Abstract
Background
About 2 million adults in Germany harbor an unruptured intracranial aneurysm (IA). Rupture can lead to a life-threatening subarachnoid hemorrhage. If an IA is detected incidentally in cranial imaging, it must be decided how to proceed.
Methods
This review includes key publications that were identified by a selective search in the PubMed database using the search term “unruptured intracranial aneurysms,” which was performed in July 2019, and based on information obtained from the German Federal Statistical Office on the frequency of the hospital discharge diagnosis “cerebral aneurysm,” excluding the diagnosis “subarachnoid hemorrhage,” in Germany from 2005 to 2017.
Results
The number of patients in Germany who were admitted or treated for an unruptured IA increased by a factor of 2.3 from 2005 to 2017. The average 5-year rupture risk of approximately 3% must be weighed against the approximately 4% risk associated with an endovascular or microneurosurgical treatment. This emphasizes the need for more precise data on the risk of rupture and for algorithms enabling individualized decision-making for patients with unruptured IA. Risk factors such as IA morphology, arterial hypertension, active smoking, and alcohol consumption (>150 g/week) can markedly increase the risk of rupture, which is generally relatively low. Growing aneurysms are 12 times more likely to rupture than stable ones. Follow-up imaging is thus essential whenever observation rather than intervention is chosen as the initial management.
Conclusion
Patients with unruptured IA should be massessed and managed individually. It is also important that risk factors should be treated, if present. Eligible patients are currently being recruited for a phase III clinical trial on the efficacy of blood pressure reduction combined with acetylsalicylic acid intake to counteract inflammatory processes in the arterial wall.
Unruptured intracranial aneurysms (IA), as we refer to them in this review, are acquired, focal, saccular outpouchings of the arterial wall that are typically found at branch points. Their prevalence among adults in Central European countries is estimated at 3.2% (95% confidence interval [1.9; 5.2]); this implies that some 2 million persons in Germany are affected (1).
This review does not concern IA in children or the various types of cerebral aneurysm that are not saccular, including mycotic (infectious), fusiform, and dissecting aneurysms, and aneurysms associated with connective-tissue diseases. These are disease entities in themselves, each with its own pathogenesis, clinical course, and favored mode of treatment, and they will not be discussed any further here.
Unruptured IA can remain asymptomatic for many years. They can also cause symptoms by by local compression of cranial nerves or rupture, leading to a life-threatening subarachnoid hemorrhage (SAH). Meta-analyses of population-based studies have shown that the incidence of aneurysmal SAH is “only” 6 per 100 000 persons per year, but the fatality of aneurysmal SAH in Europe remains 35% (2, 3). Only one-third of survivors can return to normal life (2). As aneurysmal SAH tends to affect relatively young patients, with a peak incidence between the ages of 50 and 60, it causes an overall loss of quality-adjusted life years comparable to that caused by ischemic stroke (4).
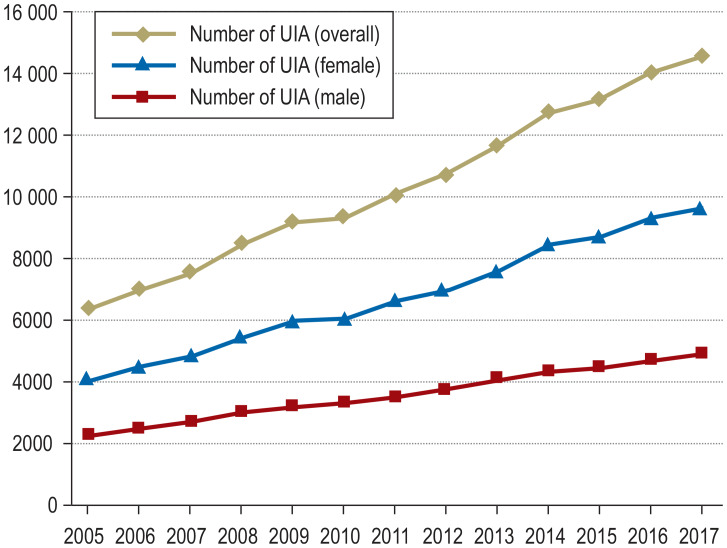
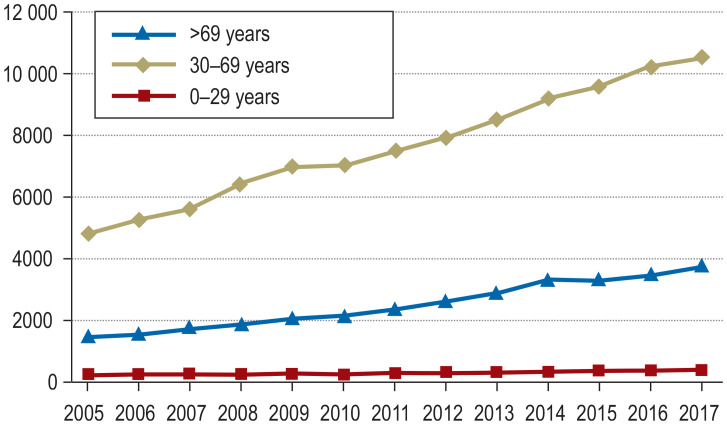
Unruptured IA are being detected more frequently because of the widespread use and increasing sensitivity of imaging modalities such as magnetic resonance imaging (MRI) and computed tomography (CT). The controversy regarding the appropriate management once an unruptured IA is detected is driven by uncertainty regarding the risks of rupture and of treatment, as well as differences in “mentality” among patients or treating physicians (5, 6). The number of patients in Germany who were admitted or treated for an unruptured IA increased by a factor of 2.3 from 2005 to 2017, with a large proportion of elderly persons (>69 years) among them (Figures 1a and b).
