Abstract
Certain skin bacteria are able to convert aromatic amino acids (AAA) into trace amines (TA) that act as neuromodulators. Since the human skin and sweat contain a comparatively high content of AAA one can expect that such bacteria are able to produce TA on our skin. Here we show that TA-producing Staphylococcus epidermidis strains expressing SadA are predominant on human skin and that TA accelerate wound healing. In wounded skin, keratinocytes produce epinephrine (EPI) that leads to cell motility inhibition by β2-adrenergic receptor (β2-AR) activation thus delay wound healing. As β2-AR antagonists, TA and dopamine (DOP) abrogate the effect of EPI thus accelerating wound healing both in vitro and in a mouse model. In the mouse model, the S. epidermidis wild type strain accelerates wound healing compared to its ΔsadA mutant. Our study demonstrates that TA-producing S. epidermidis strains present on our skin might be beneficial for wound healing.
Subject terms: Microbial genetics, Microbial communities
Arif Luqman et al. demonstrate that trace amines accelerate wound healing by antagonizing β2-adrenergic receptor whose activation inhibits cell motility. This study suggests that trace amine-producing Staphylococcus epidermidis strains present on human skin may play a beneficial role for wound healing.
Introduction
Trace amines (TA) and classical neurotransmitter (dopamine, norepinephrine, or serotonin) are stored and released together in and from the nerve terminals1,2. TA play crucial roles physiologically as neuromodulators of synaptic transmission of classical neurotransmitter in mammalian brain by potentiating their activity3,4. Beside acting as neuromodulator, TA are reported to interact with a new family of G protein-coupled receptors (GPCRs), the TA-associated receptors (TAARs), thus they act independently from classical neurotransmitter5,6. Borrowsky and colleagues reported that phenylethylamine (PEA) and tyramine (TYM) modulate the synaptic excitation of dopaminergic neurons or alter the reactivity of dopamine D2 receptor (D2R) to ligands by activating TAAR15,7. It has also been reported that TA interacts with various adrenergic receptors (ARs). TA act as agonists on α2-adrenergic receptor (α2-AR)8–10 and as partial allosteric antagonists on β2-adrenergic receptor (β2-AR)11.
Recently, it has been shown that various species of the genus Staphylococcus are able to produce TAs by using an enzyme called SadA (staphylococcal aromatic amino acid (AAA) decarboxylase)8. SadA is highly promiscuous because it not only decarboxylates all biogenic AAAs into tryptamine (TRY), PEA, and TYM but also dihydroxy phenylalanine (L-DOPA) and 5-hydroxytryptophan (5-HTP) to the neurotransmitter dopamine (DOP) and serotonin (SER).
First, it was not clear what might be the advantage to the bacteria of having SadA. The AAAs present in the medium are imported into the cell, pyridoxal phosphate (PLP)-dependent decarboxylated, and quantitatively excreted into the medium as TA8. There is no evidence that AAAs are used for protein biosynthesis or are involved in other metabolic pathways. Since energy-consuming reactions rarely occur in nature without a reason, the question arises as to what is the benefit of TA synthesis to the bacteria. As many TA-producing staphylococcal species are described as animal pathogens and skin colonizers, comparative studies with Staphylococcus pseudintermedius and its sadA deletion mutant were carried out. S. pseudintermedius is responsible for severe and necrotizing infections in humans and dogs12–14. Staphylococci are part of the human intestinal microflora and the TA-producing staphylococci are predominant among the staphylococcal genus present8. One reason for the predominance of TA-producing staphylococci in the gut could be that TA increase the internalization of bacteria into the host cells8. It is well documented that an increase in internalization of the epithelial cells protects the bacteria from both the host immune system15–17 and antibiotics18,19.
The molecular mechanism of how these neurochemicals boost bacterial internalization has been unraveled only recently20. TA ad DOP activate α2-AR and induce a reduction of the cytoplasmic cAMP level as well as an increased F-actin formation. This causes an increased internalization of the bacteria by the host cells. This internalization mechanism is independent from the FnBP-Fn-α5β1 integrin-mediated pathway20. The TA-triggered internalization of bacteria into the host cells represents a new invasion mechanism. The additional role of TA-producing bacteria play in the gut is unknown and such role is difficult to verify. However, in the meantime it emerged that neurotransmitters and neuromodulators are not only produced by the host, but also by the gut microbiota and that they play a role in the Gut-Brain axis21.
Here we show that TA produced by skin bacteria accelerate wound healing by antagonizing the EPI-induced cell migration inhibition.
Results
TA-producing Staphylococcus epidermidis strains are predominant on human skin
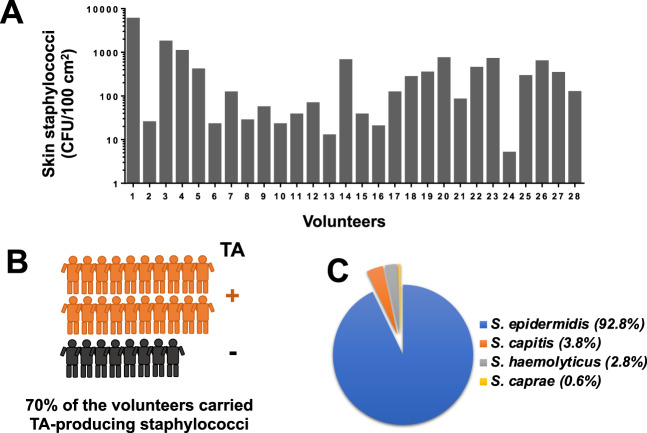
Since TA-producing staphylococci have an advantage in host cell adherence and internalization8, we investigated whether and how many TA-producing staphylococci colonize the human skin and its prevalence. We took skin swabs from the forearm (antecubital fossa) of 28 healthy volunteers as illustrated in Supplementary Fig. 1. The swabs were examined for the presence of staphylococci using SK-salt agar, a selective medium for isolating coagulase-positive and coagulase-negative staphylococci22. All volunteers were colonized with staphylococci with the degree of colonization varied from 5 to 6000 CFU/100 cm² (Fig. 1a). Twenty out of 28 volunteers were colonized by TA-producing staphylococci (70%) (Fig. 1b). In total we isolated 900 colonies and tested them for TA production by HPLC analysis (Supplementary Fig. 2). Of the 900 staphylococcal colonies/strains 185 produced TA (20%) as shown in Table 1. We identified all of 185 TA producer using 16S rRNA sequencing. The majority of the TA-producing staphylococci belonged to S. epidermidis (92,8%) followed by S. capitis, S. haemolyticus, and S. caprae (Fig. 1c).
Fig. 1. TA-producing S. epidermidis is prevalent on human skin.
a Number of staphylococci (CFU/100 cm²) found on the forearm (antecubital fossa) of 28 volunteers. b 900 random colonies from all volunteers were assayed by HPLC analysis for TA production: 70% of the volunteers carried TA producing staphylococci. c Of the 900 colonies, 185 (20%) produced TA; these colonies were identified at species level using 16s rRNA sequencing. The majority of TA producer belonged to S. epidermidis, followed by S. capitis, S. haemolyticus, and S. caprae.
Table 1.
