Abstract
In 2006, Senegal adopted artemisinin-based combination therapy (ACT) as first-line treatment in the management of uncomplicated malaria. This study aimed to update the status of antimalarial efficacy more than ten years after their first introduction. This was a randomized, three-arm, open-label study to evaluate the efficacy and safety of artemether-lumefantrine (AL), artesunate-amodiaquine (ASAQ) and dihydroartemisinin-piperaquine (DP) in Senegal. Malaria suspected patients were screened, enrolled, treated, and followed for 28 days for AL and ASAQ arms or 42 days for DP arm. Clinical and parasitological responses were assessed following antimalarial treatment. Genotyping (msp1, msp2 and 24 SNP-based barcode) were done to differentiate recrudescence from re-infection; in case of PCR-confirmed treatment failure, Pfk13 propeller and Pfcoronin genes were sequenced. Data was entered and analyzed using the WHO Excel-based application. A total of 496 patients were enrolled. In Diourbel, PCR non-corrected/corrected adequate clinical and parasitological responses (ACPR) was 100.0% in both the AL and ASAQ arms. In Kedougou, PCR corrected ACPR values were 98.8%, 100% and 97.6% in AL, ASAQ and DP arms respectively. No Pfk13 or Pfcoronin mutations associated with artemisinin resistance were found. This study showed that AL, ASAQ and DP remain efficacious and well-tolerated in the treatment of uncomplicated P. falciparum malaria in Senegal.
Subject terms: Malaria, Parasitology
Introduction
Since the abandoning of chloroquine, due to high rate of resistance, the Senegal national malaria control program (NMCP) adjusted its national guidelines for the treatment of uncomplicated malaria by introducing sulphadoxine-pyrimethamine (SP) in 2003, and artemisinin-based combination therapy (ACT) in 2006, complying with the World Health Organization (WHO) recommendations1. This policy review was adopted as ACTs were proven to be the most effective treatment in the context of resistance to chloroquine and other antimalarial drugs2. The scaling up of ACTs at national level has largely contributed to the reduction in morbidity and mortality linked to malaria in Senegal3. Artemether-lumefantrine (AL) and artesunate-amodiaquine (ASAQ) are used as first-line treatment but dihydroartemisinin-piperaquine (DP) has been widely used to compensate for antimalarial drug shortages in 2010 and 2011.
In 2008, ASAQ combination was subsidized by the government and its partners, and was made available at a very low price in public health facilities and private pharmacies4. Since May 2010, ACTs were offered free of charge in the public sector5.
However, the wide use of ACT may exert selective pressure on Plasmodium falciparum populations over time. Recent studies in Southeast Asia (SEA) have shown a decrease in the effectiveness of ACTs such as artesunate used as monotherapy6,7. Although this situation is not yet reported in Africa, it is likely that this resistance could potentially spread in Africa as history showed for chloroquine8. This is particularly important as a previous ex vivo study in Senegal showed that parasites became less sensitive to amodiaquine, artemisinin and chloroquine over time9.
Thus, simple method for monitoring antimalarial drugs is crucial for proper management of clinical cases and early detection of resistance. In accordance with WHO recommendations, the efficacy of first and second-line antimalarial drugs should be evaluated at least once every two years at all sentinel sites10.
In vivo drug clinical trials are the gold standard for assessing the therapeutic efficacy of antimalarials11. In addition, in vitro assays for the sensitivity of human malaria parasites to antimalarial drugs provide useful complementary data from drug-efficacy surveillance12.
In Senegal, the NMCP and its partners regularly conduct therapeutic efficacy study (TES) and molecular markers of antimalarial drug resistance are monitored.
Mutations in the Pfkelch13 (Pfk13) propeller domain were found to be associated with delayed parasite clearance in vitro and in vivo in SEA. These mutations were discovered in the laboratory by long-term in vitro selection using culture-adapted Plasmodium falciparum isolates by stepwise increases in artemisinin exposure13. Although the frequency of mutant alleles strongly correlated with the resistance to artemisinin, the relevance of these mutations on artemisinin resistance in other endemic areas has been subject of debates14,15. Using the same methodology as above13, but with P. falciparum isolates from Senegal, a recent study found no selected mutations on the Pfk13; instead, genetic variants (G50E, R100K, and E107V) of the gene encoding the actin-binding protein P. falciparum coronin (Pfcoronin) were found to reduce the susceptibility of the parasite15. Thus, Pfcoronin, which is structurally similar to Pfk13, is believed to be a strong predictor of potential artemisinin resistance in Senegal and even probably elsewhere in Africa. However, for those mutations to be validated as a marker for artemisinin resistance there should be, at least, a correlation with delayed clearance in clinical studies.
