Abstract
Based on a combination of the CALYPSO method for crystal structure prediction and first-principles calculations, we explore the crystal structures of VH2 under the pressure range of 0−300 GPa. The cubic Fm-3m phase with regular VH8 cubes is predicted to transform into orthorhombic Pnma structure with fascinating distorted VH9 tetrakaidecahedrons at 47.36 GPa. Both the Fm-3m phase at 0 GPa and the Pnma phase at 100 GPa are mechanically and dynamically stable, as verified with the calculations of elastic constants and phonon dispersions, respectively. Moreover, the calculated electronic band structure and density of states indicate both stable phases are metallic. Remarkably, the analyses of the Poisson’s ratio, electron localization function (ELF) and Bader charge substantiate that both stable phases are ionic crystals on account of effective charges transferring from V atom to H. On the basis of the microscopic hardness model, the Fm-3m and Pnma crystals of VH2 are potentially incompressible and hard materials with the hardness values of 17.83 and 17.68 GPa, respectively.
Subject terms: Electronic properties and materials, Phase transitions and critical phenomena
Introduction
High pressure is an interesting external condition, which can reduce interatomic separation and alter the bond angles and generate the powerful influences on the physical and chemical properties and further arouse unusual stoichiometries and crystal structures in numerous materials. Hydrides have been widely progressed under high pressure due to potential high-temperature superconductors and hydrogen storage material1–11.
Recently, the investigations on the transition metal hydrides under high pressure have also been conducted in views of two factors as follows. On the one hand, for the late transition metals, their electronegativity values (e.g., 2.2 for Ru, Pd, Os, and Ir; 2.28 for Pt; 2.54 for Au) are higher and comparable than that of hydrogen (2.2) and thus making the chemical reaction between them unfavorable and resulting in the extreme absence of the late transition metal hydrides scarce under ambient pressure. Fortunately, high pressure can effectively stimulate the chemical reactivity of elements, making the reaction between hydrogen and late transition metals come true. Previous experimental investigations have ascertained that high pressure can be utilized to synthesize new Pt, Rh and Ir hydrides12–16 and the preceding first-principles calculations have predicted the high pressure structures produced through the reaction of the late transition metals (Ru, Rh, Pd, Ag, Os, Ir, Pt, and Au) with hydrogen17,18. More interestingly, RhH2 is suggested to be a hydrogen storage compound for the highly theoretical volumetric hydrogen density of 163.7 g H2/L15. On the other hand, as for the early transition metals possessing the smaller electronegativity than hydrogen, they are found to form easily hydrides at ambient conditions, such as ScH2, YH2/YH3, TiH2, ZrH2, HfH2, VH2, NbH219,20. It is obviously seen that these early transition metal hydrides generally form transition-metal dihydrides TMH2. Upon compression, these transition-metal dihydrides that can exist under ambient conditions present fascinating properties that cannot be found at normal conditions. For example, a theoretical study indicated that TiH2 at high pressure takes a structural sequence of I4/mmm P4/nmm P21/m with the corresponding transformation pressures of 63 and 294 GPa21. A subsequent experiment22 on ZrH2 have offered a new approach with nonhydrostatic compression or shear stress for seeking higher volumetric density hydrogen structures in transition-metal hydrides. Later on, they reported another theoretical investigation on HfH2 that it undergoes pressure-induced structural phase transition I4/mmm Cmma P21/m at 180 and 250 GPa, respectively, and HfH2 is classified as a ionic crystal with the charges transferring from Hf atom to H23.
With regard to group 5 element dihydrides, the pressure-induced structural transition and related structural and electronic properties of NbH2 and TaH2 has also been deeply explored. Gao et al. predicted that NbH2 transforms from the fluorite Fm-3m structure into the orthorhombic Pnma phase at 50 GPa24. Subsequently, we also confirmed the high-pressure phase transition of NbH2 and further found a combination of ionic and metallic bonds in these two stable phases (the Fm-3m and Pnma phases)25. In the latest theoretical work, TaH2 is found to transform from the P63mc structure into the Pnma phase at about 95 GPa26. Up to now, despite few theoretical researches on the high-pressure phase transition of VH227,28, there is relatively little investigation on its new structures, mechanical properties, hardness and the chemical bonding nature under high pressure. This stimulates us to implement a comprehensive search on the crystal structures for vanadium dihydrides under high pressure and study the related structural, mechanical and electronic properties of newly stable VH2 structures.
In the current work, we explore the crystal structures of VH2 within the pressure range of 0–300 GPa by employed the developed Crystal Structure Analysis by Particle Swarm Optimization (CALYPSO) method in combination with first-principles calculations. Two stable structures (the Fm-3m and Pnma structures) are confirmed within the stable pressure ranges of 0−46.37 and 46.37−300 GPa, respectively. The Fm-3m structure is comprised of regular VH8 cubes while the Pnma structure contains highly distorted VH9 tetrakaidecahedrons. The mechanical and dynamical stabilities of both phases are verified with the calculated elastic constants and phonon dispersions, respectively. In addition, the analyses of the Poisson’s ratio, electron localization function (ELF) and Bader charge indicate that both phases are ionic crystals with the charges transferring from V atom to H. Furthermore, the mechanical properties and hardness were also investigated for both phases. The present results can provide a better understanding of the phase transformations, the structural characteristics and bonding character of VH2 and promote further experimental and theoretical investigations on the transition metal hydrides.