Harvested staphylococcal colonies per volunteer and distribution of TA producers.
| CFU range of staphylococci on volunteers’ skin (CFU/100 cm2) | Harvested colonies analyzed for TA production by HPLC | Volunteers | Total analyzed colonies by HPLC (900) | TA producing strains |
|---|---|---|---|---|
| >1000 | 120 | 1, 3, 4 | 360 | 185 = 20% |
| 500–1000 | 60 | 14, 20, 26, 23 | 240 | |
| 200–500 | 30 | 5, 18, 19, 22, 25, 27 | 180 | |
| 50–200 | 15 | 7, 9, 12, 17, 21, 28 | 90 | |
| 0–50 | ≤7 | 2, 6, 8, 10, 11, 13, 15, 16, 23 | 30 |
We also asked how prevalent the sadA gene is in the S. epidermidis species and blasted (tblastn of NCBI’s Microbial BLAST) the SadA protein sequence of ATCC 12228 against 19 complete S. epidermidis genomes. 11 of the genomes (58%) encoded a highly homologous (99–100% identity) SadA protein, while other staphylococcus species, such as of S. pseudintermedius ED99 shared only 52% identity (Table 2).
Table 2.
Presence and identity of SadA in S. epidermidis.
| S. epidermis strains | SadA | identity in % |
|---|---|---|
| O47 | + | 100 |
| ATCC 12228 | + | 100 |
| BPH0662 | + | 100 |
| DAR1907 | + | 100 |
| PM221 | + | 99,8 |
| CSF41498 | + | 99,8 |
| SEI | + | 99,8 |
| NCTC13924 | + | 99,6 |
| FDAARGOS_529 | + | 99,6 |
| CDC120 | + | 99,6 |
| CDC121 | + | 99,6 |
| ATCC 14990 | − | – |
| NBRC 100911 | − | – |
| RP62A | − | – |
| 14.1.R1 | − | – |
| FDAARGOS_153 | − | – |
| FDAARGOS_161 | − | – |
| 1457 | − | – |
| NCTC4133 | − | – |
| S. pseudintermedius ED99 | + | 52 |
TA and DOP abrogate the EPI-induced cell migration arrest—thus accelerating the wound healing
Given that human skin cells, particularly keratinocytes, express β2-adrenergic receptor (β2-AR)23–26, whereas TYM and PEA are allosteric antagonists for β2-AR11, we asked whether the TA produced by sadA-expressing staphylococci promote skin wound healing.
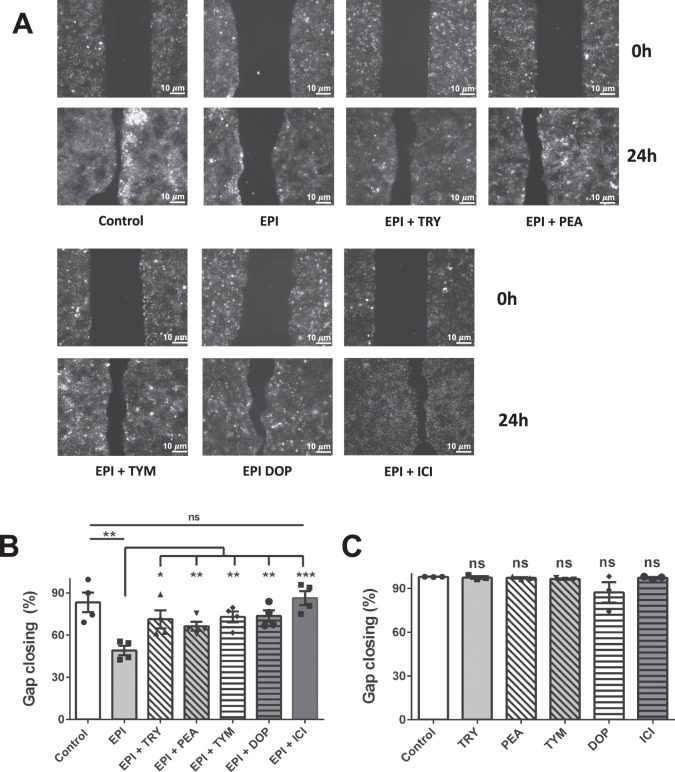
To address this question, we first carried out an in vitro migration assay with HaCaT cells, a human keratinocyte cell line that expresses β2-AR27. It is well known that the stress hormone epinephrine (EPI) acts as an agonist of the β2-AR, which lowers the migratory rate of keratinocytes and thus impairing wound healing28,29. We wanted to find out if TA and DOP, as agonists of the alpha2-adrenergic receptor (α2-AR)20, are able to abrogate the EPI effect. The wound healing assay was carried out in the presence of EPI (25 μg/ml) alone and in combination with TA (TRY, PEA, TYM) and DOP (each at 25 μg/ml). We included DOP in the experiment since the sadA-expressing staphylococci can also convert L-DOPA into DOP8. As a further control we included ICI 118551 (ICI), a selective β2-AR antagonist, also referred to as beta blocker30. For better comparison we used for all compounds at the same concentration of 25 μg/ml. At this concentration the effect of EPI on gap closing in HaCaT cells was most pronounced (Supplementary Fig. 3a).
The in vitro wound healing assay, which is mainly based on the determination of the host cell migration rate, was performed with HaCaT cells in culture-insert (2-wells format). In comparison to the control (untreated cells), EPI visibly delayed gap closing by lowering the migration of HaCaT cells. However, in the presence of TRY, PEA, TYM, DOP or ICI, the negative effect of EPI was abrogated and gap closing was almost as efficient as the control (Fig. 2a). The positive effect of ICI was mainly due to its inverse agonist effect on ß-AR31,32. The gap closing analyses using ImageJ showed that TA and DOP abrogated (p < 0.05) the negative effect of EPI on gap closing (Fig. 2b). In the absence of EPI, no effect on gap closing was seen with TA, DOP and ICI in comparison to the control (Fig. 2c). We also tested alprenolol (ALP, a neutral ß2-AR), which showed similar effect as ICI (Fig. S3b). Phentolamine (PTL) showed no effect in gap closing suggesting that α-AR does not play a role in gap closing of HaCaT cells (Supplementary Fig. 3b).
Fig. 2. TA and DOP accelerate wound healing in vitro in a wound-mimicking assay.
a TA and DOP (each at 25 μg/ml) were added into the HaCaT cell culture seeded with a gap in the presence of EPI (25 μg/ml). ICI 118551 was used as positive control. Microscopic images of the gaps were taken at time 0 h and 24 h. b The gap closing, which was calculated using ImageJ67, revealed that TA and DOP abrogated the migration-inhibiting effect of EPI. c In the absence of EPI, the TA, DOP and ICI showed similar gap closing as control. Each data point is the mean value ± SEM from 4 independent replicates for (b) and 3 independent replicates for (c), *p < 0.05; **p < 0.01; and ***p < 0.001, data were analyzed using Student's t test. Source data are provided as a Supplementary Data 1 file.
TA and DOP enhance the migration of HaCaT cells even in the presence of mitomycin C
Wound healing involves two distinct mechanisms, cell proliferation and cell migration. Therefore, we investigated cell migration and cell proliferation independently. Cell migration assay was carried out with HaCaT cells as described earlier, with the only difference that we added mitomycin C (10 μg/ml) to inhibit the cell proliferation.
