 recently elucidated the immune mechanism that directs CD103+ PDCs to modulate antiviral CD8+ T-cell immunity. Mice lacking the receptor (Ifnlr1−/−) of type III interferon (IFN-λ), which is an immune-modulatory cytokine that has the ability to promote CD8+ T-cell immunity against influenza A virus, showed a significant reduction in migratory CD103+ PDCs, along with CD8α+ DCs, in the mediastinal lymph nodes after influenza A virus infection.13 Furthermore, specific deletion of IFN-λ receptor 1 in CD11c+ DCs in a conditional knockout mouse model mirrored the global immune phenotype of Ifnlr1−/− mice, particularly the diminished CD8+ T-cell responses.13 Taken together, these findings indicate that IFN-λ signaling has an important role in programming CD103+ PDCs for migration from the lungs to the lymph nodes and induction of an optimal CD8+ T-cell response against influenza A virus. Provided the importance of the CD103+ PDC-IFN-λ-CD8+ T-cell axis in adaptive immunity, it may be prudent to exploit IFN-λ as a vaccine adjuvant against influenza infection.
Most information on how CD103+ PDCs control CD4+ T-cell responses comes from studies using bacterial and fungal pathogens.4,5,14,15 Analysis of CD103+ PDCs from Klebsiella pneumoniae-infected mice showed that the PDCs imparted enhanced antigen-specific CD4+ T-cell responses in an in vitro DC-T-cell coculture system with OVA TCR transgenic CD4+ T helper cells.5 Recently, we assessed the immune function of classical major PDC subsets, CD103+ and CD11bhi PDCs, in a mouse model of pulmonary chlamydial infection.4 Intranasal transfer of CD103+ PDCs isolated from Chlamydia muridarum-infected mice into naive mice induced better protection against C. muridarum challenge infection than that in CD11bhi PDC recipients.4 Analysis of cytokines in CD103+ PDC recipients clearly showed a stronger bias toward Th1 (IFN- γ) and Th17 (IL-17) responses than that in mice receiving CD11bhi PDCs. These data suggest a predominant role for the CD103+ PDC subset over CD11bhi PDCs in conferring protective Th1/Th17 immunity to chlamydial infection. In accordance with these findings, depletion of CD103+ PDCs in mice resulted in increased bacterial burdens in the lungs and lymph nodes following Mycobacterium tuberculosis infection, which was associated with consistently reduced levels of total and activated CD4+ and CD8+ T cells and Th1-related cytokines (IFN-γ and TNF-α).14 Using a mouse model of pulmonary Aspergillus fumigatus infection, Zelante et al.15 deciphered the mechanisms by which CD103+ PDCs control CD4+ cell function in the lungs. A. fumigatus triggers CD103+ PDCs to secrete IL-2 through the receptor dectin-1, phagocytosis, and Ca2+-calmodulin-dependent NFAT signaling pathway, thus eliciting IL-17+ CD4+ T-cell responses that induce protective immunity.15 On the other hand, CD103+ PDCs incompetent for IL-2 production produce IL-23, which in turn causes lethal Th17-driven hyperinflammation.15 In summary, the equilibrium between CD103+ PDC-expressed IL-2 and IL-23 is critical for modulating mucosal pulmonary responses to infection.
CD103+ PDCs are critical to adaptive immunity against pulmonary infections. They not only excel in cross-priming CD8+ T cells to generate antigen-specific cytotoxic responses against viruses but also induce effective CD4+ T-cell immunity (Th1/Th17) to bacterial and fungal pathogens (Fig. 1). Indeed, a multifaceted role for CD103+ PDCs in pathogen defense is becoming clearer in light of new knowledge originating from various mouse studies. The indispensability of CD103+ PDCs for the generation and maintenance of protective antiviral T cells suggests that vaccination strategies should target these DCs to achieve optimal immunity. Modulation of CD103+ PDC function may have implications for both ensuing protective immunity and suppressing infection-driven hyperinflammation in the lungs. The relevance of data accumulated regarding murine CD103+ PDCs for humans warrants further exploration. The similarity of human CD141+ DCs to murine CD103+ DCs presents an opportunity to establish a functional correlation between these two DC subsets in mice and humans during infection. Moving in this direction, humanized mice have emerged as an attractive model to study CD141+ DC function in vivo under naive and infectious conditions. This advance is owing mainly to the striking functional similarities of CD141+ DCs from humanized mice, such as cross-priming and IFN-λ expression, with those found in the human blood.16
Fig. 1.