Figure 1.
Frequency of the hospital discharge diagnosis “incidental aneurysm” in Germany, 2005–2017.
a) Overall and sex-specific distribution of the diagnosis-related groups (DRG) I67.* (cerebral aneurysm) excluding I60.* (subarachnoid hemorrhage) from 2005 to 2017. UIA, unruptured intracranial aneurysm.
b) Age distribution of the DRG I67.* I67.* (cerebral aneurysm) excluding I60.* (subarachnoid hemorrhage) from 2005 to 2017 (source: German Federal Statistical Office, DRG statistics 2019, www.destatis.de). The chosen treatment is unknown.
This review includes key publications that were identified by a selective search in the PubMed database using the search term “unruptured intracranial aneurysms,” which was performed in July 2019, without any restriction on language or date of publication, and based on data provided by the German Federal Statistical Office on the frequency of the hospital discharge diagnosis “cerebral aneurysm” excluding the diagnosis “subarachnoid hemorrhage” in Germany from 2005 to 2017. In this review, we aim to show how rational decisions can be made in the management of unruptured IA that are based on the scientific understanding of this condition, which has improved markedly in recent years.
Formation of intracranial aneurysms
Intracranial aneurysms are a complex condition that originates from a variety of risk factors—genetic and acquired, known and unknown—which presumably interact with one another. Intracranial aneurysms are associated with connective tissue diseases such as Marfan or Ehlers-Danlos syndrome, autosomal dominant polycystic kidney disease, and congenital cardiovascular malformations such as aortic coarctation or a biscuspid aortic valve, but not with extracranial (e.g., aortic) aneurysms (7, 8).
Familial preponderance seems to point toward a genetic predisposition, even if the genome-wide studies and meta-analyses performed to date have revealed no more than weak associations of sporadic IA with particular genetic loci. The latter appear mainly to be responsible for basic mechanisms of endothelial repair and maintenance of vascular wall structure (9). It also remains unclear whether the observed familial preponderance of IA (table) truly represents a predisposition to aneurysms as such, or rather a hereditary tendency toward the development of, or vulnerability to, the major risk factors that promote aneurysm formation, i.e. hypertension and cigarette smoking. A recently published Finnish study revealed that these two risk factors are themselves highly prevalent among children born to two parents with sporadic or familial IA, with a high concordance rate in monozygotic twins born to such parents (10).
Table. Overview of all patient– and aneurysm-related risk factors for aneurysm rupture.
| Risk factor | Change in rupture risk (95% CI) | Evidence level | Geographic region*2 |
| Patient-related | |||
| Modifiable | |||
| Arterial hypertension | HR 1.4 [1.1; 1.8] | IIa | Europe (incl. Finland), Japan, North America (15) |
| HR 1.3 [0.9; 1.9] | IIa | Japan (30) | |
| HR 7.9 [1.3; 47.4] | IIb | Japan (UIA size <5 mm) (31) | |
| Smoking (active) | RR 2.2 [1.3; 3.6] | IIa | Europe (incl. Finland), Asia (incl. Japan), North America (32) |
| HR 3.2 [1.3; 7.6] | IIb | Finland (33) | |
| Alcohol (>150 g/week) | RR 2.2 [1.5; 2.8] | IIa | Europe (incl. Finland), Asia (incl. Japan), North America (32) |
| Not modifiable | |||
| Age (≥ 70 years) | HR 1.44 [1.05; 1.97] | IIa | Europe (incl. Finland), Japan, North America (15) |
| Age (<50 years) | HR 5.23 [1.03; 26.52] | IIb | Japan (UIA size <5 mm) (31) |
| Age (per 10 years) | HR 0.62 [0.39; 0.99] | IIb | Finland (33) |
| Geographic region | IIa | Europa (inkl. Finnland), Japan, North America (15) | |
| USA, Europe except Finland | Reference | ||
| Japan | HR 2.8 [1.8; 4.2] | ||
| Finland | HR 3.6 [2.0; 6.3] | ||
| Prior subarachnoid hemorrhage from another aneurysm | HR 1.4 [0.9; 2.2] | IIa | Europe (incl. Finland), Japan, North America (15) |
| Female sex | RR 1.6 [1.1; 2.4] | IIa | Europe (except Finland) (34) |
| Multiple aneurysms | HR 4.9 [1.6; 14.7] | IIb | Japan (UIA size <5 mm) (31) |
| Family history (≥ 2 first-degree relatives with UIA or SAH) | 17-fold elevation | IIb | North America (35) |
| Aneurysm-related | |||
| Size | IIa | Europe (incl. Finland), Japan, North America (15) | |
| <5.0 mm | Reference | ||
| 5.0–6.9 mm | HR 1.1 [0.7; 1.7] | ||
| 7.0–9.9 mm | HR 2.4 [1.6; 3.6] | ||
| 10.0–19.9 mm | HR 5.7 [3.9; 8.3] | ||
| ≥ 20.0 mm | HR 21.3 [13.5; 33.8] | ||
| Localization | IIa | Europe (incl. Finland), Japan, North America (15) | |
| ICA | HR 0.5 [0.3; 0.9] | ||
| MCA | Reference | ||
| ACA incl. AComA | HR 1.7 [0.7; 2.6] | ||
| PCA | HR 1.9 [1.2; 2.9] | ||
| PComA | HR 2.1 [1.4; 3.0] | ||
| Irregular aneurysm shape/lobulation | HR 1.5 [1.0; 2.2] | IIa | Japan (17, 30) |
| OR 4.8 [2.7; 8.7] | IIb | Europe (36) | |
| Aneurysm growth >1 mm on serial scans | 12-fold | IIIb | USA (19) |
| Gadolinium uptake in the aneurysm wall | HR 9.2 [2.9; 29.0]*1 | IIIb | Europe (37) |
*1 This value relates to aneurysm instability, i.e., either growth or rupture of the aneurysm.