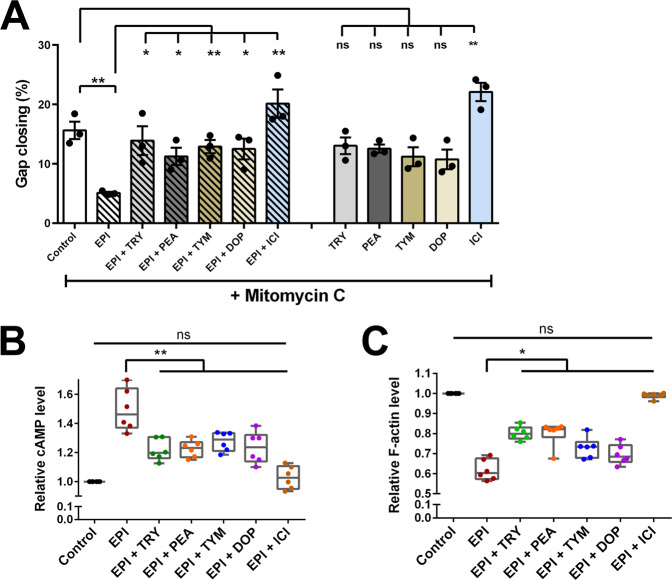
In the absence of mitomycin C the gap closing was about 85% after 24 h (Fig. 2b, control). However, in the presence of mitomycin C, however the gap closing in the control decreased to only 15% after 24 h (Fig. 3a). In the presence of EPI gap closing decreased further to only 5%, while the addition of TA, DOP, and ICI abrogated the negative effect of EPI (Fig. 3a). In the presence of mitomycin C and the absence of EPI, TA, and DOP showed a similar gap closing as observed in the control (Fig. 3a, second half). In the presence of ICI, the gap closing was always higher. Here we show that the inhibition of cell proliferation by mitomycin C decreased the gap closing but had no principal effect on the activities of TA, and DOP, indicating that TA and DOP only affect cell migration but not proliferation.
Fig. 3. TA and DOP partially abrogate the effect of EPI in inhibiting HaCaT cells migration by affecting cAMP level and F-actin formation.
a HaCaT cells migration was examined using a similar method as the wound healing assay with the addition of Mitomycin C (10 µg/ml). TA, DOP and ICI (25 µg/ml) enhanced the HaCaT cells migration in the presence of EPI (25 µg/ml). TA and DOP alone did not show any significant effect while ICI boosted the HaCaT cells migration. b HaCaT cells was incubated with EPI (25 µg/ml) and TA, DOP and ICI as positive control (25 µg/ml) separately for 2 h. TA, DOP and ICI were added 30 min prior to the addition of EPI. EPI increased the intracellular cAMP level of HaCaT cells but the addition of TA, DOP and ICI, decreased the cAMP level compared to HaCaT cells treated with EPI alone. c EPI (25 µg/ml) decreased the F-actin level compared to the control. The addition of TA, DOP and ICI (25 µg/ml) in the presence of EPI, increased the F-actin level compared to the treatment with EPI only. For all graphs, each data point is the mean value ± SEM from 3 independent replicates for (a) and from 6 independent replicates for (b), *p < 0.05; **p < 0.01, data were analyzed using Student's t test. Source data are provided as a Supplementary Data 1 file.
TA and DOP counteract the EPI-induced activation of β2-AR
Activation of the β-AR by EPI inhibits the wound closing by inhibiting the migration of keratinocytes29,33–35. Since the activation of β-AR is followed by an increase in the intracellular cAMP level which decreases cell migration, we investigated whether TA, DOP, and ICI can counteract this activity. Indeed, in the presence of EPI alone, the relative cAMP level was increased by approximately 50% compared to the control, while the addition of TA, DOP, and ICI caused a decrease (p < 0.01) in the cAMP level (Fig. 3b).
In contrast, the relative F-actin level, which positively correlates to the migration36, was decreased by the addition of EPI and this effect was partially abrogated by TA and DOP (Fig. 3c). In the absence of EPI, TA, and DOP showed no effect on cAMP and F-actin level, while ICI decreased the cAMP level and increased the F-actin significantly (p < 0.01) compared to the control. This is due to the inverse agonist effect of ICI31,32 (Supplementary Fig. 4a, b). As expected, in the presence of the β-AR blocker, ICI, the effect of EPI was almost completely abrogated. Our data show that TA and DOP are only able to partially abrogate the EPI effect.
TA and DOP affect neither effect cell proliferation nor intracellular Ca++ level
We performed HaCaT proliferation assay in the presence of EPI (25 μg/ml) alone and together with TA, DOP, and ICI (each at 25 μg/ml). EPI alone caused an increase in cell proliferation by about 50%. The addition of TA and DOP to the EPI treated cells had no effect (Fig. 4a). TA and DOP showed no difference to the control level in the absence of EPI (Fig. 4a, second half).
Fig. 4. TA and DOP did not inhibit the HaCaT cell proliferation and the increase of intracellular Ca++.
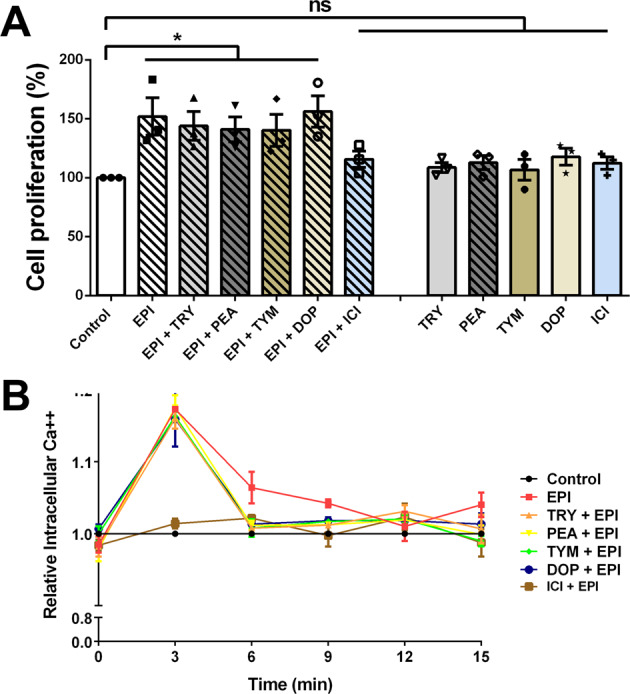
a HaCaT cells proliferations were measured using MTT assay. The presence of EPI (25 µg/ml) increased the cell proliferations compared to control. The addition of TAs and DOP (25 µg/ml) showed no significant effect. b The intracellular Ca++ was measured using fluorescence-based assay. HaCaT cells was incubated in the presence of EPI (25 µg/ml) alone, and EPI with TA, DOP, and ICI. EPI increased the intracellular Ca++ level with and without TA and DOP at 3 min ICI nullified the effect of EPI both in terms of cells proliferation and increase in intracellular Ca++. For all graphs, each data point is the mean value ± SEM from three independent replicates, *p < 0.05; **p < 0.01; and ***p < 0.001, data were analyzed using Student's t test. Source data are provided as a Supplementary Data 1 file.
Considering that one of the factors that regulate the cell proliferation is intracellular Ca++ level37–39 we investigated the potential effect of TA and DOP on intracellular Ca++ level. As shown in Fig. 4b, EPI increased the intracellular Ca++ level after 3 min, whereas the addition of TA and DOP did not alter the Ca++ level. Only the ß-blocker ICI decreased the Ca++ level to the control value (Fig. 4b). The lack of responsiveness of TA and DOP to cell proliferation and the intracellular Ca++ level in the presence of EPI confirms that TA and DOP do not affect cell proliferation. In the absence of EPI, the Ca++ level was not affected by TA, DOP, and ICI (Supplementary Fig. 4c).
These results show that EPI impairs wound healing (gap closing) in keratinocytes and that TA and DOP abrogate the effect of EPI. Furthermore, the effect of the TA and DOP is not based on cell proliferation but rather on cell migration.