Thus, we aimed to analyze simultaneously the sequences of Pfk13 and Pfcoronin on samples that were subject to treatment failure, along with the routine TES of ACTs.
Results
Baseline characteristics
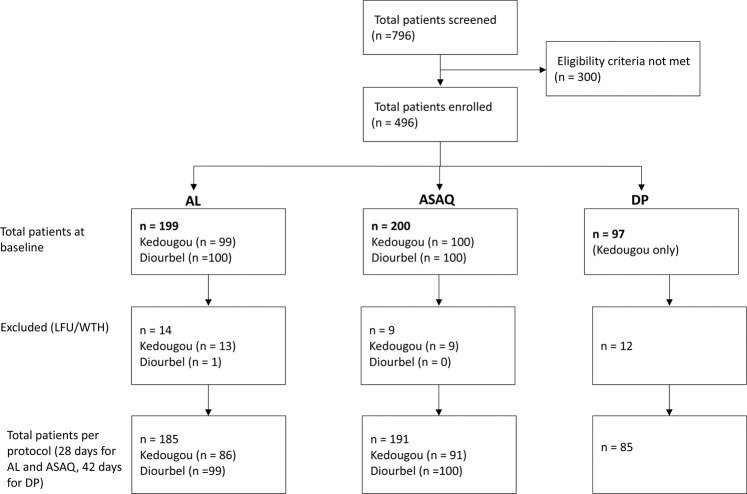
A total of 796 patients suspected for malaria were screened during the study period. Of these, 496 (62.3%) met the inclusion criteria and were enrolled accordingly as shown in Fig. 2.
Figure 2.
Flow chart showing screen and enrollment of study patients.
At baseline (day 0), the male to female sex ratio was largely dominated by males in Diourbel (5.1) while in Kedougou this was balanced (0.9). Thirty-six (12.2%) were under-five years old children, while this age group was not found in patients enrolled in Diourbel. Age group 5–15 years represented 117 patients (58.5%) and 163 patients (55.1%) in Diourbel and Kedougou respectively. Adult patients represented 83 (41.5%) and 97 (32.8%) in Diourbel and Kedougou respectively. Mean weight was 39.3 kg and 36.1 kg in Diourbel and Kedougou respectively. Mean body temperature was 38.3 °C and 38.1 °C in Diourbel and Kedougou respectively. Mean parasitemia was estimated at 11939 parasites/ul and 12991 parasites/ul in Diourbel and Kedougou respectively (Table 1).
Table 1.
Baseline characteristics of the study participants.
| Diourbel | Kedougou | |
|---|---|---|
| Number of enrolled patients | 200 | 296 |
| Ratio male/female | 167/33 | 143/153 |
| Age group (years) | ||
| adults | 83 | 97 |
| 5 to 15 years | 117 | 163 |
| under 5 years | 0 | 36 |
| Age (years) | ||
| mean (sd) | 17.1 (11.9) | 13.3 (9.7) |
| range (min-max) | 5–64 | 1–49 |
| Weight (kg), day 0 | ||
| mean weight (sd) | 39.3 (18.8) | 36.1 (18.8) |
| range (min-max) | 13–93 | 9–93 |
| Temperature (°C), day 0 | ||
| mean temperature (sd) | 38.3 (1) | 38.1 (0.8) |
| range (min-max) | 36.2–41.0 | 36.0–42.0 |
| Parasitemia (density/µl), day 0 | ||
| mean (geometric) parasitemia | 11939 | 12991 |
| range (min-max) | 1000–99750 | 1010–99828 |
Clinical and parasitological outcomes
Of the 496 patients enrolled, 199, 200 and 97 were assigned to AL, ASAQ and DP arms respectively. For AL and ASAQ arms, 185 out of 199 and 191 out of 200 completed the follow-up at day-28 respectively while for the DP arm, 85 out of 97 completed the follow-up at day-42 (Fig. 2).
Table 2 presents the treatment outcome by sites and drugs based on per protocol analysis.
Table 2.