*2 The significant differences in the risk of rupture depending on geographic region (North America and Europe excluding Finland versus Finland versus Japan) have the consequence that data from Japan or FInland, for example, are not necessarily applicable to patients in Germany. Multiple studies are listed here to demonstrate the variability of risk depending on geographic region.
ACA incl. AComA, anterior cerebral arteries incl. anterior communicating artery; CI, confidence interval; HR, hazard ratio; ICA, internal carotid artery; MCA, middle cerebral artery;
OR, odds ratio; PCA, posterior cerebral arter“; PComA, posterior communicating artery; RR, relative risk; SAH, subarachnoid hemorrhage; UIA, unruptured intracranial aneurysm
Intracranial aneurysms form at branching arteries, in relation to the branching angle and from the presence of anatomical variants such as hypoplasia or fenestration (11). Such congenital changes probably predispose to the subsequent development of the IA; presumably, an aneurysm will or will notform subsequently depending on the presence of further cardiovascular risk factors, local hemodynamic factors, and other inducers of endothelial damage, such as non-laminar flow, the increased effect of pulsatile pressure in arterial hypertension, and increased shear stress due to non-physiological flow profiles at bifurcations. The combined effect of such risk factors may cause a disruption the internal elastic membrane, a structure that physiologically maintains the integrity of the vascular wall. This disruption are a key event in the pathogenesis of IA (7).
Following disruption of the internal elastic lamina and in the presence of the risk factors detailed above, the vascular wall distenses on a microscopic level. Local endothelial dysfunction, changes in smooth muscle cells, apoptosis, inflammation, and disturbances in the extracellular matrix ultimately lead to vascular remodeling and to the macroscopic outpouching that constitutes an IA (7).
Clinical presentation and radiological diagnosis
In the authors’ clinical experience, most unruptured IA are diagnosed incidentally. Chronic headache and dizziness are the most common symptoms leading to the MRI and CT scans on which IA are incidentally found (5). Less common clinical correlates of larger unruptured IA, in particular, include neurological deficits due to local mass effect, e.g. palsies of the cranial nerves supplying the ocular muscles (ptosis, mydriasis, or diplopia). IA that cause such compressive deficits over a very short period of time (days or weeks) are presumably rapidly enlarging in size and are therefore at markedly higher risk of rupture than unruptured IA overall. They require urgent treatment.
MR angiography (MRA) has a sensitivity of 95% [89; 98] for the detection of an IA, with a specificity of 89% [80; 95]; the corresponding figures for CT angiography (CTA) are 95% [93; 96] and 96% [93; 98], respectively—always in comparison to intra-arterial digital subtraction angiography (DSA) as the gold standard (12, 13). The non-invasive angiographic techniques are much less sensitive for the detection of aneurysms with diameter less than 3 mm: the relevant sensitivity figures are 61% [51; 70] for CTA, and 38% [25; 53] for MRA (14). Intra-arterial DSA, with its high resolution, therefore remains the gold standard for the precise assessment of IA, yet the indication for it should always be viewed critically, as it involves not only invasive access to the vascular system, but also a burden of ionizing radiation.
For follow-up imaging of IA, non-invasive modalities are preferred, above all MRA.
Risk of aneurysm rupture
The estimation of the individidual risk of aneurysm rupture is a very controversial topic in neurovascular medicine. This is partly due to the heterogeneity of the available studies (cohort and case-control studies) and partly because of patient selection due to preventive IA repair outside of the studies. The geographical disparity of the studies further complicates the analysis.
The largest meta-analysis for the estimation of the individual risk of rupture is based on six prospective cohort studies, among them the large-scale International Study of Unruptured Intracranial Aneurysms (ISUIA) and the Unruptured Cerebral Aneurysms Study (UCAS Japan). The meta-analysis includes data from a total of 8382 patients with 10 272 aneurysms who were followed for 29 166 patient-years (15– 17).
Six independent risk factors for IA rupture were identified:
aneurysm size
aneurysm location
prior rupture of another aneurysm
age
arterial hypertension
geographic origin
The PHASES scoring system was developed on the basis of these six factors to estimate the risk of rupture of an unruptured IA in the next five years. The estimated risk can range from 0.3% to 17.8% (mean 3.4%); some of the confidence intervals are wide. Because of incomplete data collection in the underlying studies, the PHASES score takes no account of further relevant factors such as smoking, irregular aneurysm shape, family history of IA or subarachnoid hemorrhage, or the quality of risk factor treatment. All of these factors are thought to lead to a higher (additive) risk of rupture (7).
The risk of rupture differs markedly depending on geographical location (North America and Europe other than Finland, vs. Finland, vs. Japan). Thus, for example, data from Japan or Finland cannot be assumed to apply to patients in Germany (15).