Computational method
To search for the ground-state structures for VH2 under high pressure, we performed an unbiased structure prediction based on the particle swarm optimization algorithm as implemented with the CALYPSO code29,30. The superior efficiency of this methodology has been verified on various systems25,31–39. Our structure searches with unit cells containing up to six formula units (f.u.) per simulation cell were conducted within pressures of 0−300 GPa. The detailed description for this search algorithm can be found elsewhere29,30. The structural optimization and electronic structure calculations were carried out using density functional theory within the Perdew−Burke−Ernzerhof (PBE) exchange-correlation functional as implemented in the Vienna ab initio simulation package (VASP)40,41. Plane-wave basis sets and the projector augmented wave (PAW) method42 were adopted with 4s23d3 and 1s1 treated as valence electron space for V and H atoms, respectively. The cutoff energy of 800 eV for the expansion of the expansion of the wave function into plane waves and appropriate Monkhorst−Pack k-meshes43 were selected to ensure that all the enthalpy calculations were well converged to better than 1 meV/atom. The phonon dispersion curves were calculated within the direct supercell approach as implemented in the Phonopy code44,45. The electron localization function (ELF)46,47 and Bader charge48 were also computed using VASP. In addition, all the crystal structures and electron localization functions (ELF) diagrams were generated using VESTA 3.4.449. The elastic constants were determined by calculating stress tensor generated by applying a small strain to an optimized unit cell. Besides, the bulk modulus (B), shear modulus (G), Young’s modulus (E), and Poisson’s ratio were thus derived from the Voigt−Reuss−Hill (VRH) approximation50.
Results and Discussions
Crystal structure searching and phase transition under pressure
Firstly, on the basis of the chemical constitution, we have conducted the structure prediction simulations of VH2 in the pressure range of 0−300 GPa. Subsequently, the analysis of the predicted structures provides eight candidate structures with space groups Fm-3m, Pnma, P21/m, P-62m, P63mc, P63/mmc, P4/nmm and I4/mmm. The candidate crystal structures diagrams are depicted in Fig. S1 in the Supplementary Information and their lattice parameters and atomic coordinates under ambient pressure are summarized in Table S1. The fact that all the earlier known structures (Fm-3m, Pnma, P63mc, P63/mmc, I4/mmm and P4/nmm)27,28,51–53 were successfully reproduced in specific pressure ranges supports the validity of CALYPSO methodology used in structure searches of VH2. The enthalpy−pressure (H − P) relations of the candidate structures under the pressure range of 0−300 GPa for VH2 are presented in Fig. 1a. Under ambient pressure, the cubic CaF2 structure (space group Fm-3m) possesses the lowest ground state energy, which is consistent with the experimental consequence51. With increasing pressure, the orthorhombic Pnma structure is favored over the Fm-3m phase above 46.37 GPa, which remains stable in the ground state up to at least 300 GPa. This Pnma structure is also the high-pressure configuration of NbH225,54, WH255 and certain alkaline-earth dihydrides such as CaH2, SrH2, and BaH256,57. This phase transformation is also supported by the volume-pressure relations (see Fig. 1b), as we observed a volume collapse of 4.1% at the transition pressure. Furthermore, the transition pressure (46.37 GPa) is in good agreement with the relevant theoretical calculations27,28. Below we concentrate on the relevant properties of the Fm-3m phase at 0 GPa and the Pnma phase at 100 GPa, which are most stable and accessible to experiment at the corresponding pressures.
Figure 1.

(a) Enthalpy-pressure relation with respect to the Fm-3m phase and (b) volume-pressure relations for VH2.