TA and DOP produced by skin staphylococci accelerate the wound healing in a murine model
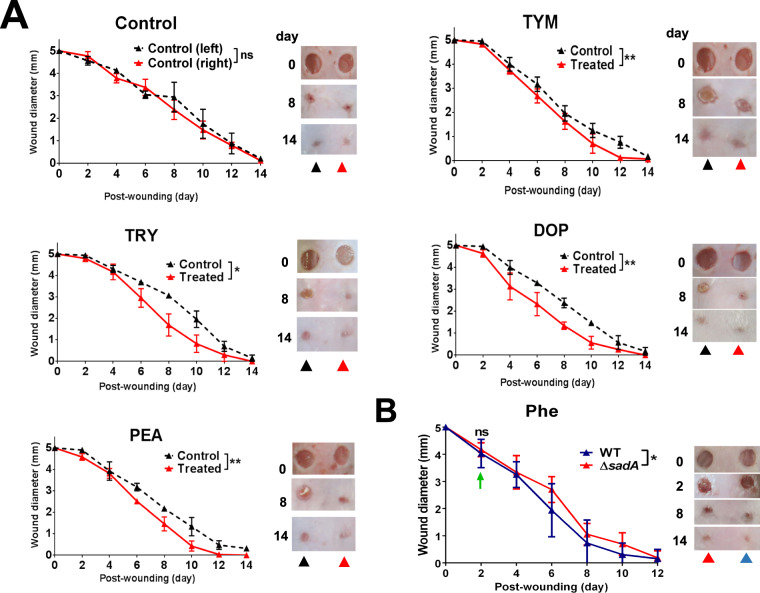
Our next question was whether the positive effect of TA and DOP on wound healing can also be observed in vivo. It is well known that one of the stress responses to the wound is the systemic and local increase of EPI, which impairs wound healing40,41. Therefore, we investigated the effect of TA, DOP, and ICI on wound healing in a murine model. Cutaneous wound healing experiments were performed in DDY mice with two circular full-thickness wounds on the back of each mouse. Each mouse was treated both on one side with TA or DOP and the other side with water as control starting from day 0 post-wounding. ICI was used as a positive control. The wound diameter was measured by calculating the mean of diameters measured from two sides of one wound (Supplementary Fig. 6). This procedure was chosen because the wound area was not always circular. The wounds treated with TA, DOP or ICI (25 μg/ml) closed faster than the controls (Fig. 5a and Supplementary Fig. 4). This confirms that the positive wound healing effect observed with keratinocytes also applies for the mouse model.
Fig. 5. Effect of TA on wound healing in mice.
Mice were shaved and wounded on their back with 2 biopsy punches for each mouse. a TRY, PEA, TYM, and DOP (each 25 μg/ml) with total volume of 10 μl were applied topically daily starting from day 0. The diameter of the wounds was measured every 2 days. The wounds treated with TRY, PEA, TYM, and DOP showed a faster wound closing than the untreated wounds (control). b S. epidermidis O47 WT and ΔsadA were applied on and around the wound at cell density 5000 CFU/100 cm2 on 2 days post-wounding (green arrow). In addition, 10 μl phenylalanine (Phe) 50 μg/ml was also applied daily. The diameter of the wounds on 2 days post-wounding before application of S. epidermidis O47 were not significantly different. Wounds treated with S. epidermidis O47 WT showed faster wound closure than those treated with S. epidermidis O47 ΔsadA. For all graphs, each data point is the mean value ± SEM from three independent replicates for (a) and six independent replicates for (b), *p < 0.05 and **p < 0.01, data were analyzed using paired Student's t test. Source data are provided as a Supplementary Data 1 file.
To mimic the natural condition where TA-producing staphylococci and AAA are present on the skin, we applied S. epidermidis O47 as a model for skin staphylococci on the wound murine model with the application of phenylalanine. It resulted that wounds with S. epidermidis O47 wild type closed faster than wounds with S. epidermidis O47 ΔsadA (Fig. 5b). This result suggests that the TA-producing skin staphylococci play a role in accelerating wound healing.
Discussion
The fact that many staphylococcal species are able to produce TA with the help of the enzyme SadA is a new insight8. Originally, we investigated the occurrence of TA-producing staphylococci in the gut of human volunteers and found that the TA-producing strains were predominant among the isolated staphylococcal strains. This finding suggests that such strains most likely have an advantage, which to this time is unclear.
Here we addressed the question whether TA-producing staphylococci are prevalent on our skin and what effect TA production has for our skin. The skin is a classical habitat for members of the Staphylococcacea as already described by Kloos and Schleifer. The authors mapped and characterized the staphylococcal species on human skin42,43, and presented a body atlas with the distribution of live staphylococcal species on various regions of the adult skin44. They already described that staphylococci, coryneforms, micrococci, and bacilli were the most predominant and persistent bacteria isolated from the head, legs, and arms. Later characterizations using genomic approaches revealed a much greater diversity of bacteria than that by the culture-based methods, but nevertheless, they come to a similar topographical distribution of bacteria on skin sites45,46. Due to the high abundance of staphylococci on the skin, we choose to take samples from the forearm (antecubital fossa) of 28 volunteers and plated them on a staphylococcal selective SK-salt agar. 20% of the 900 isolated staphylococcal colonies produced TA and the majority belonged to S. epidermidis, a species in which we found that >50% of the strains carried the sadA gene.
The key question in this study was whether TA can be produced by the skin bacteria and if so, the possible role they play on the skin. In staphylococci, we have found that TA are produced if the environment contains AAAs. There is no evidence that AAA are used for protein biosynthesis, instead they are imported, decarboxylated by SadA and excreted as TA8. Therefore, TA should be produced if sufficient AAAs are present on the skin. This is indeed the case as human sweat contains a comparatively high content (in the range of 300 μM or about 60 μg/ml) of AAAs47–51. In this context, approximately 10 µg/100 cm2 of AAA and 5 µg/100 cm2 of TA can be detected on our skin (Supplementary Table 1). For this reason, it is expected that sadA-expressing staphylococci are able to produce TA on our skin. During the course of the investigation of the possible role TA–DOP–ICI might play on our skin, we found out that they accelerate wound healing.
After an injury, keratinocytes migrate over the wound bed to repair a wound. However, a wound triggers a stress reaction that leads to the production of various stress hormones such as EPI and cortisol which delay wound healing28,29,41. The enzymes necessary for both cortisol and EPI synthesis are located in the epidermis. Keratinocytes synthesize both hormones locally41,52,53. Keratinocytes also express beta-2-adrenergic-receptor (ß2-AR), a receptor for the stress hormone EPI, also known as adrenaline. Local cortisol and EPI synthesis impair keratinocyte migration and delay re-epithelialization52,54. Activation of β2-AR inhibits keratinocyte migration in a cAMP-independent manner55, while antagonists of the ß2-AR accelerate wound healing56. The concentration of EPI that we used (25 μg/ml = 136 μM) is above the physiological concentrations found in plasma of mice with a wounded skin (18 ng/ml or 0.1μM)41. However, it could be that HaCaT cells express lower concentrations of ß2-AR. If this is the case, they would need a higher concentration of ligands to be responsive. On the other hand, we show in our mouse model (with the endogenously produced EPI) the effectiveness of TA.
The interaction of TA with the TA-associated receptors (TAARs), suggests that TA may also have functions that are entirely independent of the classical neurotransmitters5,6. Indeed, in co-stimulation studies have found that TYR and PEA are partial allosteric antagonists of ß1- and ß2-AR11. However, if TA are ß2-AR antagonists, then they should also accelerate wound healing.