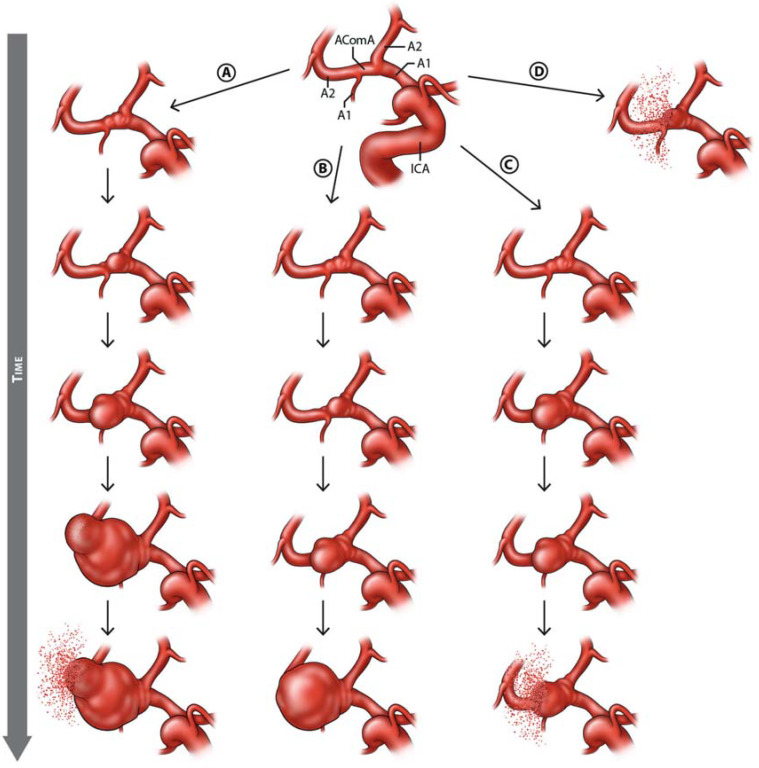
Further risk factors that can be considered when estimating the risk of rupture, which were discussed in other population-based or case-control studies, are summarized in the table. The formation and potential progression of IA are depicted in Figure 2.
Figure 2.
The formation and potential progression over time of an unruptured intracranial aneurysm, illustrated by the example of an aneurysm of the anterior communicating artery (AcomA) complex in the setting of unilateral hypoplasia of the A1 segment:
a) formation and progressive growth leading to rupture b) formation and progressive growth, without rupture during the period of observation c) formation and rupture without any growth in the period before the rupture d) rupture of a recently formed, very small de novo aneurysm (modified from Etminan, Rinkel [7])
The growth of intracranial aneurysms
Documented growth of an unruptured aneurysm, if present, has no effect on the PHASES score despite being highly relevant to the patient’s further course. The risk factors for aneurysm growth are similar to those for de novo aneurysm formation: above all, cigarette smoking and arterial hypertension. A meta-analysis reported that 9% of unruptured IA enlarged in size over a mean period of observation of 2.8 years (18). The long-term risk of aneurysm growth was estimated at 45% in 19 years. The risk of rupture of a growing aneurysm is larger by a factor of 12 (7, 19). Aneurysm growth is thus a highly important risk factor that must strongly influence clinical decision-making in favor of preventive aneurysm repair.
The largest analysis of the individual risk of growth of unruptured IA is based on ten prospective cohort studies with data from 1507 patients who had 1909 aneurysms and were observed for 5782 patient-years (20). The following independent risk factors for growth were identified:
prior rupture of another aneurysm
aneurysm location
age
geographical origin
aneurysm size
aneurysm shape
The ELAPPS score, based on these six easily ascertained variables, provides an estimate of the risk of aneurysm growth in the next three or five years (20).
The risk of aneurysm treatment
The available methods of treating an IA to prevent rupture include microsurgical clipping and the endovascular treatment options—above all, coil embolization, but also newer techniques including balloon-assisted coiling, stent-supported coiling, and the use of flow diverters. In general, microsurgical treatment is considered more invasive, while the endovascular techniques carry a higher risk of aneurysm recurrence.
To date, there have been many retrospective, single-center studies and industry-sponsored registry studies, but only a single independent, randomized and controlled trial on the outcomes of treatment, including the treatment-related risk, in patients with unruptured IA. This trial, the Canadian Unruptured Endovascular versus Surgery (CURES) trial, is still recruiting patients; preliminary findings were published in 2017 (21). Patients with unruptured IA were randomly allotted to either clipping or endovascular treatment (any kind). The primary composite endpoint was treatment failure, which was defined as aneurysm rupture, inability to treat with the assigned method, or a radiologically demonstrated aneurysm remnant within one year. The main secondary endpoints were neurological morbidity (Modified Rankin Scale >2) and mortality at one year, new neurologic deficits 30 days after treatment, and hospitalization for more than five days. The primary endpoint was reached by 5 of 48 patients in the microsurgical arm and by 10 of 56 patients in the endovascular arm (odds ratio [OR] 0.54 [0.13; 1.9]). New neurologic deficits were more common in the surgical arm (OR 3.12 [1.05; 10.57]), as was hospitalization for more than five days (OR 8.85 [3.22; 28.59]). The two modalities did not differ significantly in combined morbidity and mortality at one year (3.6% for coiling vs. 4.2% for clipping Thus, there is currently no robust, evidence-based answer to the everlasting question “clipping or coiling?” in the setting of unruptured IA
Data on the risk of treating unruptured IA and its determinants were recently reported in a large-scale meta-analysis of 114 non-randomized studies, with data from 106 433 patients who had 108 263 IA (22). A complication was defined as any neurologic deficit after treatment. In 74 studies on endovascular treatment, the complication rate, thus defined, was 4.96% [4.00; 6.12], with 0.30% mortality [0.20; 0.40]. The corresponding figures derived from the 54 studies on microsurgical treatment were 8.34 % [6.25; 11.10] and 0.10% [0.00; 0.20], respectively. It must be pointed out as a limitation of this analysis that many of the included studies were performed in single centers or were retrospective, and that the data do not permit any direct comparison of the microsurgical and endo-vascular treatment methods.