Structural properties and the lattice parameter behavior under pressure
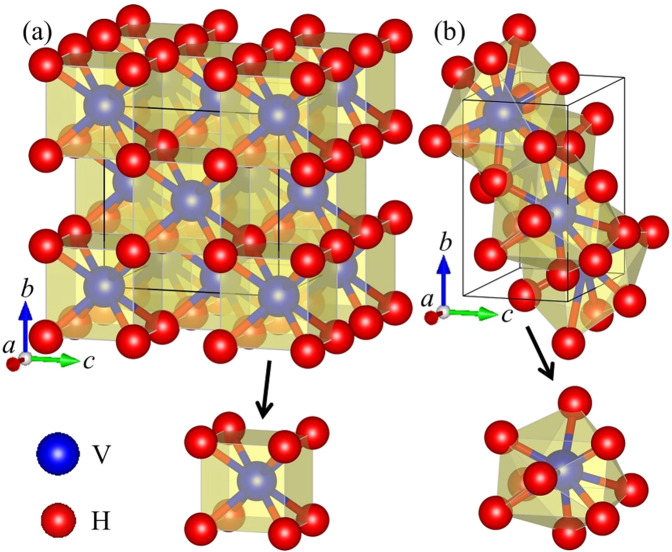
The crystal structures of the Fm-3m phase at 0 GPa and the Pnma phase at 100 GPa for VH2 are illustrated in Fig. 2 and their structural parameters are presented in Table 1. For the Fm-3m phase at 0 GPa, as displayed in Table 1, the lattice parameter (a = b = c = 4.215 Å) is in excellent agreement with the previous results (a = b = c = 4.217 Å) calculated by Chen et al.27 In the Fm-3m phase at 0 GPa, each V atom is at the center of a regular VH8 cube with eight equal V-H separation of 1.825 Å and identical H-V-H angle of 71°. Therefore, the V environment in the Fm-3m configuration can be described as highly symmetrical VH8 cube. However, in the Pnma phase at 100 GPa, each V atom is surrounded by one H atom at a separation of 1.634 Å, two H atoms at a separation of 1.644 Å, one H atom at a separation of 1.654 Å, one H atom at a separation of 1.701 Å, two H atoms at a separation of 1.825 Å and two further H atoms at a separation of 1.899 Å. Besides, the H-V-H angles vary between 46° and 106°. These results describe that the V environment in the Pnma phase are strongly distorted VH9 tetrakaidecahedron. It is obviously found that the V-H environments in VH2 transform from the regular VH8 cube to the highly distorted VH9 tetrakaidecahedron with increasing pressure. More interestingly, the coordination number of the V atoms in VH2 increase monotonously upon further compression. This phenomenon can also be found in other systems25,31,58.
Figure 2.
Crystal structures of two considered VH2 phases, together with metal coordination polyhedra: (a) the Fm-3m phase at 0 GPa and (b) the Pnma phase at 100 GPa. The blue and red spheres represent vanadium and hydrogen atoms, respectively, and VHn polyhedra are shaded in two phases.
Table 1.
Lattice constants and atomic coordinates of VH2 for the Fm-3m phase at 0 GPa and the Pnma phase at 100 GPa.
| Pressure (GPa) | Space group | a | b | c | Atomic coordinates (fractional) | |||
|---|---|---|---|---|---|---|---|---|
| Atom | x | y | z | |||||
| 0 | Fm-3m | 4.215 | 4.215 | 4.215 | V1(4a) | 0.00000 | 0.00000 | 0.00000 |
| H1(8c) | 0.25000 | 0.25000 | 0.25000 | |||||
| 0 | Fm-3m | 4.217a | 4.217a | 4.217a | V1(4a) | 0.00000 | 0.00000 | 0.00000 |
| H1(8c) | 0.25000 | 0.25000 | 0.25000 | |||||
| 100 | Pnma | 4.268 | 2.622 | 4.721 | V1(4c) | 0.23761 | 0.75000 | 0.09160 |
| H1(4c) | 0.52689 | 0.75000 | 0.71430 | |||||
| H2(4c) | 0.12491 | 0.75000 | 0.42227 | |||||
aref. 27.
It is essential to calculate the phonon spectra of two stable VH2 structures for the purpose of ascertaining their dynamical stabilities. The calculated phonon dispersion curves and projected phonon density of states (PHDOS) for the Fm-3m phase at 0 GPa and the Pnma phase at 100 GPa are depicted in Fig. 3. The absence of any imaginary frequencies in the entire Brillouin zone confirms the dynamical stabilities of both structures. Notably, the phonon bands can be divided into two separate regions. The higher frequency modes are mainly correspond to the vibration of the H atoms while the lower frequency region is mostly related to the V atoms, which can be attributed to the much heavier atom mass of vanadium atom than hydrogen atom.
Figure 3.

Phonon dispersion curves and projected phonon density of states (PHDOS) for two stable VH2 phases: (a) the Fm-3m phase at 0 GPa and (b) the Pnma phase at 100 GPa.
For the purpose to investigate the effect of the external pressure on the lattice parameters and the volume of VH2, the normalized parameters a/a0, b/b0, c/c0 and volume V/V0 of two considered phases as a function of pressure are displayed in Fig. S2, where a0, b0, c0 and V0 are the equilibrium structural parameters at zero pressure, respectively. It is obviously found that the values a/a0, b/b0, c/c0 and V/V0 decrease gradually with the increasing pressure. This can be attributed to the reason that the distance between atoms is reduced as the pressure increases, which make the repulsive interaction among atoms strengthened and lead to the higher incompressibility of the crystal under pressure.
Mechanical stability, mechanical properties and hardness
To inspect the mechanical stabilities of two stable VH2 structures, we computed the elastic constants of the Fm-3m phase at 0 GPa and the Pnma phase at 100 GPa by using the strain−stress means59, as summarized in Table S2. As given in Table S2, both phases satisfy their respectively mechanical stability standards60, which states that both structures are mechanically stable.