Summary of treatment outcomes based on Per Protocol analysis in Diourbel and Kedougou, 28 days follow up of AL and ASAQ and 42 follow up of DP.
| Sites | drug | N | LFU/WTH n (%) | PD day 3 n (%) | ETF % (IC95%) | LCF\(IC95%) | LPF%(IC95%) | ACPR%(IC95%) | Recrudescence n (%) | Re-infection n (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PCR non-corrected | Diourbel | AL | 100 | 1 (1) | 0 | 0 | 0 | 0 | 100.0 (96.3–100.0) | ||
| ASAQ | 100 | 1 (1) | 0 | 0 | 0 | 0 | 100 (96.4–100.0) | ||||
| Kedougou | AL | 99 | 13 (13.1) | 0 | 1.2 (0.0–6.3) | 1.2 (0.0–6.3) | 1.2 (0.0–6.3) | 96.5 (90.1–99.3) | |||
| ASAQ | 100 | 9 (9.0) | 1 (1) | 0 | 0 | 0 | 100 (96.0–100.0) | ||||
| DP | 97 | 11 (11.6) | 0 | 0 | 0 | 3.6(0.7–10.1) | 96.4 (89.9–99.3) | ||||
| PCR corrected | Diourbel | AL | 100 | 1 (1%) | 0 | 0 | 0 | 0 | 100 (96.3–100.0) | ||
| ASAQ | 100 | 1 (1%) | 0 | 0 | 0 | 0 | 100 (96.4–100.0) | ||||
| Kedougou | AL | 98 | 14 (14.3) | 0 | 1.2 (0.0–6.5) | 0 | 0 | 98.8 (93.5–100.0) | 1 (1.2) | 2 (2.4) | |
| ASAQ | 100 | 9 (9.0) | 1 (1) | 0 | 0 | 0 | 100 (96.0–100.0) | 0 | 0 | ||
| DP | 97 | 12 (12.6) | 0 | 0 | 0 | 2.4 (0.3–8.4) | 97.6 (91.6–99.7) | 2 (2.5) | 1 (1.2) |
Abbreviations: LFU/WTH lost to follow-up/withdrawn, PD3 positive on day 3, LCF late clinical failure, LPF late parasitological failure, ACPR adequate clinical and parasitological response, ASAQ artesunate + amodiaquine, AL artemether + lumefantrine, DP dihydroartemisinin + piperaquine.
In Diourbel, PCR non-corrected ACPR was 100.0% (95%CI: 96.3–100.3) in both AL and ASAQ arms.
In Kedougou, PCR non-corrected ACPR were 96.5% (95%CI: 90.1–99.3), 100% (95%CI: 96.0–100.0) and 96.4% (95%CI: 89.9–99.3) in AL, ASAQ and DP arms respectively; when PCR-corrected, ACPR became 98.8% (95%CI: 93.5–100.0), 100% (95%CI: 96.0–100.0) and 97.6% (95%CI: 91.6–99.7) respectively.
Treatment failures (TF) were noted in Kedougou, as following: a) one ETF at day 3 in the ASAQ arm; b) three TF in the AL arm: 1 LCF at day 7 and 1 LPF at day 21, giving PCR-uncorrected failure rate of 3.5% (95% CI: 0.0–6.3%); c) three TF in the DP arm: one LPF at day 14, one LPF at day 35, and one LPF at day 42. After PCR correction, one sample in the AL arm and two in the DP arm (at day 14 and 35) were confirmed to be recrudescence cases.
Considering the 10% of AL and ASAQ patients followed up to 42 days, there was three LPF in the AL arm and when PCR-corrected this yielded to one confirmed recrudescence (day 35) as shown in Table 3.
Table 3.
Summary of treatment outcomes based on Per Protocol analysis of the 10% extra patients of AL and ASAQ arms followed up to 42 days.
| Sites | drug | N | LFU/WTH | PD day 3 n | ETF n | LCF n | LPF n | ACPR n/n | Pf recrudescence n | Pf re-infection n | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PCR uncorrected | Diourbel | AL | 10 | 0 | 0 | 0 | 0 | 0 | 10/10 | ||
| ASAQ | 10 | 2 | 0 | 0 | 0 | 0 | 8/8 | ||||
| Kedougou | AL | 10 | 2 | 0 | 1 | 0 | 3 | 4/5 | |||
| ASAQ | 10 | 1 | 0 | 0 | 0 | 0 | 9/9 | ||||
| PCR corrected | Diourbel | AL | 10 | 0 | 0 | 0 | 0 | 0 | 10/10 | ||
| ASAQ | 10 | 2 | 0 | 0 | 0 | 0 | 8/8 | ||||
| Kedougou | AL | 10 | 4 | 0 | 1 | 0 | 1 | 4/5 | 1 | 2 | |
| ASAQ | 10 | 1 | 0 | 0 | 0 | 0 | 9/9 |
Abbreviations: LFU/WTH lost to follow-up/withdrawal, PD3 positive on day 3, LCF late clinical failure, LPF late parasitological failure, ACPR adequate clinical and parasitological response, ASAQ artesunate + amodiaquine, AL artemether + lumefantrine, DP dihydroartemisinin + piperaquine.