The question which of the two “active” treatment methods for unruptured IA is less risky and more effective will thus remain open at least until the conclusion of the CURES trial. This trial will not be able to account for further, poorly quantifiable factors that may vary locally, such as the individual experience of the treating personnel. Nor does the CURES trial at all address the issue of the indications for treating an unruptured aneurysm, which is prior to any decision on the particular method of “active” treatment.
Observation or preventive aneurysm treatment?
For every patient with an unruptured IA, this question should be dealt with individually and discussed by an interdisciplinary, specialized cerebrovascular team. In specific cases, it may be challenging to discuss this on the basis of the currently available evidence on the risk of rupture versus the risk of a complication of treatment. In the past, such decisions were generally based on threshold values for preventive aneurysm treatment; these have largely been abandoned in recent years in favor of the newly developed rating systems that enable more objective and individualized counseling.
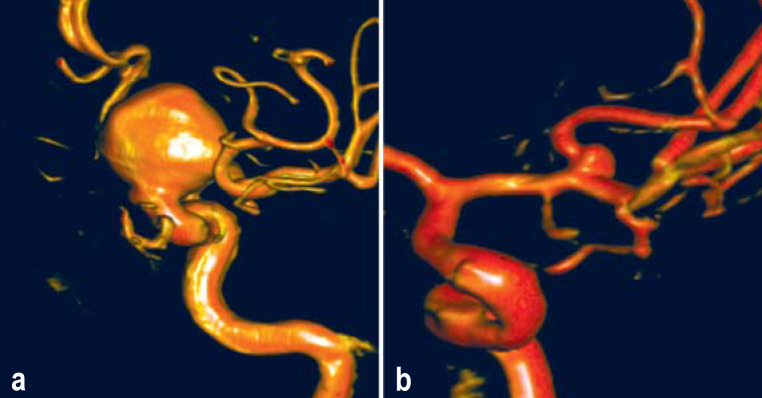
The PHASES score enables estimation of the individual 5-year risk that an unruptured IA will rupture, on the basis of a small number of risk factors; however, the estimated risk of treatment is not included in PHASES. In contrast, the Unruptured Intracranial Aneurysm Treatment Score (UIATS) enables complementary consideration of the risks of both rupture and treatment complications, while also taking psychological aspects, such as the fear of aneurysm rupture, into account (case illustrations in Figure 3). The UIATS is based on a consensus project of 39 aneurysm specialists representing multiple clinical disciplines and subsequent validation by 30 further aneurysm specialists (23).
Figure 3.
Case illustrations and complementary use of the PHASES score and the UIATS (Unruptured Intracranial Aneurysms Treatment Score)
a) Three-dimensional reconstruction of the digital subtraction angiogram of a 43-year-old man with an incidentally discovered aneurysm of the left internal carotid artery and a further incidentally discovered aneurysm of the right internal carotid artery (4 mm in diameter, not shown); he had undergone cerebral imaging because of headache. Risk factors: smoking and positive family history for intracranial aneurysms and subarachnoid hemorrhage. Aneurysm size 20.1 mm, irregular shape, aspect ratio >1.6. These data yield a PHASES score of 10, corresponding to a 5-year rupture risk of 5.3%. The UIATS supports preventive over conservative treatment (17 points to 14). The three-point difference in UIATS implies a clear recommendation for the preventive treatment of the aneurysm.
b) Three-dimensional reconstruction of the digital subtraction angiogram of a 64-year-old woman with an incidentally discovered aneurysm of the right middle cerebral artery; she had undergone cerebral imaging because of paranoid schizophrenia. Risk factor: arterial hypertension. Aneurysm size 2.5 mm, regular shape. PHASES score: 3 points, corresponding to a 5-year rupture risk of 0.7%. The UIATS support conservative over preventive treatment (10 points to 4). The six-point difference in UIATS implies a clear recommendation for the conservative treatment of the aneurysm.
Once a decision has been made to treat an unruptured IA preventively, the safest and most effective modality should be discussed by the interdisciplinary team. The currently available evidence from non-randomized studies suggests that simple coiling is generally preferable to microsurgical clipping. The main exceptions to this statement are unruptured IA of the middle cerebral artery or unruptured IA in patients under age 40 (the latter because clipping is associated with a lower rate of aneurysm recurrence). Older age and pre-treatment comorbidities increase the perioperative risk and tend to make endovascular coiling the better option. On the other hand, particularly for morphologically complex unruptured IA or those with a broad base, clipping is preferred, because of the higher rates of complete occlusion and the more durable results. Finally, for IA of the posterior circulation, endovascular treatment carries a distinctly lower risk than surgery and is preferred. A “center effect” has been shown to date only for ruptured IA, but the decision on the treatment modality should always take the individual expertise of the treating specialists and of their institution into account (24).
If a choice has been made to observe the aneurysm rather than treat it immediately, the ELAPSS score can be used to estimate the probability that it will grow in the next three or five years; this may be helpful in determining the interval for serial follow-up imaging (20). Further elements of the counseling of patients with unruptured IA are found in the Box.
BOX. The dos und don’ts of counseling patients with unruptured aneurysms.
Inform the patient of the risk of rupture, the risk factors for rupture, and the fact that the short-term risk of rupture is low (7).
Motivate the patient to abstain from smoking, and counsel about additional help for smoking reduction and cessation (6).
Treat arterial hypertension (6).
Consult a specialized neurovascular center with an interdisciplinary treatment team.
Consider the possibility of inclusion in the PROTECT-U trial, in case the aneurysm is judged not to require treatment (29).
Don’ts
Avoid emotional formulations such as “a time-bomb in your head” (7).
Avoid assessing risk on the basis of any single criterion, such as the diameter or location of the aneurysm (6).