In addition, to get further knowledge of mechanical properties for two stable configurations, the elastic constants of the Fm-3m and Pnma structures of VH2 at 0 GPa were calculated with the identical strain−stress means59. At the same time, the bulk modulus (B) and shear modulus (G), B/G, Young′s modulus E (GPa) and Poisson’s ratio (ν) can be derived from the calculated elastic constants on the basis of the Voigt–Reuss–Hill method61. The computed consequences above are tabulated in Table 2. Interestingly, the value of C11 for two considered crystals is larger than the others. It is well known that a material with a high bulk modulus indicates its strong ability to resist volume deformation caused by an applied load. According to Table 2, the calculated bulk moduli of the Fm-3m and Pnma structures are 174 and 173 GPa, respectively, illustrating the incompressible feature of these two phases to a certain degree. In addition, the shear modulus of a material can be used to quantify its resistance to the shear deformation. From Table 2, these two crystals possess the equal the shear modulus of 118 GPa, suggesting that both configurations can withstand the shear strain to a large extent. The ratio value of B/G is commonly used to describe the ductility or brittleness of materials with 1.75 as the critical value60. From Table 2, the calculated identical ratio value of B/G for both phases is 1.47, indicating their brittle characteristic. Poisson’s ratio is an important parameter to describe the bond nature in a material. For covalent materials, the value of ν should be small (typically ν = 0.1), and shear modulus G should be slightly different with bulk modulus B with G = 1.1B. The typical value of ν for ionic materials is 0.25 and G = 0.6B. For metallic materials, ν is typically 0.33 and G = 0.4B; in the extreme case where ν is 0.5, G is zero62. From Table 2, the equal ν (0.22) for both structures extremely approach the typical value ν (0.25) of ionic materials. Further, shear modulus G is equal to 0.68B. These two conditions above demonstrate that the Fm-3m and Pnma phases of VH2 can be classified into ionic materials. In order to estimate the hardness (Hv) of VH2, an empirical hardness equation is applied as follows: Hv = 2(K2G)0.585 − 3, where K = G/B63. As shown in Table 2, the calculated hardness (Hv) values of the Fm-3m and Pnma phases are 17.83 and 17.68 GPa, respectively, declaring that both phases of VH2 could be classified as incompressible and hard materials.
Table 2.
Calculated elastic constants Cij (GPa), bulk modulus B (GPa), shear modulus G (GPa), B/G, Young’s modulus E (GPa), Poisson’s ratio ν and Vickers hardness Hv of VH2 for the Fm-3m and Pnma phases under 0 GPa.
| Phase | C11 | C22 | C33 | C44 | C55 | C66 | C12 | C13 | C23 | B | G | B/G | E | ν | Hv |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fm-3m | 293 | 143 | 115 | 174 | 118 | 1.47 | 290 | 0.22 | 17.83 | ||||||
| Pnma | 340 | 332 | 337 | 106 | 121 | 119 | 101 | 81 | 93 | 173 | 118 | 1.47 | 288 | 0.22 | 17.68 |
Electronic properties and bonding features
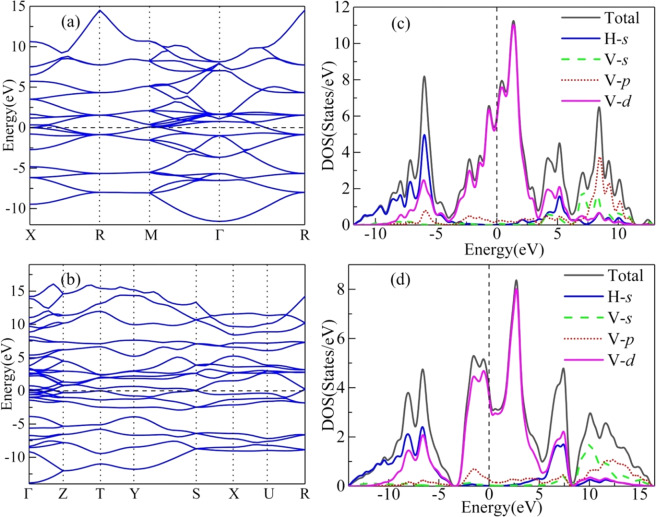
In order to further gain insight into the electronic properties of both stable VH2 configurations, we calculated their electronic band structures and densities of states (DOS) (see Fig. 4). Both structures are found to exhibit metallic character to the overlap between the conduction bands and the valence bands, as displayed in Fig. 4a,b. This is also confirmed by the finite electronic DOS at the Fermi level (EF) in Fig. 4c,d. For both compounds, the total densities of states (TDOS) near the Fermi level is largely contributed by the V-d states, so the metallic properties are mainly due to partially filled V 3d shell.
Figure 4.