Genotyping and molecular markers of drug resistance (Pfk13 and Pfcoronin)
msp1 and msp2 genotyping and 24 SNP-based barcoding results were in agreement on all tested samples. Among the nine treatment failures, one was classified as ETF (recurrence at day 2), six samples were successfully genotyped and two samples gave negative PCR results on the day of recurrence. Thus, the successfully genotyped samples showed three cases of recrudescence and three re-infections. Out of the three recrudescence cases, two samples were successfully sequenced and gave interpretable sequences at day 0 and the day of respective recurrence for both Pfk13 and Pfcoronin. As well, the sequencing of the ETF sample was successful for the two molecular markers. For one recrudescence sample, the sequencing was obtained only at day 0 and this was unsuccessful for the day of recurrence.
All sequences had wild-type Pfk13 allele and wild-type Pfcoronin allele for both day 0 and day of recurrence (Table 4).
Table 4.
Summary of genotyping of parasites and molecular markers analysis.
| Drug | Sample ID | Day of recurrence | Parasitemia at day of recurrence | PCR correction | Pfk13 allele | Pfcoronin allele |
|---|---|---|---|---|---|---|
| AL | 303 | 2 | 4910 (vs 1148 at day 0) | NA | WT | WT |
| AL | 344 | 7 | 55 | negativea | ||
| AL | 378 | 21 | 8730 | re-infection | ||
| AL | 305 | 35 | 72632 | recrudescence | WT | WT |
| AL | 306 | 35 | 44100 | re-infection | ||
| AL | 317 | 35 | 28700 | re-infection | ||
| DP | 278 | 14 | 2974 | recrudescence | WT | WT |
| DP | 260 | 35 | 40 | recrudescence | WT* | WT* |
| DP | 216 | 42 | 495 | negativea |
*Only sample from day 0 was successfully sequenced.
WT: wild-type
NA: Not applicable.
aThere were two samples for which microscopy was positive while DNA amplification (PCR correction) was negative, probably due to either false positive microscopy or low-quality DNA. Thus, it was not possible to classify those samples into recrudescence or re-infection.
Safety outcomes
Generally, drug regimens were well-tolerated. Few minor adverse events were recorded, which did not require a rescue treatment. This included only vomiting in four patients under the ASAQ arm.
Discussion
More than 10 years after ACT use in Senegal, this study has shown the efficacy and tolerance of these antimalarial drugs in Senegal.
Efficacy and safety of antimalarial drugs has been monitored since the 80 s as highlighted by the finding of chloroquine resistance in this period, yielding to the abandoning of this molecule in 20031. As well, ACT has been evaluated since their first introduction in 2006 in the country and annual TES following WHO protocol is now regularly conducted.
TES has the advantage to prospectively and directly evaluate the clinical and parasitological responses after antimalarial treatment and thus has the potential of early detection of delayed clearance. A change in the national malaria treatment policy should be initiated if the total treatment failure rate is ≥10%, as assessed through TES16.
In this study, PCR-corrected ACPR greater than 98% was observed for the three antimalarial combinations drugs. As well, parasitemia cleared rapidly on day-3 in this study.
This demonstrates that ACTs remains highly efficacious, more than ten years after their implementation, consistent with previous studies showing high cure rates and well tolerance of these drugs17–24.
Previously, artemether-lumefantrine given as four doses has been associated with a failure rate of 3.6%25. However, when this regimen was reviewed by switching to six doses, the high cure rate was restored26. This denotes the role of incorrect dosage. In addition, poor patient compliance and poor drug quality can play a major role in causing treatment failure. Thus, in its 2016–2020 Strategic Plan, the Senegal NMCP planned to strengthen coordination in the analytical control of drugs before any marketing authorization is issued, before any distribution of drugs and to monitor drug quality at operational level27.