To analyze the impact of TA–DOP–ICI on wound healing we have chosen two classical approaches. We carried out an in vitro migration or wound healing assay with HaCaT cells, a human keratinocyte cell line that expresses β2-AR27. In the presence of EPI, TA–DOP–ICI accelerated gap closing (p < 0.05) (Fig. 2a, b). No effect of TA–DOP–ICI was observed in the absence of EPI (Fig. 2c).
It is important to discriminate between the effect of proliferation and migration of adjacent cells on wound closure. Therefore, we investigated the wound healing of HaCaT cells in the presence of mitomycin C, a potent DNA crosslinker and inhibitor of cell proliferation. Comparison of Fig. 2 and Fig. 3 shows that in the presence of mitomycin C the gap closing was generally decreased from 85% to about 15%. However, independent of the presence or absence of EPI, TA–DOP–ICI always increased gap closing to almost the control level (Fig. 3a).
These result show two things: firstly, EPI worsens wound healing in the presence of mitomycin C. In addition to the inhibition of cell proliferation by mitomycin, cell migration is now inhibited by EPI. Similar effects were reported for catecholamines (isoproterenol, EPI, and norepinephrine), which delay wound closure by inhibiting epidermal cell migration57. Secondly, TA–DOP–ICI counteracted the negative effect of EPI on gap closing, indicating that they activate cell migration.
To further confirm that TA–DOP–ICI abrogate the negative effect of EPI on cell migration, we investigated the cAMP and F-actin levels in cytoplasm. It has been the accepted dogma since the 1960s that β2-receptor activation causes an increased intracellular cAMP levels due to the stimulation of adenylate cyclase58,59. As expected, EPI increased the plasma cAMP level in HaCaT cells, and this increase was counteracted by TA–DOP–ICI, indicating that they antagonize the EPI effect (Fig. 3b). Similar effect was observed with F-actin (Fig. 3c). EPI decreased the F-actin level, while TA–DOP–ICI relieved this blockade. The EPI-induced decrease of the F-actin level is responsible for the inhibition of cell migration.
It has been reported that EPI stimulates cell proliferation60 and increases the intracellular calcium level61. Therefore, we tested TA–DOP–ICI whether they negate EPI's activity on cell proliferation and intracellular calcium level (Fig. 4a, b). However, there was no such activity observed, indicating that TA–DOP are not involved in cell proliferation. In our studies, ICI was also included as a control since it is a selective ß2-AR antagonist or beta blocker31. Its effect to counteract EPI-induced activation of ß2-AR was always stronger than that of TA–DOP, suggesting that ICI is a full ß2-AR antagonist, while TA–DOP are partial antagonists.
As all these studies were done in vitro with keratinocytes we examines the activity of TA–DOP in vivo using a mouse model. In agreement with in vitro studies, the wound healing was accelerated by TRY, DOP, PEA, TYM, and ICI (p < 0.05) (Fig. 5a and Supplementary Fig. 5).
Cogen et al. 62 stated that skin microbiota are usually regarded as pathogens, potential pathogens or innocuous symbiotic organisms. Indeed, it has long been assumed that a normal skin microbiota has rather a protective rather than destructive function on our skin. For example, a commensal strain of S. epidermidis protects again skin neoplasia63. Here we show that the TA produced by skin bacteria accelerate wound healing (Fig. 5b). A wound represents a stress situation which is accompanied by the production of various stress hormones such as EPI and cortisol which delay wound healing by inhibiting cell migration due to activation of ß2-AR. Members of Staphylococcaceae, particularly the classical skin bacterium S. epidermidis, produces TA, which act as neurotransmitter or neuromodulators. While studying the function of TA on our skin, we found that they abrogate the negative effect of EPI on wound healing thus accelerate wound healing. Surprisingly, when we applied the S. epidermidis strains 2 days post-wounding at and around the wound area, 95% of the mice showed no infection symptoms such as inflammation or necrosis, suggesting that commensal S. epidermidis strains evolved strategies to co-exist rather than being a symbiote on our skin.
Methods
Skin swab samples collection, sadA-expressing staphylococci screening and AAA and TA quantification of skin swab samples
We collected the skin swab samples from 28 healthy and random subjects by swabbing the antecubital fossa part using sterile swab sample collector and sterile phosphate buffer saline (PBS). Collected skin swab samples were then spread on SK salt agar medium8 and incubated at 37 °C for 48 h. The colonies were then picked, inoculated in basic medium (BM) in 96-well plates and incubated at 37 °C for 24 h with shaking at 150 rpm. The collected supernatants were analyzed by reversed-phase HPLC (RP-HPLC) at room temperature with an Eclipse XDB-C18 column (4.6 × 150 mm; 5 μm) (Agilent) installed together with an analytical guard column for Eclipe XDB-C-18 (4.6 × 12.5 mm; 5 μm) (Agilent), with a 15-min linear gradient of 0.1% phosphoric acid to acetonitrile and 5 min post time washing with 0.1% phosphoric acid. The flow rate used was 1.5 mL/min; the sample volume that was injected was 10 μl. We used diode array detector (DAD) at 210 nm and 360 nm as reference. The simplified workflow is illustrated in Fig. S1. While for AAA and TA quantification on skin swab samples, we resuspended the samples in sterile PBS and analyzed using RP-HPLC with the same method as for screening.
Skin staphylococci identification
We identified the species of the isolated TA producing staphylococci by growing the strains on the selective agar separately and incubated overnight at 37 °C. We then picked the colonies, resuspended in PBS and isolated the genomic DNA using Quick-DNA Microprep Kit (Zymo Research). We then amplified the 16s rRNA gene using specific primers (Supplementary Tabel 1) and Q5 polymerase (New England Biolabs). We purified the PCR product using Illustra GFX DNA and Gel Band Purification Kit (GE healthcare) and sequenced it (GATC). We identified the species by performing BLASTN from the obtained sequence of amplified 16s rRNA gene against the 16s rRNA database in NCBI.
Construction of sadA deletion mutant in S. epidermidis O47
Construction of S. epidermidis O47 ΔsadA was carried out using plasmid pBASE664. The 1 kb upstream downstream region of sadA were amplified using appropriate primers (Supplementary Table 2) and ligated with linearized pBASE6 (EcoRV restriction site) using Hi-Fi DNA Assembly Master Mix (New England Biolabs) and transformed into E. coli C2987 (New England Biolabs) and then into E. coli DC10B chemically competent cells. Colonies with the desired plasmid were picked and the respective plasmid was transformed into S. epidermidis O47 by protoplast transformation. Mutagenesis was conducted as reported by65. Mutants were confirmed using PCR for the gene deletion and HPLC analyses for the overnight supernatants.
Bioinformatic analysis
Homologs of SadA from S. pseudintermedius ED99 were identified within the species S. epidermidis. To find homologs on the strain level tblastn of NCBI’s Microbial BLAST was used and all complete genomes of S. epidermidis were used as a database. As a query the SadA homolog of the S. epidermidis strain ATCC 12228 (NC_004461.1, genomic position 107418-108839) was used as a query sequence.