Calculated band structures and densities of states (DOS) for two stable VH2 phases. The electronic band structures for (a) the Fm-3m phase at 0 GPa and (b) the Pnma phase at 100 GPa. The total and partial density of states for (c) the Fm-3m phase at 0 GPa and (d) the Pnma phase at 100 GPa.
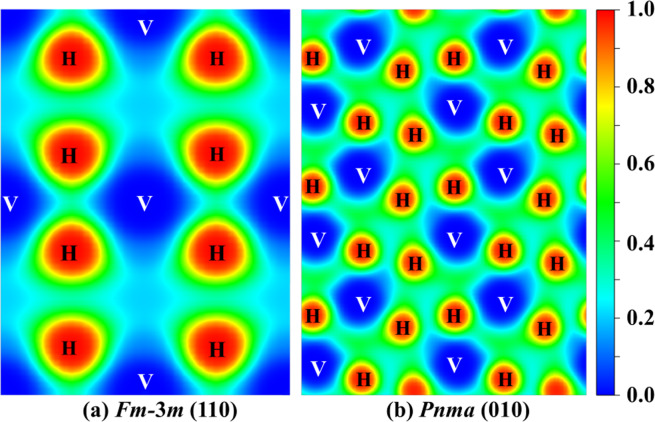
To further visualize the chemical bonding nature in the polymeric sublattice for two considered VH2 structures, we calculate the electron localization function (ELF) which can make a description of the bond type between atoms, as shown in Fig. 5. Generally speaking, large ELF values (>0.5) correspond to covalent bonds and lone pair or inner shell electrons while smaller ELF values (<0.5) correspond to ionic or metallic bonds46,47. For two stable VH2 structures, the analyses of ELF toward the neighboring V − H and H − H connections obviously reveal no electron localization, thus suggesting that no covalent interaction exists for neighboring V − H and H − H. Moreover, the ELF values between V and the nearest H atom in both stable phases are smaller than 0.5, implying the ionic or metallic bonds are present between V and H atoms. Furthermore, in order to illuminate the chemical bonding features between V and H atoms, Bader charge analysis48 was implemented, as summarized in Table 3. For the Fm-3m phase at 0 GPa, each H atom gains 0.54 e while the V atoms lose 1.08 e. In addition, for the Pnma phase at 100 GPa, a charge of 0.92 e is stripped off the V atom and transferred completely to the H atoms (0.46 e each). The current prediction of the charge transferring from V to H atoms can also be corroborated by the element electronegativity. From the periodic law64, the electronegativity value of the V element (1.63) is smaller than that of the H element (2.2), meaning that the charge should transfer from V to H atoms. The calculated consequences above apparently show that there are large amounts of charge transferring from V to H atoms, demonstrating the ionic character of the V−H bonding in two stable VH2 crystals. This ionic bonding character is similar in AlH3, LiHn, HfH2, TaHn, VH, VH3 and VH523,26,65–67. However, the ionic characteristic for the H atoms is different from that of many hydrogen-rich polymorphs (i.e., SiH4, GeH4, SnH4, InH3, OsH6 and OsH8)68–72, where the H atoms are either bonded to the nearest H atoms to form an H2 or H3 unit and/or they are covalently bonded to M (M = Si, Ge, Sn, In, or Os) atoms to form M − H bonds. In short, a combination analysis of ELF and Bader charge reveals that the ionic bonds are formed between V and H atoms and VH2 should be classified as an ionic crystal, which is accord with the analysis of Poisson’s ratio above.
Figure 5.
Electron localization function (ELF) of two stable VH2 phases: (a) (110) plane for the Fm-3m phase at 0 GPa and (b) (010) plane for the Pnma phase at 100 GPa.
Table 3.
Calculated Bader charges of V and H atoms in two stable VH2 crystals.
| Phase | Pressure(GPa) | Atom | Charge value (e) | δ(e) |
|---|---|---|---|---|
| Fm-3m | 0 | V | 3.92 | 1.08 |
| H | 1.54 | −0.54 | ||
| Pnma | 100 | V | 4.08 | 0.92 |
| H | 1.46 | −0.46 |
δ represents the amount of charge transferred from V atom to H atom.
Conclusions
In summary, we conducted the systematic structure evolutionary searches of the VH2 compound based on the CALYPSO method for crystal structure prediction combined with first-principles calculations under the pressure range between 0 and 300 GPa. This material undergoes a pressure-induced structural phase transformation from the cubic structure Fm-3m structure to the orthorhombic Pnma structure at 46.37 GPa. Intriguing, the Fm-3m structure is comprised of regular VH8 cubes while the Pnma structure contains highly distorted VH9 tetrakaidecahedron. The calculations of elastic constants and phonon dispersions confirm the mechanical and dynamical stabilities of both configurations, respectively. Both phases possess the metallic properties as verified by the band structures and densities of states for two stable VH2 structures. Further analyses of the Poisson’s ratio, ELF and Bader charge indicate that VH2 should be classified into an ionic crystal with large amounts of charge transferring from V atom to H. Remarkably, the estimated hardness values of the Fm-3m and Pnma phases are 17.83 and 17.68 GPa, respectively. The current work will stimulate further high pressure experiments to synthesize these vanadium hydrides and carry out the structural, mechanical and hardness measurements.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 11747139 and 11804031), the Scientific Research Project of Education Department of Hubei Province (No. Q20191301), Talent and High Level Thesis Development Fund of Department of Physics and Optoelectronic Engineering of Yangtze University (C. Z. Z.), the Youth Fund of Yangtze University (No. 2016cqn54, Y. Y. J) and Yangtze University Innovation and Entrepreneurship Project (No. 2018180 and 2018176).