Similarly, the efficacy of ACTs monitored in most African malaria-endemic countries showed overall average efficacy rates of dihydroartemisinin-piperaquine, artesunate-amodiaquine and artemether- lumefantrine of 98.7%, 98.3% and 97.9%, respectively10,28–35. Some reports of delayed parasite clearance have been reported in Africa but not consistent over time10.
Although treatment failure rate was very low in this study, it is important to analyze those particular samples at the molecular level. In fact, one mechanism of drug resistance emergence is through selection of a resistant clone which may then spread if successful transmission occurs8. In this study, Pfk13 and Pfcoronin were analyzed simultaneously. However, in this study, it was not intended to perform a comprehensive analysis of molecular markers of resistance. Instead, previous studies conducted from field isolates in Senegal showed no Pfk13 mutation associated with artemisinin delayed parasite clearance36. Pfcoronin is a novel marker that has been discovered using the same approach as that of Pfk13 but using Senegalese parasites background15. In this study, no Pfcoronin mutations associated with treatment failure were observed. To date, none of the Pfcoronin mutations conferring resistance have been observed in field isolates in Africa37,38. Unless slow clearance is observed, as measured by microscopy, the implication of Pfcoronin as a genetic basis of artemisinin resistance remains to be elucidated. Further investigations including protein dynamics and intracellular trafficking need to be addressed to better understand the mechanism of artemisinin resistance at the biochemical level39. However, consideration should be given in the future to Pfcoronin along with Pfk13 in monitoring molecular markers of artemisinin resistance during efficacy studies.
Conclusion
This study showed that AL, ASAQ and DP remain efficacious in the treatment of uncomplicated falciparum malaria in Senegal. Continued monitoring of treatment efficacy and for molecular markers of resistance throughout the country is recommended to allow early detection of emerging and spreading resistance and to provide updates for malaria control measures. In addition, it is important that both Pfk13 and Pfcoronin are monitored in sub-Saharan Africa studies.
Materials and Methods
Study design
This was a randomized, three-arm, open-label study to evaluate the efficacy and safety of artemether-lumefantrine (AL), artesunate-amodiaquine (ASAQ) and dihydroartemisinin-piperaquine (DP), following a modified WHO 2009 protocol11. The study was carried out in two different areas of malaria transmission level (Fig. 1): (1) the health post of Tomboronkoto (Kedougou region), a rural area, located in south where the transmission is high and 2) the health post of Keur Serigne Mbaye Sarr (Diourbel region), an urban area, in the centre where the transmission is moderate. In Tomboronkoto, the malaria incidence rate was estimated at 1049 per 1000 population in 2017. At the same time, in Keur Serigne Mbaye Sarr health post, the incidence rate was 168 per 1000 population40.
Figure 1.
Map of Senegal showing the two NMCP sentinel sites covered in the 2018 therapeutic efficacy study (TES). This map was created using QGIS v. 3.8.3-Zanzibar.
Sample size calculation
According to the WHO protocol11, a minimum of 73 patients should be recruited to detect a failure rate ≤5%, at confidence level of 95% and an estimate precision of 5%. An additional of 20% was added to take into account lost to follow-up and withdrawals. By applying this guideline, 88 patients were targeted for each drug combination in each site.
Patients screen and enrolment
Malaria suspected patients were screened using both malaria rapid diagnostic test (RDT) (SD Bioline Pf/HRP2) and microscopy. Patients were enrolled if they were aged 6 months or above, had an axillary temperature ≥37.5 °C at presentation or history of fever during the last 24 hours, a positive P. falciparum mono-infection parasitemia of 1,000 to 100,000 asexual forms/μl, ability to swallow oral medication, ability and willingness to comply with the study protocol and visit schedule for the duration of the study and written informed consent from the patient or from the parent or guardian for enrolled children. Infected patients who did not meet inclusion criteria were treated according to the NMCP guideline.
In addition, dried blood spots (DBS) were collected on Whatman filter paper for molecular analysis including parasite genotyping (PCR correction) to differentiate re-infections from recrudescence and to determine molecular markers of artemisinin resistance on samples that were subject to treatment failure.
Key exclusion criteria were any of the following: presence of general danger signs in children aged under 5 years or signs of severe falciparum malaria according to the definitions of WHO41, mixed or mono-infection with another Plasmodium species detected by microscopy, positive pregnancy test, severe malnutrition, febrile conditions due to diseases other than malaria or other known underlying chronic or severe diseases, regular medication, which may interfere with anti-malarial treatment, history of hypersensitivity reactions or contraindications to any of the medicine(s) being tested or used as alternative treatment(s).