In vitro wound healing assay with HaCaT cells
The in vitro wound healing assay with HaCaT cells was carried out essentially as described by Jonkman et al.66 HaCaT cells were seeded in culture-insert 2 well in μ-dish35 mm,low (Ibidi) with a cell density 6 × 105 cells/ml and in 70 μl volume in DMEM medium with 10% fetal bovine serum (FBS) and antibiotic mix and incubated at 37 °C in 5% CO2 for 24 h prior to the assay. After 24 h incubation, the culture-inserts were gently removed, and the cell culture was washed with PBS twice and 1 ml of DMEM medium was added into the μ-dish. Gap closing was microscopically monitored (Leica DMLB) with ×100 magnification (×10 objective lens and ×10 ocular lens magnification). The TA, DOP and ICI were added at a concentration of 25 μg/ml each and incubated for 30 min prior to the addition of EPI (25 μg/ml). Cell cultures were then incubated for 24 h and microscopic images were taken. The gaps were analyzed using ImageJ as described in ref. 67 and wound closing was calculated by subtracting the final gap area from initial gap area. Cell migration assays were carried out in a similar way as described for the in vitro wound healing assay, but 10 μg/ml of Mitomycin C was added as a cell proliferation inhibitor. We performed at least three times independent replications for each experiments.
Cell proliferation assay
Prior to the cell proliferation assay, HaCaT cells were seeded in a 96-well microtiter flat-bottom plate with 5 × 104 cells/well and incubated 24 h at 37 °C in 5% CO2. The HaCaT cells were treated with the neurochemicals (TRY, PEA, TYM, and DOP) and ICI 118155 at a final concentration of 25 μg/ml, with and without EPI (25 μg/ml). The cell proliferation assay was performed using the Cell Proliferation Kit I (MTT; Roche, Germany) according to the protocol provided by the company. We performed at least 3 times independent replications for each experiment.
cAMP level measurement of HaCaT cells
Prior the cAMP measurement, HaCaT cells were seeded in black 96-well plate flat bottom with 1 × 105 cells per well and incubated overnight in DMEM medium with 10% FBS and antibiotic mix and incubated at 37 °C in 5% CO2. Cells were then treated with TA, DOP and ICI (25 μg/ml) for 30 min prior to the addition of EPI (25 μg/ml) and incubated further for 1.5 h. cAMP levels were measured using cAMP GloTM Assay (Promega) according to the protocol provided by the company. We performed at least 3 times independent replications for each experiment.
F-actin level
Prior the cAMP measurement, HaCaT cells were seeded in black 96-well plate flat bottom with 1 × 105 cells per well and incubated overnight in DMEM medium with 10% FBS and antibiotic mix and incubated at 37 °C in 5% CO2. Cells were then treated with TA, DOP and ICI (25 μg/ml) for 30 min prior to the addition of EPI (25 μg/ml) and incubated further for 1.5 h. The treated cells were then washed with DPBS, permeabilized with 0.1% (v/v) Triton X-100, washed again with DPBS, stained with ActinGreen™ 488 ReadyProbes® (Thermo Fischer) for 30 min and washed again 3–5 times with DPBS with soaking duration of 5 min for each washing step. The relative fluorescence intensity was measured at 495 nm for the excitation and 518 nm for the emission using Tecan Infinite M200. We performed at least 3 times independent replications for each experiment.
Intracellular calcium assay
We seeded the HaCaT cells in 96-well microtiter flat bottom plate with 5 × 104 cells/well and incubated for 24 h at 37 °C in 5% CO2. The intracellular calcium measurement assays were performed using Fluo-8 calcium flux assay kit—no wash (Abcam) according to the protocol provided by the company. The HaCaT cells were treated with the neurochemicals (TRY, PEA, TYM, and DOP) and ICI, with and without EPI. We performed at least 3 times independent replications for each experiment.
In vivo murine model
For wound healing experiments we followed essentially the protocol for cutaneous wound healing in a murine model as described Ganuli-Indra68. Six to eight weeks old male of DDY mice were used in wound healing experiments. Prior to the wounding, mice were anesthetized with ketamine/xylazine (10:1) with a dose of 0.04 mg/g mouse weight. The back of the mice was shaved and two circular full-thickness wounds (5 mm in diameter) were made on the back skin of each mouse using a skin biopsy punch. Two wounds were made on each mouse (right and left) so that the control and treatment were on the same mouse. The neurochemicals TRY, PEA, TYM, and DOP as well as ICI 118551 at a concentration of 25 μg/ml, respectively, and a total volume of 10 μl each were applied topically on one wound (right), water was applied as control on the other wound (left). The application of the neurochemicals was done daily, and the wound diameters were measured every two days until 14 days post-wounding. We used three mice for each variation in these experiments. For wound healing experiments using bacteria, we used S. epidermidis O47 WT and its ΔsadA deletion mutant as model bacteria. At two days post-wounding, we applied S. epidermidis O47 WT (on the right wound) and ΔsadA (on the left wound) with a cell density of 5000 CFU/100 cm2 at the wound area. At the same time, we applied phenylalanine (50 μg/ml) with a total volume of 10 μl on wound daily. The wound diameters were measured every two days until 14 days post-wounding. We used six mice in this experiment.
Statistics
Normal distributions were analyzed by Student’s t test. Statistical analyses were performed with GraphPad Prism software, with significance defined as p < 0.05; n represents independent biological replicates.
Ethical statement
The collection of human skin swab samples was approved by the Ethic Commission of the University of Tübingen (Approval no. 320/2017BO2). Skin swab samples were obtained from 28 adult volunteers. All samples were anonymized and obtained with written consent from the volunteers. The wound healing experiment using mice were approved by the Ethic Commission of Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia (Approval no. 2.KE.172.09.2019).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
The authors thank Dr. Sook-Ha Fan for proofreading the manuscript and Dr. Surasak Jittavisutthikul and Mulugeta Nega for the valuable suggestions. This work was supported by the Deutsche Forschungsgemeinschaft (DFG) SFB766 and SFB/TRR24 to F.G., and by infrastructural funding from the Deutsche Forschungsgemeinschaft (DFG), Cluster of Excellence EXC 2124 Controlling Microbes to Fight Infections. It was also supported by the Ministry for Science, Research and the Arts of Baden-Wuerttemberg (MWK) “AntibioPPAP”. PMT held a postdoctoral Alexander von Humboldt fellowship.
Author contributions
F.G., D.H. and A.L. designed the study. A.L., P.E., M.M., P.M.T., K.N., D.H. and F.G. designed the experiments. A.L. performed all experiments except the in vivo wound healing which was carried out by M.Z.M. and S.Y., the bioinformatic analysis was performed by S.Z. and F.G., A.L. and S.Z. wrote the manuscript.
Data availability
All relevant data are available from the authors upon request. The 16s rDNA sequences are uploaded to NCBI with accession number MT445230-MT445414. Source data underlying plots shown in figures are provided in Supplementary Data 1.
Competing interests
The authors declare no competing interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dewi Hidayati, Email: dewi_hidayati@ymail.com.
Friedrich Götz, Email: friedrich.goetz@uni-tuebingen.de.
Supplementary information
Supplementary information is available for this paper at 10.1038/s42003-020-1000-7.