Do not advise any restriction on exercise, sports, sexual intercourse, pregnancy if desired, or air travel (6, 38, 39).
Do not recommend the screening of family members unless two or more of the patient’s first-degree relatives are known to be affected (6).
Conservative treatment to lower the risk of rupture
The risk of rupture of an unruptured IA is relatively low, provided that there are no additive risk constellations beyond the six factors considered in the PHASES score (table): in the PHASES cohort, the average risk was about 3% in five years, and thus much lower than the risk associated with preventive treatment of any modality. Such patients should generally be observed at first, with MR angiography as the method of choice. We recommend performing follow-up MRA at intervals of 6–12 months initially, which can be gradually increased to three-year intervals if the aneurysm does not grow in size. Aside from following the IA with serial scans, it is important to treat the risk factors for rupture: above all, high blood pressure and smoking.
The importance of treating risk factors for rupture is underscored by a recent meta-analysis of data from more than 8000 persons in 23 countries, showing that the incidence of aneurysmal SAH decreased by approximately 40% around the world from 1980 to 2010 in parallel with systolic blood pressure and the prevalence of smoking (3).
Moreover, the use of drugs for the preventive treatment of inflammation in the aneurysm wall has been gaining increasing scientific interest as a novel therapeutic target for the reduction of the risk of rupture. A number of experimental and clinical studies indicate that acetylsalicylic acid (ASA) in particular may have a protective on aneurysms because of its anti-inflammatory properties (25, 26). In a cohort study, 1691 persons with IA who were being treated with ASA for other reasons sustained an aneurysm rupture much less commonly than persons in the group not taking ASA (OR 0.27 [0.11; 0.67]) (25). The hypothesis is further supported by population-based studies on more than 200 000 persons with IA who took low-dose ASA over the long term: in the overall cohort, ASA use was associated with a more than 20% reduction of the incidence of aneurysmal bleeding (27). Importantly, prior ASA treatment in patients with subarachnoid hemorrhage is not associated with more severe hemorrhages or poorer outcome (28).
Future perspectives
These two elements of the conservative treatment of patients with unruptured IA (blood-pressure reduction, ASA) need further scientific validation. They are now being studied in a prospective, randomized, non-commercial phase III trial titled PROTECT-U, which is being carried out in Germany, the Netherlands, Canada, and Finland (www.protect-u-trial.com, clinicaltrials.gov: NCT03063541) (etable) (29).
eTable. Key features of the PROTECT-U trial.
| Sponsor and Principal Clinical Investigator Germany (PCI): | Universität Heidelberg PCI: Prof. Dr. med. Nima Etminan, Klinik für Neurochirurgie, Universitätsklinikum Mannheim, Ruprecht-Karls-Universität Heidelberg, Theodor-Kutzer-Ufer 1–3, D-68167 Mannheim, Germany Tel: +49-(0)621–3832360. E-Mail: nima.etminan@umm.de |
| Objective of trial | To determine whether intensified blood pressure normalization combined with ASA lowers the risk of aneurysm rupture or of aneurysm growth (an established surrogate for elevated risk of rupture) compared to standard blood-pressure normalization alone. |
| Type of trial | Multi-center, randomized, controlled phase III trial with PROBE design (prospective, randomized, ‧open-label trial with blinded outcome assessment) |
| Intervention(s) | Experimental intervention: intensified blood pressure normalization (target: <120 mm hg) plus 100 mg asa daily Control intervention: standard blood pressure normalization (target: <140 mm hg) Follow-up observation per patient: at least three years under treatment Duration of intervention per patient: all patients continue to receive the intervention for three years after inclusion of the last patient, or until a primary endpoint is reached |
| Inclusion and exclusion criteria | Main inclusion criterion: patients with intradural, saccular, unruptured aneurysms that are judged not to require primary preventive treatment with surgery or an endovascular intervention. Main exclusion criterion: patients who 1) are already taking ASA daily, 2) have a contraindication to ASA intake, including pregnancy, or 3) suffer from severe chronic renal failure |
| Endpoints | Primary endpoint: aneurysm rupture or growth (increase of diameter in any axis by ≥ 1 mm) on serial imaging (2 magnetic resonance or computed tomography studies after ≥ 3 years) Secondary endpoints: –changes in absolute aneurysm volume (automatized analyses) or aneurysm shape (development of outpouchings) –development of de novo aneurysms, as seen on serial images –neurosurgical or endovascular aneurysm treatment during follow-up –cardiovascular events (any ischemic or hemorrhagic stroke, heart attack, or death of any vascular cause) –spontaneous extracranial hemorrhages requiring hospitalization –death of any other cause –safety: adverse or severe adverse events |
Key messages.
Intracranial aneurysms form through an interaction of genetic predisposing factors and vascular risk factors, particularly high blood pressure and smoking. These vascular risk factors are also highly important risk factors for the growth and rupture of intracranial aneurysms, and they are treatable.
The evidence-based PHASES score permits an individualized estimate of the risk of rupture of an incidentally discovered intracranial aneurysm. It is calculated from easily ascertained factors: the size and location of the aneurysm, whether there was a prior rupture of another aneurysm, the patient’s age, and arterial hypertension.
The UIATS, which is based on a consensus among experts, provides assistance with treatment decisions by balancing the risk of rupture against the risk associated with treatment, under additional consideration of the patient’s life expectancy and comorbid diseases.
For most incidentally discovered unruptured aneurysms, the five-year risk of rupture (median, ca. 3%) is lower than the risk associated with prophylactic treatment.
If the initial management decision is against surgical or endovascular treatment, conservative risk-reducing treatment should be provided, and the size of the aneurysm should be periodically rechecked on serial radiological follow-up examinations, optimally with MR angiography. The ELAPSS score provides guidance for the timing of the interval between follow-up scans.