Treatment and follow-up
Enrolled patients were randomly allocated into the three drug arms: oral administration of AL, ASAQ or DP using the WHO recommended therapeutic dose regimens11. The allocation sequence was generated using a randomization table created by an Excel-based application. Patients were observed for 30 min after the drug administration for adverse events or vomiting the study drugs. Any patient who vomited was re-treated with the same dose of medicine and observed for an additional 30 min. If vomiting occurred again, the patient was withdrawn and treated with IV quinine and if necessary, the patient was referred to the nearest hospital. Paracetamol was offered to all feverish patients.
All daily doses were administered at the health facility level under the supervision of the medical staff.
Follow-up visits were scheduled on days 1, 2, 3, 7, 14, 21, and 28 for treatment with AL and ASAQ and additional follow up on days 35 and 42 for DP treatment (the partner drug, piperaquine, has a longer elimination half-life). During the follow-up visit, the patients were subject to clinical assessment and parasitological examination of blood smear to seek for malarial parasites, and DBS was also collected.
Safety assessment
A physician from the research staff was responsible for monitoring the safety of ACTs. Adverse events were documented through interviews about previous symptoms and about symptoms that have emerged since the previous follow-up visit. A clinical examination was performed to determine any adverse event. The reported events were recorded and any serious adverse event was to be reported to the sponsor.
Laboratory assessment
Microscopic blood examination
Thick and thin smears were performed and slides were examined on days 0 to confirm the meeting criteria and during subsequent scheduled follow-up day or any unscheduled day if the patient returned for being unwell.
Microscopy examination of Giemsa-stained thick and thin blood films was performed to identify parasite species and determine density according to the WHO procedure42. Parasite density (per μl) was calculated assuming a white blood cell count of 8000/μl. All slides were read independently by two level 1 malaria microscopists and the average of the two counts was calculated. If any discrepancy was noted (either in species identification or difference of parasite density of >30%), slides were checked by a third independent reader, and parasite densities were calculated by averaging the two most close counts.
Genotyping of malaria parasites
In order to differentiate a recrudescence (same parasite strain) from a newly acquired infection (different parasite strain), a genotype analysis was performed. DNA was extracted from the DBS on day 0 (before treatment) and during recurrence of parasitemia on day 7 onwards (cases of treatment failure). All DNA samples from patients undergoing parasitemia recurrence were analyzed for genotyping of the highly polymorphic regions msp1 and msp2 loci, as recommended by WHO11. In addition, a 24 SNP-based molecular barcode using High Resolution Melting qPCR43,44 was used. The WHO 2009 protocol requires sequential genotyping of msp1, msp2 and glurp genes to discriminate re-infections from recrudescence11. The latter is confirmed only when all three markers yield to the same genotype. However, comparable results from msp1 and msp2 genotyping and the 24 SNP-based barcodes have been reported. Unlike msp genotyping, the SNP-based barcodes present the advantage to be faster and less intensive45.
Molecular markers for antimalarial drug resistance
In the case of confirmed recrudescence as described above, Pfk13 propeller domain (codon positions: 440–600) and a portion of the Pfcoronin gene (codon positions: 31–186) were amplified using a nested PCR assay. Amplicons were sequenced using Sanger method as previously described13,15 from extracted DNA on day 0 (before treatment) and during recrudescence of parasitemia on day 7 onwards.
DNA sequences were analyzed using the Geneious Prime software (version 2020.1.2) to identify specific single-nucleotide polymorphism (SNP) related to artemisinin resistance. Sequences generated in this study were submitted into the European Nucleotide Archive (EBI) database under the accession numbers LR782242, LR782250, LR782259, LR782260, LR782261 and LR782262.
Treatment outcome
Treatment outcomes were classified according to the WHO protocol as early treatment failure (ETF), late clinical failure (LCF), late parasitological failure (LPF) and adequate clinical and parasitological response (ACPR)11.
Data analysis
Data for each study participant were entered into the WHO standardized Microsoft Excel data collection form. This WHO excel sheet was specifically design for the classification of treatment outcome with and without PCR correction11.
Ethical clearance
The study protocol received ethical clearance from the Ethic Committee of the Senegalese Ministry of Health. Written informed consent was obtained from all participants or parents/guardians of the children. The authors have complied with all relevant regulations for work with human participants. This study was registered at the Pan African Clinical Trials Registry on 09 March 2020 under the number PACTR 202003802011316.