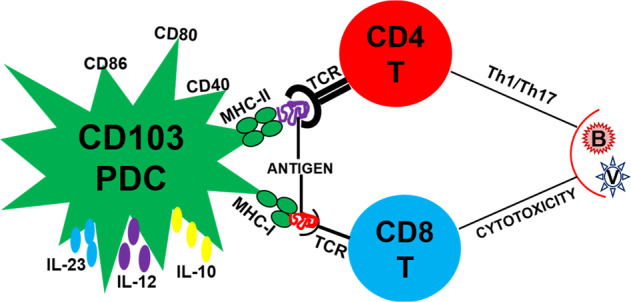
CD103+ PDC-mediated T-cell responses to pathogens. Following a respiratory infection with pathogens, CD103+ PDCs acquire and process microbial antigens, express costimulatory molecules (e.g., CD80, CD86, and CD40), secrete multiple cytokines (e.g., IL-23, IL-12, and IL-10), and migrate to the lung-draining lymph nodes to present the antigens on MHC-I/MHC-II molecules to the TCR of naive CD8+/CD4+ T cells, inducing cytotoxic/T helper (Th1/17) responses against viral and bacterial pathogens. PDC pulmonary dendritic cell, IL Interleukin, MHC major histocompatibility complex, TCR T-cell receptor, B bacterium, V virus
Author contributions
SS and XY wrote the manuscript and gave approval of its last version to be published.
Competing interests
The authors declare no competing interests.
References
- 1.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.del Rio ML, Bernhardt G, Rodriguez-Barbosa JI, Forster R. Development and functional specialization of CD103+ dendritic cells. Immunol. Rev. 2010;234:268–281. doi: 10.1111/j.0105-2896.2009.00874.x. [DOI] [PubMed] [Google Scholar]
- 3.Kessel CHG, Lambrecht BN. Division of labor between dendritic cell subsets of the lung. Mucosal Immunol. 2008;1:442–450. doi: 10.1038/mi.2008.39. [DOI] [PubMed] [Google Scholar]
- 4.Shekhar S, Peng Y, Wang S, Yang X. CD103+ lung dendritic cells (LDCs) induce stronger Th1/Th17 immunity to a bacterial lung infection than CD11b(hi) LDCs. Cell Mol. Immunol. 2018;15:377–87.. doi: 10.1038/cmi.2016.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hackstein H, et al. Modulation of respiratory dendritic cells during Klebsiella pneumonia infection. Resp. Res. 2013;14:91. doi: 10.1186/1465-9921-14-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helft J, et al. Cross-presenting CD103(+) dendritic cells are protected from influenza virus infection. J. Clin. Invest. 2012;122:4037–4047. doi: 10.1172/JCI60659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutierrez-Martinez E, et al. Cross-presentation of cell-associated antigens by MHC class I in dendritic cell subsets. Front. Immunol. 2015;6:363. doi: 10.3389/fimmu.2015.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Keeffe M, Mok WH, Radford KJ. Human dendritic cell subsets and function in health and disease. Cell. Mol. Life Sci. 2015;72:4309–4325. doi: 10.1007/s00018-015-2005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.GeurtsvanKessel CH, et al. Clearance of influenza virus from the lung depends on migratory langerin(+)CD11b(−) but not plasmacytoid dendritic cells. J. Exp. Med. 2008;205:1621–34.. doi: 10.1084/jem.20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai P, Tahiliani V, Abboud G, Stanfield J, Salek-Ardakani S. Batf3-dependent dendritic cells promote optimal CD8 T cell responses against respiratory poxvirus infection. J. Virol. 2018;92:e00495–18. doi: 10.1128/JVI.00495-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho AW, et al. Lung CD103+ dendritic cells efficiently transport influenza virus to the lymph node and load viral antigen onto MHC class I for presentation to CD8 T cells. J. Immunol. 2011;187:6011–6021. doi: 10.4049/jimmunol.1100987. [DOI] [PubMed] [Google Scholar]
- 12.Ng SL, Teo YJ, Setiagani YA, Karjalainen K, Ruedl C. Type 1 conventional CD103(+) dendritic cells control effector CD8(+) T cell migration, survival, and memory responses during influenza infection. Front. Immunol. 2018;9:3043. doi: 10.3389/fimmu.2018.03043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemann EA, et al. Interferon-lambda modulates dendritic cells to facilitate T cell immunity during infection with influenza A virus. Nat. Immunol. 2019;20:1035–45.. doi: 10.1038/s41590-019-0408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koh VH, et al. Role and contribution of pulmonary CD103(+) dendritic cells in the adaptive immune response to Mycobacterium tuberculosis. Tuberculosis (Edinb.) 2017;102:34–46. doi: 10.1016/j.tube.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Zelante T, et al. CD103(+) dendritic cells control Th17 cell function in the lung. Cell Rep. 2015;12:1789–1801. doi: 10.1016/j.celrep.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 16.Minoda Y, et al. Human CD141(+) dendritic cell and CD1c(+) dendritic cell undergo concordant early genetic programming after activation in humanized mice in vivo. Front Immunol. 2017;8:1419. doi: 10.3389/fimmu.2017.01419. [DOI] [PMC free article] [PubMed] [Google Scholar]