Acknowledgments
Transmitted from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Prof. Etminan has received third-party funding from the Dr. Rolf M. Schwiete Foundation (Mannheim, Germany) for carrying out the PROTECT-U trial.
Prof. Dörfler has received lecture honoraria from Medtronic, Microvention, Penumbra, und Batt.
Prof. Steinmetz states that he has no conflict of interest.
References
- 1.Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 2011;10:626–636. doi: 10.1016/S1474-4422(11)70109-0. [DOI] [PubMed] [Google Scholar]
- 2.Rinkel GJE, Algra A. Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2011;10:349–356. doi: 10.1016/S1474-4422(11)70017-5. [DOI] [PubMed] [Google Scholar]
- 3.Etminan N, Chang HS, Hackenberg K, et al. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and meta-analysis. JAMA Neurol. 2019;76:588–597. doi: 10.1001/jamaneurol.2019.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston SC, Selvin S, Gress DR. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology. 1998;50:1413–1418. doi: 10.1212/wnl.50.5.1413. [DOI] [PubMed] [Google Scholar]
- 5.Gabriel RA, Kim H, Sidney S, et al. Ten-year detection rate of brain arteriovenous malformations in a large, multiethnic, defined population. Stroke. 2010;41:21–26. doi: 10.1161/STROKEAHA.109.566018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Etminan N, Beseoglu K, Barrow DL, et al. Multidisciplinary consensus on assessment of unruptured intracranial aneurysms: proposal of an international research group. Stroke. 2014;45:1523–1530. doi: 10.1161/STROKEAHA.114.004519. [DOI] [PubMed] [Google Scholar]
- 7.Etminan N, Rinkel GJ. Unruptured intracranial aneurysms: development, rupture and preventive management. Nat Rev Neurol. 2016;12:699–713. doi: 10.1038/nrneurol.2016.150. [DOI] [PubMed] [Google Scholar]
- 8.Kurtelius A, Vantti N, Rezai Jahromi B, et al. Association of intracranial aneurysms with aortic aneurysms in 125 patients with fusiform and 4253 patients with saccular intracranial aneurysms and their family members and population controls. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.013277. e013277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alg VS, Sofat R, Houlden H, Werring DJ. Genetic risk factors for intracranial aneurysms: ameta-analysis in more than 116,000 individuals. Neurology. 2013;80:2154–2165. doi: 10.1212/WNL.0b013e318295d751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurtelius A, Kurki MI, von und zu Fraunberg M, et al. Saccular intracranial aneurysms in children when both parents are sporadic or familial carriers of saccular intracranial aneurysms. Neuroepidemiology. 2019;52:47–54. doi: 10.1159/000493856. [DOI] [PubMed] [Google Scholar]
- 11.Bor ASE, Velthuis BK, Majoie CB, Rinkel GJE. Configuration of intracranial arteries and development of aneurysms: a follow-up study. Neurology. 2008;70:700–705. doi: 10.1212/01.wnl.0000302176.03551.35. [DOI] [PubMed] [Google Scholar]
- 12.Sailer AM, Wagemans BA, Nelemans PJ, de Graaf R, van Zwam WH. Diagnosing intracranial aneurysms with MR angiography: systematic review and meta-analysis. Stroke. 2014;45:119–126. doi: 10.1161/STROKEAHA.113.003133. [DOI] [PubMed] [Google Scholar]
- 13.Menke J, Larsen J, Kallenberg K. Diagnosing cerebral aneurysms by computed tomographic angiography: meta-analysis. Ann Neurol. 2011;69:646–654. doi: 10.1002/ana.22270. [DOI] [PubMed] [Google Scholar]
- 14.White PM, Wardlaw JM, Easton V. Can noninvasive imaging accurately depict intracranial aneurysms? A systematic review. Radiology. 2000;217:361–370. doi: 10.1148/radiology.217.2.r00nv06361. [DOI] [PubMed] [Google Scholar]
- 15.Greving JP, Wermer MJ, Brown RD Jr., et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol. 2014;13:59–66. doi: 10.1016/S1474-4422(13)70263-1. [DOI] [PubMed] [Google Scholar]
- 16.Wiebers DO, Whisnant JP, Huston J 3rd, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–110. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 17.Morita A, Kirino T, Hashi K, et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012;366:2474–2482. doi: 10.1056/NEJMoa1113260. [DOI] [PubMed] [Google Scholar]
- 18.Backes D, Rinkel GJ, Laban KG, Algra A, Vergouwen MD. Patient- and aneurysm-specific risk factors for intracranial aneurysm growth: a systematic review and meta-analysis. Stroke. 2016;47:951–957. doi: 10.1161/STROKEAHA.115.012162. [DOI] [PubMed] [Google Scholar]
- 19.Villablanca JP, Duckwiler GR, Jahan R, et al. Natural history of asymptomatic unruptured cerebral aneurysms evaluated at CT angiography: growth and rupture incidence and correlation with epidemiologic risk factors. Radiology. 2013;269:258–265. doi: 10.1148/radiol.13121188. [DOI] [PubMed] [Google Scholar]
- 20.Backes D, Rinkel GJE, Greving JP, et al. ELAPSS score for prediction of risk of growth of unruptured intracranial aneurysms. Neurology. 2017;88:1600–1606. doi: 10.1212/WNL.0000000000003865. [DOI] [PubMed] [Google Scholar]