Acknowledgements
We are grateful to Daba Zoumarou and Younouss Diedhiou (Laboratory of Parasitology and Mycology at Aristide Le Dantec hospital) for their logistic support during fieldwork; Stéphane Ranque and Aly Kodio (Aix Marseille Univ, IRD, APHM, SSA, VITROME, Marseille, France; IHU Méditerranée Infection, Marseille, France) for PCR and sequencing facilities; Naomi W Lucchi (Malaria Branch, Division of Parasitic Diseases and Malaria, Center for Global Health, Centers for Disease Control and Prevention, Atlanta, GA, USA) for reviewing this manuscript; the study participants and field teams for their involvement in this study. This work was supported by the PMI/USAID under the Senegal Fixed Amount Reimbursement Agreement (FARA) NMCP.
Author contributions
M.A.D. and M.S.Y. wrote the main manuscript and contributed equally to this work. Y.D.N. and A.B.D. performed genotyping. D.S. prepared figure 1. I.D., A.S.B. and D.N. conceived the study protocol. J.F.G. did the data management. K.D., S.A.S., M.S.Y., B.D. and M.A.D. performed microscopy and supervised the field study. M.A.D. performed Sanger sequencing. M.N. and M.C.S. did the data analysis. M.S. created the revised version of the map in figure 1. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Mamadou Alpha Diallo and Mamadou Samb Yade.
References
- 1.Trape, J. et al. The rise and fall of malaria in a west African rural community, Dielmo, Senegal, from 1990 to 2012: a 22 year longitudinal study. Lancet Infect. Dis.14, 476–488 (2014). [DOI] [PubMed]
- 2.Sinclair, D., Zani, B., Donegan, S., Olliaro, P. & Garner, P. Artemisinin-based combination therapy for treating uncomplicated malaria. Cochrane Database Syst. Rev. 10.1002/14651858.CD007483.pub2 (2009). [DOI] [PMC free article] [PubMed]
- 3.USAID. Evaluation of the Impact of the Scale-up of Malaria Control Interventions on All- Cause Mortality in Children under Five Years of Age in Senegal. USAID (2015).
- 4.Kone, G. K., Audibert, M., Lalou, R., Lafarge, H. & Le Hersan, J.-Y. Subsidized antimalarial drugs in Dakar (Senegal): Do the poor benefit? Etudes Doc. 1–25 (2017).
- 5.Thiam S, et al. Scale-up of home-based management of malaria based on rapid diagnostic tests and artemisinin-based combination therapy in a resource-poor country: results in Senegal. Malar. J. 2012;11:334. doi: 10.1186/1475-2875-11-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naing C, Whittaker MA, Htet NH, Aye SN, Mak JW. Efficacy of antimalarial drugs for treatment of uncomplicated falciparum malaria in Asian region: A network meta-analysis. Plos one. 2019;14:1–16. doi: 10.1371/journal.pone.0225882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashley EA, et al. Spread of Artemisinin Resistance in Plasmodium falciparum Malaria. N. Engl. J. Med. 2014;371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White NJ. Antimalarial drug resistance. J. Clin. Invest. 2004;113:1084–1092. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Tyne D, et al. Changes in drug sensitivity and anti-malarial drug resistance mutations over time among Plasmodium falciparum parasites in Senegal. Malar. J. 2013;12:1–10. doi: 10.1186/1475-2875-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. Artemisinin resistance and artemisinin-based combination therapy efficacy: status report. (World Health Organization, 2018).
- 11.WHO. Methods for surveillance of antimalarial drug efficacy. World Health Organization doi:ISBN 978 92 4 159753 1 (2009).
- 12.Basco, L. K. Field application of in vitro assays for the sensitivity of human malaria parasites to antimalarial drugs. World Heal. Organ. 191 (2007).
- 13.Ariey F, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muwanguzi J, et al. Lack of K13 mutations in Plasmodium falciparum persisting after artemisinin combination therapy treatment of Kenyan children. Malar. J. 2016;15:36. doi: 10.1186/s12936-016-1095-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demas AR, et al. Mutations in Plasmodium falciparum actin-binding protein coronin confer reduced artemisinin susceptibility. Proc. Natl. Acad. Sci. 2018;115:12799–12804. doi: 10.1073/pnas.1812317115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Status report on artemisinin resistance and ACT efficacy. World Health Organization (2018).