References
- 1.Premont RT, Gainetdinov RR, Caron MG. Following the trace of elusive amines. Proc. Natl Acad. Sci. USA. 2001;98:9474–9475. doi: 10.1073/pnas.181356198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dewar KM, Dyck LE, Durden DA, Boulton AA. Involvement of brain trace amines in the behavioural effects of phenelzine. Neurochem. Res. 1988;13:113–119. doi: 10.1007/BF00973322. [DOI] [PubMed] [Google Scholar]
- 3.Burchett SA, Hicks TP. The mysterious trace amines: protean neuromodulators of synaptic transmission in mammalian brain. Prog. Neurobiol. 2006;79:223–246. doi: 10.1016/j.pneurobio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Branicky R, Schafer WR. Tyramine: a new receptor and a new role at the synapse. Neuron. 2009;62:458–460. doi: 10.1016/j.neuron.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Borowsky B, et al. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc. Natl Acad. Sci. USA. 2001;98:8966–8971. doi: 10.1073/pnas.151105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zucchi R, Chiellini G, Scanlan TS, Grandy DK. Trace amine-associated receptors and their ligands. Br. J. Pharmacol. 2006;149:967–978. doi: 10.1038/sj.bjp.0706948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espinoza S, et al. Functional interaction between trace amine-associated receptor 1 and dopamine D2 receptor. Mol. Pharmacol. 2011;80:416–425. doi: 10.1124/mol.111.073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luqman A, Nega M, Nguyen MT, Ebner P, Götz F. SadA-expressing staphylococci in the human gut show increased cell adherence and internalization. Cell Rep. 2018;22:535–545. doi: 10.1016/j.celrep.2017.12.058. [DOI] [PubMed] [Google Scholar]
- 9.Cornil CA, Ball GF. Interplay among catecholamine systems: dopamine binds to alpha(2)-adrenergic receptors in birds and mammals. J. Comp. Neurol. 2008;511:610–627. doi: 10.1002/cne.21861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma GY, Bavadekar SA, Schaneberg BT, Khan IA, Feller DR. Effects of synephrine and beta-phenethylamine on human alpha-adrenoceptor subtypes. Planta Med. 2010;76:981–986. doi: 10.1055/s-0029-1240884. [DOI] [PubMed] [Google Scholar]
- 11.Kleinau G, et al. Differential modulation of beta-adrenergic receptor signaling by trace amine-associated receptor 1 agonists. PLoS ONE. 2011;6:e27073. doi: 10.1371/journal.pone.0027073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben Zakour NL, Bannoehr J, van den Broek AH, Thoday KL, Fitzgerald JR. Complete genome sequence of the canine pathogen Staphylococcus pseudintermedius. J. Bacteriol. 2011;193:2363–2364. doi: 10.1128/JB.00137-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kmieciak W, Szewczyk EM. Are zoonotic Staphylococcus pseudintermedius strains a growing threat for humans? Folia Microbiol. (Praha) 2018;63:743–747. doi: 10.1007/s12223-018-0615-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maali Y, et al. Understanding the Virulence of Staphylococcus pseudintermedius: a major role of pore-forming toxins. Front Cell Infect. Microbiol. 2018;8:221. doi: 10.3389/fcimb.2018.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ricciardi BF, et al. Staphylococcus aureus evasion of host immunity in the setting of prosthetic joint infection: biofilm and beyond. Curr. Rev. Musculoskelet. Med. 2018;11:389–400. doi: 10.1007/s12178-018-9501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hornef MW, Wick MJ, Rhen M, Normark S. Bacterial strategies for overcoming host innate and adaptive immune responses. Nat. Immunol. 2002;3:1033–1040. doi: 10.1038/ni1102-1033. [DOI] [PubMed] [Google Scholar]
- 17.Josse J, Laurent F, Diot A. Staphylococcal Adhesion and host cell invasion: fibronectin-binding and other mechanisms. Front Microbiol. 2017;8:2433. doi: 10.3389/fmicb.2017.02433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dusane DH, et al. Targeting intracellular Staphylococcus aureus to lower recurrence of orthopaedic infection. J. Orthop. Res. 2018;36:1086–1092. doi: 10.1002/jor.23723. [DOI] [PubMed] [Google Scholar]
- 19.Easmon CS. The effect of antibiotics on the intracellular survival of Staphylococcus aureus in vitro. Br. J. Exp. Pathol. 1979;60:24–28. [PMC free article] [PubMed] [Google Scholar]
- 20.Luqman, A. et al. A new host cell internalisation pathway for SadA-expressing staphylococci triggered by excreted neurochemicals. Cell Microbiol. e13044, 10.1111/cmi.13044 (2019). [DOI] [PMC free article] [PubMed]
- 21.Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat. Rev. Neurosci. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schleifer KH, Krämer E. Selective medium for isolating staphylococci. Zentralbl. Bakteriol. 1980;1:270–280. [Google Scholar]
- 23.Steinkraus V, Steinfath M, Korner C, Mensing H. Binding of beta-adrenergic receptors in human skin. J. Investigative Dermatol. 1992;98:475–480. doi: 10.1111/1523-1747.ep12499860. [DOI] [PubMed] [Google Scholar]
- 24.Steinkraus V, et al. Autoradiographic mapping of beta-adrenoceptors in human skin. Arch. Dermatol. Res. 1996;288:549–553. doi: 10.1007/BF02505253. [DOI] [PubMed] [Google Scholar]
- 25.Sivamani RK, Lam ST, Isseroff RR. Beta adrenergic receptors in keratinocytes. Dermatol. Clin. 2007;25:643–653. doi: 10.1016/j.det.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gazith J, Cavey MT, Cavey D, Shroot B, Reichert U. Characterization of the beta-adrenergic receptors of cultured human epidermal keratinocytes. Biochem. Pharm. 1983;32:3397–3403. doi: 10.1016/0006-2952(83)90368-4. [DOI] [PubMed] [Google Scholar]
- 27.Steinkraus V, Korner C, Steinfath M, Mensing H. High density of beta 2-adrenoceptors in a human keratinocyte cell line with complete epidermal differentiation capacity (HaCaT) Arch. Dermatol. Res. 1991;283:328–332. doi: 10.1007/BF00376622. [DOI] [PubMed] [Google Scholar]
- 28.Stojadinovic O, Gordon KA, Lebrun E, Tomic-Canic M. Stress-induced hormones cortisol and epinephrine impair wound epithelization. Adv. Wound Care (N. Rochelle) 2012;1:29–35. doi: 10.1089/wound.2011.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pullar CE, Grahn JC, Liu W, Isseroff RR. Beta2-adrenergic receptor activation delays wound healing. FASEB J. 2006;20:76–86. doi: 10.1096/fj.05-4188com. [DOI] [PubMed] [Google Scholar]
- 30.Arnold JM, et al. Effects of the beta 2-adrenoceptor antagonist ICI 118,551 on exercise tachycardia and isoprenaline-induced beta-adrenoceptor responses in man. Br. J. Clin. Pharm. 1985;19:619–630. doi: 10.1111/j.1365-2125.1985.tb02689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bond RA, et al. Physiological effects of inverse agonists in transgenic mice with myocardial overexpression of the beta 2-adrenoceptor. Nature. 1995;374:272–276. doi: 10.1038/374272a0. [DOI] [PubMed] [Google Scholar]
- 32.Azzi M, et al. Allosteric effects of G protein overexpression on the binding of beta-adrenergic ligands with distinct inverse efficacies. Mol. Pharm. 2001;60:999–1007. doi: 10.1124/mol.60.5.999. [DOI] [PubMed] [Google Scholar]
- 33.Kim MH, et al. Catecholamine stress alters neutrophil trafficking and impairs wound healing by beta(2)-adrenergic receptor-mediated upregulation of IL-6. J. Investigative Dermatol. 2014;134:809–817. doi: 10.1038/jid.2013.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pullar CE, Manabat-Hidalgo CG, Bolaji RS, Isseroff R. R. beta-adrenergic receptor modulation of wound repair. Pharmacol. Res. 2008;58:158–164. doi: 10.1016/j.phrs.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Pullar CE, et al. beta 2AR antagonists and beta 2AR gene deletion both promote skin wound repair processes. J. Investigative Dermatol. 2012;132:2076–2084. doi: 10.1038/jid.2012.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olson MF, Sahai E. The actin cytoskeleton in cancer cell motility. Clin. Exp. Metastasis. 2009;26:273–287. doi: 10.1007/s10585-008-9174-2. [DOI] [PubMed] [Google Scholar]
- 37.Pinto MCX, et al. Calcium signaling and cell proliferation. Cell Signal. 2015;27:2139–2149. doi: 10.1016/j.cellsig.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Berridge MJ. Calcium signaling and cell-proliferation. Bioessays. 1995;17:491–500. doi: 10.1002/bies.950170605. [DOI] [PubMed] [Google Scholar]
- 39.Munaron L, Antoniotti S, Lovisolo D. Intracellular calcium signals and control of cell proliferation: how many mechanisms? J. Cell. Mol. Med. 2004;8:161–168. doi: 10.1111/j.1582-4934.2004.tb00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romana-Souza B, et al. Rotational stress-induced increase in epinephrine levels delays cutaneous wound healing in mice. Brain Behav. Immun. 2010;24:427–437. doi: 10.1016/j.bbi.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 41.Sivamani RK, et al. Stress-mediated increases in systemic and local epinephrine impair skin wound healing: potential new indication for beta blockers. PLoS Med. 2009;6:e12. doi: 10.1371/journal.pmed.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kloos WE, Musselwhite MS. Distribution and persistence of Staphylococcus and Micrococcus species and other aerobic bacteria on human skin. Appl. Microbiol. 1975;30:381–385. doi: 10.1128/am.30.3.381-395.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kloos WE, Schleifer KH. Simplified scheme for routine identification of human Staphylococcus species. J. Clin. Microbiol. 1975;1:82–88. doi: 10.1128/jcm.1.1.82-88.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kloos, W. E. in McGraw-Hill Book 1986 Yearbook of Science and Technology (ed Parker, S. P.) 431–434 (McGraw-Hill Book, 1985).