- 21.Darsaut TE, Findlay JM, Magro E, et al. Surgical clipping or endovascular coiling for unruptured intracranial aneurysms: a pragmatic randomised trial. J Neurol Neurosurg Psychiatry. 2017;88:663–668. doi: 10.1136/jnnp-2016-315433. [DOI] [PubMed] [Google Scholar]
- 22.Algra AM, Lindgren A, Vergouwen MDI, et al. Procedural clinical complications, case-fatality risks, and risk factors in endovascular and neurosurgical treatment of unruptured intracranial aneurysms: asystematic review and meta-analysis. JAMA Neurol. 2019;76:282–293. doi: 10.1001/jamaneurol.2018.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Etminan N, Brown RD Jr, Beseoglu K, et al. The unruptured intracranial aneurysm treatment score: a multidisciplinary consensus. Neurology. 2015;85:881–889. doi: 10.1212/WNL.0000000000001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindgren A, Burt S, Bragan Turner E, et al. Hospital case-volume is associated with case-fatality after aneurysmal subarachnoid hemorrhage. Int J Stroke. 2019;14:282–289. doi: 10.1177/1747493018790073. [DOI] [PubMed] [Google Scholar]
- 25.Hasan DM, Mahaney KB, Brown RD Jr, et al. Aspirin as a promising agent for decreasing incidence of cerebral aneurysm rupture. Stroke. 2011;42:3156–3162. doi: 10.1161/STROKEAHA.111.619411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasan DM, Chalouhi N, Jabbour P, et al. Evidence that acetylsalicylic acid attenuates inflammation in the walls of human cerebral aneurysms: preliminary results. J Am Heart Assoc. 2013;2 doi: 10.1161/JAHA.112.000019. e000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cea Soriano L, Gaist D, Soriano-Gabarro M, Bromley S, Garcia Rodriguez LA. Low-dose aspirin and risk of intracranial bleeds: an observational study in UK general practice. Neurology. 2017;89:2280–2287. doi: 10.1212/WNL.0000000000004694. [DOI] [PubMed] [Google Scholar]
- 28.Dasenbrock HH, Yan SC, Gross BA, et al. The impact of aspirin and anticoagulant usage on outcomes after aneurysmal subarachnoid hemorrhage: a nationwide sample analysis. J Neurosurg. 2016:1–11. doi: 10.3171/2015.12.JNS151107. [DOI] [PubMed] [Google Scholar]
- 29.Vergouwen MD, Rinkel GJ, Algra A, et al. Prospective randomized open-label trial to evaluate risk factor management in patients with unruptured intracranial aneurysms: study protocol. Int J Stroke. 2018;13:992–998. doi: 10.1177/1747493018790033. [DOI] [PubMed] [Google Scholar]
- 30.Tominari S, Morita A, Ishibashi T, et al. Prediction model for 3-year rupture risk of unruptured cerebral aneurysms in Japanese patients. Ann Neurol. 2015;77:1050–1059. doi: 10.1002/ana.24400. [DOI] [PubMed] [Google Scholar]
- 31.Sonobe M, Yamazaki T, Yonekura M, Kikuchi H. Small unruptured intracranial aneurysm verification study: SUAVe study, Japan. Stroke. 2010;41:1969–1977. doi: 10.1161/STROKEAHA.110.585059. [DOI] [PubMed] [Google Scholar]
- 32.Feigin V, Parag V, Lawes CM, et al. Smoking and elevated blood pressure are the most important risk factors for subarachnoid hemorrhage in the Asia-Pacific region: an overview of 26 cohorts involving 306,620 participants. Stroke. 2005;36:1360–1365. doi: 10.1161/01.STR.0000170710.95689.41. [DOI] [PubMed] [Google Scholar]
- 33.Juvela S, Poussa K, Lehto H, Porras M. Natural history of unruptured intracranial aneurysms: a long-term follow-up study. Stroke. 2013;44:2414–2421. doi: 10.1161/STROKEAHA.113.001838. [DOI] [PubMed] [Google Scholar]
- 34.Wermer MJ, van der Schaaf IC, Algra A, Rinkel GJ. Risk of rupture of unruptured intracranial aneurysms in relation to patient and aneurysm characteristics: an updated meta-analysis. Stroke. 2007;38:1404–1410. doi: 10.1161/01.STR.0000260955.51401.cd. [DOI] [PubMed] [Google Scholar]
- 35.Broderick JP, Brown RD Jr., Sauerbeck L, et al. Greater rupture risk for familial as compared to sporadic unruptured intracranial aneurysms. Stroke. 2009;40:1952–1957. doi: 10.1161/STROKEAHA.108.542571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kleinloog R, de Mul N, Verweij BH, Post JA, Rinkel GJE, Ruigrok YM. Risk factors for intracranial aneurysm rupture: a systematic review. Neurosurgery. 2018;82:431–440. doi: 10.1093/neuros/nyx238. [DOI] [PubMed] [Google Scholar]
- 37.Edjlali M, Guedon A, Ben Hassen W, et al. Circumferential thick enhancement at vessel wall MRI has high specificity for intracranial aneurysm instability. Radiology. 2018;289:181–187. doi: 10.1148/radiol.2018172879. [DOI] [PubMed] [Google Scholar]
- 38.Macleod MR, White PM. Not tonight, darling, I might get a headache. Stroke. 2011;42:1807–1808. doi: 10.1161/STROKEAHA.111.617787. [DOI] [PubMed] [Google Scholar]
- 39.Tiel Groenestege AT, Rinkel GJE, van der Bom JG, Algra A, Klijn CJ. The risk of aneurysmal subarachnoid hemorrhage during pregnancy, delivery, and the puerperium in the Utrecht population. Case-crossover study and standardized incidence ratio estimation. Stroke. 2009;40:1148–1151. doi: 10.1161/STROKEAHA.108.539700. [DOI] [PubMed] [Google Scholar]