Author contributions
C.Z.Z., Y.Y.J., S.L. and W.B.Z. conceived the idea. W.J.W., P.L.K., C.W.X., C.Z.D., Q. L. and C.H.Z. performed the calculations. W.J.W., C.Z.Z., Y.Y.J. and S.L. wrote the manuscript and all authors contributed to revisions.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chuanzhao Zhang, Email: zcz19870517@163.com.
Yuanyuan Jin, Email: scujyy@163.com.
Supplementary information
is available for this paper at 10.1038/s41598-020-65910-4.
References
- 1.Eremets MI, et al. Superconductivity in hydrogen dominant materials: silane. Science. 2008;319:1506–1509. doi: 10.1126/science.1153282. [DOI] [PubMed] [Google Scholar]
- 2.Pickard CJ, Needs RJ. High-pressure phases of silane. Phys. Rev. Lett. 2006;97:045504. doi: 10.1103/PhysRevLett.97.045504. [DOI] [PubMed] [Google Scholar]
- 3.Jin X, et al. Superconducting high-pressure phases of disilane. Proc. Natl. Acad. Sci. U. S. A. 2010;107:9969–9973. doi: 10.1073/pnas.1005242107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao G, et al. Superconducting high pressure phase of germane. Phys. Rev. Lett. 2008;101:107002. doi: 10.1103/PhysRevLett.101.107002. [DOI] [PubMed] [Google Scholar]
- 5.Tse J, Yao Y, Tanaka K. Novel Superconductivity in Metallic SnH4 under high pressure. Phys. Rev. Lett. 2007;98:117004. doi: 10.1103/PhysRevLett.98.117004. [DOI] [PubMed] [Google Scholar]
- 6.Duan D, et al. Pressure-induced metallization of dense (H2S)2H2 with high-Tc superconductivity. Sci. Rep. 2014;4:6968. doi: 10.1038/srep06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drozdov P, et al. Conventional superconductivity at 203 kelvin at high pressures in the sulfur hydride system. Nature. 2015;525:73–76. doi: 10.1038/nature14964. [DOI] [PubMed] [Google Scholar]
- 8.Einaga M, et al. Crystal structure of the superconducting phase of sulfur hydride. Nat. Phys. 2016;12:835–838. doi: 10.1038/nphys3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mao WL, Mao HK. Hydrogen storage in molecular compounds. Proc. Natl. Acad. Sci. U. S. A. 2004;101:708–710. doi: 10.1073/pnas.0307449100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ying J, et al. Synthesis and stability of tantalum hydride at high pressures. Phys. Rev. B. 2019;99:224504. [Google Scholar]
- 11.Li H, et al. Superconducting TaH5 at high pressure. New J. Phys. 2019;21:123009. [Google Scholar]
- 12.Degtyareva O, et al. Formation of transition metal hydrides at high pressures. Solid State Commun. 2009;149:1583–1586. [Google Scholar]
- 13.Scheler T, et al. Synthesis and properties of platinum hydride. Phys. Rev. B. 2011;83:214106. [Google Scholar]
- 14.Antonov V. Phase transformations, crystal and magnetic structures of high-pressure hydrides of d-metals. J.Alloys Compd. 2002;330:110–116. [Google Scholar]
- 15.Li B, et al. Rhodium dihydride (RhH2) with high volumetric hydrogen density. Proc. Natl. Acad. Sci. U. S. A. 2011;108:18618–18621. doi: 10.1073/pnas.1114680108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheler T, et al. High-pressure synthesis and characterization of iridium trihydride. Phys. Rev. Lett. 2013;111:215503. doi: 10.1103/PhysRevLett.111.215503. [DOI] [PubMed] [Google Scholar]
- 17.Zaleski-Ejgierd P. Formation of transition metal hydrides at high pressures. Phys. Chem. Chem. Phys. 2014;16:3220–3229. doi: 10.1039/c3cp54300e. [DOI] [PubMed] [Google Scholar]
- 18.Gao G. Pressure-induced formation of noble metal hydrides. J. Phys. Chem. C. 2012;116:1995–2000. [Google Scholar]
- 19.Smithson H, et al. First-principles study of the stability and electronic structure of metal hydrides. Phys. Rev. B. 2002;66:144107. [Google Scholar]
- 20.Miwa K, Fukumoto A. First-principles study on 3d transition-metal dihydrides. Phys. Rev. B. 2002;65:155114. [Google Scholar]
- 21.Gao G, et al. Pressure induced phase transitions in TiH2. J. Appl. Phys. 2013;113:103512. [Google Scholar]
- 22.Huang X, et al. Structural stability and compressive behavior of ZrH2 under hydrostatic pressure and nonhydrostatic pressure. RSC Adv. 2014;4:46780–46786. [Google Scholar]