- 45.Grice EA, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324:1190–1192. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grice EA, Segre JA. The skin microbiome. Nat. Rev. Microbiol. 2011;9:244–253. doi: 10.1038/nrmicro2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gitlitz PH, Sunderman FW, Hohnadel DC. Ion-exchange chromatography of amino-acids in sweat collected from healthy subjects during sauna bathing. Clin. Chem. 1974;20:1305–1312. [PubMed] [Google Scholar]
- 48.Hadorn B, Hanimann F, Anders P, Curtius HC, Halverson R. Free amino-acids in human sweat from different parts of body. Nature. 1967;215:416–417. doi: 10.1038/215416a0. [DOI] [PubMed] [Google Scholar]
- 49.Dunstan RH, et al. Sweat facilitated amino acid losses in male athletes during exercise at 32-34 degrees C. PLoS ONE. 2016;11:e0167844. doi: 10.1371/journal.pone.0167844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dunstan RH, et al. Sex differences in amino acids lost via sweating could lead to differential susceptibilities to disturbances in nitrogen balance and collagen turnover. Amino Acids. 2017;49:1337–1345. doi: 10.1007/s00726-017-2431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hungerland H, Liappis N. Study of free amino-acids in human eccrine sweat. Klinische Wochenschr. 1972;50:973. doi: 10.1093/ajcn/25.7.661. [DOI] [PubMed] [Google Scholar]
- 52.Vukelic S, et al. Cortisol synthesis in epidermis is induced by IL-1 and tissue injury. J. Biol. Chem. 2011;286:10265–10275. doi: 10.1074/jbc.M110.188268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cirillo N, Prime SS. Keratinocytes synthesize and activate cortisol. J. Cell Biochem. 2011;112:1499–1505. doi: 10.1002/jcb.23081. [DOI] [PubMed] [Google Scholar]
- 54.Steenhuis P, et al. Adrenergic signaling in human oral keratinocytes and wound repair. J. Dent. Res. 2011;90:186–192. doi: 10.1177/0022034510388034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen J, Hoffman BB, Isseroff RR. Beta-adrenergic receptor activation inhibits keratinocyte migration via a cyclic adenosine monophosphate-independent mechanism. J. Invest. Dermatol. 2002;119:1261–1268. doi: 10.1046/j.1523-1747.2002.19611.x. [DOI] [PubMed] [Google Scholar]
- 56.Pullar CE, Rizzo A, Isseroff R. R. beta-Adrenergic receptor antagonists accelerate skin wound healing: evidence for a catecholamine synthesis network in the epidermis. J. Biol. Chem. 2006;281:21225–21235. doi: 10.1074/jbc.M601007200. [DOI] [PubMed] [Google Scholar]
- 57.Donaldson DJ, Mahan JT. Influence of catecholamines on epidermal cell migration during wound closure in adult newts. Comp. Biochem. Physiol. C. 1984;78:267–270. doi: 10.1016/0742-8413(84)90081-1. [DOI] [PubMed] [Google Scholar]
- 58.Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 59.Johnson M. Molecular mechanisms of beta(2)-adrenergic receptor function, response, and regulation. J. Allergy Clin. Immunol. 2006;117:18–24. doi: 10.1016/j.jaci.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 60.Liu Y, Yu X, Zhuang J. Epinephrine stimulates cell proliferation and induces chemoresistance in myeloma cells through the beta-adrenoreceptor in vitro. Acta Haematol. 2017;138:103–110. doi: 10.1159/000478517. [DOI] [PubMed] [Google Scholar]
- 61.Kenny AD. Effect of catecholamines on serum calcium and phosphorus levels in intact and parathyroidectomized rats. Naunyn Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1964;248:144–152. doi: 10.1007/BF00246669. [DOI] [PubMed] [Google Scholar]
- 62.Cogen AL, Nizet V, Gallo RL. Skin microbiota: a source of disease or defence? Br. J. Dermatol. 2008;158:442–455. doi: 10.1111/j.1365-2133.2008.08437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakatsuji T, et al. A commensal strain of Staphylococcus epidermidis protects against skin neoplasia. Sci. Adv. 2018;4:eaao4502. doi: 10.1126/sciadv.aao4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Geiger T, et al. The stringent response of Staphylococcus aureus and its impact on survival after phagocytosis through the induction of intracellular PSMs expression. Plos Pathog. 2012;8:e1003016. doi: 10.1371/journal.ppat.1003016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bae T, Schneewind O. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid. 2006;55:58–63. doi: 10.1016/j.plasmid.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 66.Jonkman JE, et al. An introduction to the wound healing assay using live-cell microscopy. Cell Adh. Migr. 2014;8:440–451. doi: 10.4161/cam.36224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bigliardi PL, Neumann C, Teo YL, Pant A, Bigliardi-Qi M. Activation of the delta-opioid receptor promotes cutaneous wound healing by affecting keratinocyte intercellular adhesion and migration. Br. J. Pharm. 2015;172:501–514. doi: 10.1111/bph.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ganguli-Indra G. Protocol for cutaneous wound healing assay in a murine model. Methods Mol. Biol. 2014;1210:151–159. doi: 10.1007/978-1-4939-1435-7_12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All relevant data are available from the authors upon request. The 16s rDNA sequences are uploaded to NCBI with accession number MT445230-MT445414. Source data underlying plots shown in figures are provided in Supplementary Data 1.