- 23.Liu Y, et al. First-principles study on the structural and electronic properties of metallic HfH2 under pressure. Sci. Rep. 2015;5:11381. doi: 10.1038/srep11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao G, et al. Theoretical study of the ground-state structures and properties of niobium hydrides under pressure. Phys. Rev. B. 2013;88:184104. [Google Scholar]
- 25.Zhang C, et al. Prediction of novel high-pressure structures of magnesium niobium dihydride. ACS Appl. Mater. Interfaces. 2017;9:26169–26176. doi: 10.1021/acsami.7b06143. [DOI] [PubMed] [Google Scholar]
- 26.Zhuang Q, et al. Pressure-stabilized superconductive ionic tantalum hydrides. Inorg. Chem. 2017;56:3901–3908. doi: 10.1021/acs.inorgchem.6b02822. [DOI] [PubMed] [Google Scholar]
- 27.Chen C, et al. Pressure induced phase transition in MH2 (M = V, Nb), J. Chem. Phys. 2014;140:114703. doi: 10.1063/1.4866179. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Peng F. Superconductivity of pressure-stabilized vanadium hydrides. Inorg. Chem. 2017;56:13759–13765. doi: 10.1021/acs.inorgchem.7b01686. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Lv J, Zhu L, Ma Y. Crystal structure prediction via particle-swarm optimization. Phys. Rev. B: Condens. Matter Mater. Phys. 2010;82:094116. [Google Scholar]
- 30.Wang Y, Lv J, Zhu L, Ma Y. CALYPSO: A method for crystal structure prediction. Comput. Phys. Commun. 2012;183:2063–2070. [Google Scholar]
- 31.Lu C, Miao MS, Ma YM. Structural evolution of carbon dioxide under high pressure. J. Am. Chem. Soc. 2013;135:14167–14171. doi: 10.1021/ja404854x. [DOI] [PubMed] [Google Scholar]
- 32.Zhu L, et al. Reactions of xenon with iron and nickel are predicted in the earth’s inner core. Nat. Chem. 2014;6:644–648. doi: 10.1038/nchem.1925. [DOI] [PubMed] [Google Scholar]
- 33.Lu SH, et al. Self-assembled ultrathin nanotubes on diamond (100) surface. Nat. Commun. 2014;5:3666. doi: 10.1038/ncomms4666. [DOI] [PubMed] [Google Scholar]
- 34.Gao B, et al. Interface structure prediction via CALYPSO method. Sci. Bull. 2019;64:301–309. doi: 10.1016/j.scib.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Zhang CZ, et al. Prediction of stable ruthenium silicides from first-principles calculations: stoichiometries, crystal structures, and physical properties. ACS Appl. Mater. Interfaces. 2015;7:26776–26782. doi: 10.1021/acsami.5b08807. [DOI] [PubMed] [Google Scholar]
- 36.Lu C, et al. Extraordinary indentation strain stiffening produces superhard tungsten nitrides. Phys. Rev. Lett. 2017;119:115503. doi: 10.1103/PhysRevLett.119.115503. [DOI] [PubMed] [Google Scholar]
- 37.Lu C, Chen CF. High-pressure evolution of crystal bonding structures and properties of FeOOH. J. Phys. Chem. Lett. 2018;9:2181–2185. doi: 10.1021/acs.jpclett.8b00947. [DOI] [PubMed] [Google Scholar]
- 38.Lu C, Amsler M, Chen CF. Unraveling the structure and bonding evolution of the newly discovered iron oxide FeO2. Phys. Rev. B. 2018;98:054102. [Google Scholar]
- 39.Tang X, et al. CoB6 monolayer: A robust two-dimensional ferromagnet. Phys. Rev. B. 2019;99:045445. [Google Scholar]
- 40.Kresse G, Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B: Condens. Matter Mater. Phys. 1996;54:11169–11186. doi: 10.1103/physrevb.54.11169. [DOI] [PubMed] [Google Scholar]
- 41.Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996;77:3865–3868. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 42.Kresse G, Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B: Condens. Matter Mater. Phys. 1999;59:1758–1775. [Google Scholar]
- 43.Monkhorst HJ, Pack JD. Special points for brillouin-zone integrations. Phys. Rev. B. 1976;13:5188–5192. [Google Scholar]
- 44.Parlinski K, Li ZQ, Kawazoe Y. First-principles determination of the soft mode in cubic ZrO2. Phys. Rev. Lett. 1997;78:4063–4066. [Google Scholar]
- 45.Togo A, Oba F, Tanaka I. First-principles calculations of the ferroelastic transition between rutile-type and CaCl2-type SiO2 at high pressures. Phys. Rev. B. 2008;78:134106. [Google Scholar]
- 46.Becke AD, Edgecombe KE. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 1990;92:5397–5403. [Google Scholar]
- 47.Savin A, et al. Electron localization in solid-state structures of the elements: the diamond structure. Angew. Chem., Int. Ed. Engl. 1992;31:187–188. [Google Scholar]
- 48.Bader, R. F. Atoms in Molecules: A Quantum Theory (1994).
- 49.Momma, K. & Izumi, F. VESTA 3 for Three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276, http://jp-minerals.org/vesta/en/ (2011).
- 50.Hill R. The elastic behavior of a crystalline aggregate. Proc. Phys. Soc. London, Sect. A. 1952;65:349. [Google Scholar]
- 51.Reilly JJ, Wiswall RH. The higher hydrides of vanadium and niobium. Inorg. Chem. 1970;9:1678–1682. [Google Scholar]
- 52.Smithson H, et al. First-principles study of the stability and electronic structure of metal hydrides. Phys. Rev. B. 2002;66:144107. [Google Scholar]
- 53.Miwa K, et al. First-principles study on 3d transition-metal dihydrides. Phys. Rev. B. 2002;65:155114. [Google Scholar]
- 54.Gao G, et al. Theoretical study of the ground-state structures and properties of niobium hydrides under pressure. Phys. Rev. B: Condens. Matter Mater. Phys. 2013;88:184104. [Google Scholar]
- 55.Scheler T, et al. Nanocrystalline tungsten hydrides at high pressures. Phys. Rev. B: Condens. Matter Mater. Phys. 2013;87:184117. [Google Scholar]
- 56.Li Y, et al. High-pressure phase transformations in CaH2. J. Phys.: Condens. Matter. 2008;20:045211. [Google Scholar]
- 57.El Gridani AH, et al. Elastic, electronic and crystal structure of BaH2: A pseudopotential study. J. Mol. Struct.: Theochem. 2002;577:161–175. [Google Scholar]
- 58.Zhong X, et al. Ten-fold coordinated polymorph and metallization of TiO2 under high pressure. RSC Adv. 2015;5:54253. [Google Scholar]
- 59.Lu C, et al. Theoretical investigation on the high-pressure structural transition and thermodynamic properties of cadmium oxide. Europhys. Lett. 2010;91:16002. [Google Scholar]
- 60.Wu Z, et al. Crystal structures and elastic properties of superhard IrN2 and IrN3 from first principles. Phys. Rev. B: Condens. Matter Mater. Phys. 2007;76:054115. [Google Scholar]
- 61.Connétable D, Thomas O. First-principles study of the structural, electronic, vibrational, and elastic properties of orthorhombic NiSi. Phys. Rev. B. 2009;79:094101. [Google Scholar]
- 62.Haines J, Léger JM, Bocquillon G. Synthesis and design of superhard materials. Annu. Rev. Mater. Res. 2001;31:1–23. [Google Scholar]
- 63.Chen XQ, et al. Modeling hardness of polycrystalline materials and bulk metallic glasses. Intermetallics. 2011;19:1275–1281. [Google Scholar]
- 64.Lide, D. R. Handbook of Chemistry and Physics, 82th Edition, CRC Press, Boca Raton, FL, (2002).
- 65.Pickard J, Needs RJ. Metallization of aluminum hydride at high pressures: A first-principles study. Phys. Rev. B: Condens. Matter Mater. Phys. 2007;76:144114. [Google Scholar]
- 66.Zurek E, et al. A little bit of lithium does a lot for hydrogen. Proc. Natl. Acad. Sci. U. S. A. 2009;106:17640–17643. doi: 10.1073/pnas.0908262106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhuang Q, et al. Investigation of superconductivity in compressed vanadium hydrides. Phys. Chem. Chem. Phys. 2017;19:26280–26284. doi: 10.1039/c7cp03435k. [DOI] [PubMed] [Google Scholar]
- 68.Zhang H, et al. High-temperature superconductivity in compressed solid silane. Sci. Rep. 2015;5:8845. doi: 10.1038/srep08845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang H, et al. Investigation of stable germane structures under high-pressure. Phys. Chem. Chem. Phys. 2015;17:27630–27635. doi: 10.1039/c5cp03807c. [DOI] [PubMed] [Google Scholar]
- 70.Zhang H, et al. Pressure-induced phase transition of SnH4: a new layered structure. RSC Adv. 2016;6:10456–10461. [Google Scholar]
- 71.Liu Y, et al. Pressure-induced structures and properties in indium hydrides. Inorg. Chem. 2015;54:9924–9928. doi: 10.1021/acs.inorgchem.5b01684. [DOI] [PubMed] [Google Scholar]
- 72.Liu Y, et al. Structures and properties of osmium hydrides under pressure from first principle calculation. J. Phys. Chem. C. 2015;119:15905–15911. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.