- 17.Dieye B, et al. West Africa International Centers of Excellence for Malaria Research: Drug Resistance Patterns to Artemether-Lumefantrine in Senegal, Mali, and The Gambia. Am. J. Trop. Med. Hyg. 2016;95:1054–1060. doi: 10.4269/ajtmh.16-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faye B, et al. Efficacy and tolerability of artesunate-amodiaquine (Camoquin plus) versus artemether-lumefantrine (Coartem) against uncomplicated Plasmodium falciparum malaria: Multisite trial in Senegal and Ivory Coast. Trop. Med. Int. Heal. 2010;15:608–613. doi: 10.1111/j.1365-3156.2010.02487.x. [DOI] [PubMed] [Google Scholar]
- 19.Yavo W, et al. Multicentric assessment of the efficacy and tolerability of dihydroartemisinin-piperaquine compared to artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in sub-Saharan Africa. Malar. J. 2011;10:198. doi: 10.1186/1475-2875-10-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menan H, et al. Comparative study of the efficacy and tolerability of dihydroartemisinin - Piperaquine - trimethoprim versus artemether - Lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Cameroon, Ivory Coast and Senegal. Malar. J. 2011;10:185. doi: 10.1186/1475-2875-10-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Souares A, Lalou R, Sene I, Sow D, Le Hesran JY. Adherence and effectiveness of drug combination in curative treatment among children suffering uncomplicated malaria in rural Senegal. Trans. R. Soc. Trop. Med. Hyg. 2008;102:751–758. doi: 10.1016/j.trstmh.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 22.Tine RC, et al. Efficacy and tolerability of a new formulation of artesunate-mefloquine for the treatment of uncomplicated malaria in adult in Senegal: Open randomized trial. Malar. J. 2012;11:1. doi: 10.1186/1475-2875-11-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faye B, et al. A randomized trial of artesunate mefloquine versus artemether lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in Senegalese children. Am. J. Trop. Med. Hyg. 2010;82:140–144. doi: 10.4269/ajtmh.2010.09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sow D, et al. Evaluation of the efficacy and safety of three 2-drug combinations for the treatment of uncomplicated Plasmodium falciparum malaria in Senegal: artesunate-amodiaquine, dihydroartemisinin-piperaquine, and artemether-lumefantrine. Med. Sante Trop. 2019;26:45–50. doi: 10.1684/mst.2015.0524. [DOI] [PubMed] [Google Scholar]
- 25.Faye B, et al. Efficacy and tolerability of four antimalarial combinations in the treatment of uncomplicated Plasmodium falciparum malaria in Senegal. Malar. J. 2007;6:1–9. doi: 10.1186/1475-2875-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sylla, K. et al. Monitoring the efficacy and safety of three artemisinin based-combinations therapies in Senegal: Results from two years surveillance. BMC Infect. Dis. 13, (2013). [DOI] [PMC free article] [PubMed]
- 27.Programme National de Lutte contre le Paludisme (PNLP). Plan stratégique national de lutte contre le paludisme 2016–2020 (2015).
- 28.Kakolwa MA, et al. Efficacy and safety of artemisinin-based combination therapy, and molecular markers for artemisinin and piperaquine resistance in Mainland Tanzania. Malar. J. 2018;17:1–10. doi: 10.1186/s12936-018-2524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nhama A, et al. In vivo efficacy of artemether-lumefantrine and artesunate-amodiaquine for the treatment of uncomplicated falciparum malaria in children: A multisite, open-label, two-cohort, clinical trial in Mozambique. Malar. J. 2014;13:1–9. doi: 10.1186/1475-2875-13-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warsame M, et al. High therapeutic efficacy of artemether-lumefantrine and dihydroartemisinin-piperaquine for the treatment of uncomplicated falciparum malaria in Somalia. Malar. J. 2019;18:1–11. doi: 10.1186/s12936-019-2864-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Getnet G, et al. Therapeutic efficacy of artemether-lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in Enfranze, north-west Ethiopia. Malar. J. 2015;14:1–7. doi: 10.1186/s12936-015-0775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nega D, et al. Therapeutic efficacy of artemether-lumefantrine (Coartem) in treating uncomplicated P. falciparum malaria in Metehara, Eastern Ethiopia: Regulatory clinical study. Plos one. 2016;11:1–14. doi: 10.1371/journal.pone.0154618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teklemariam M, Assefa A, Kassa M, Mohammed H, Mamo H. Therapeutic efficacy of artemether-lumefantrine against uncomplicated Plasmodium falciparum malaria in a high-transmission area in northwest Ethiopia. Plos one. 2017;12:1–18. doi: 10.1371/journal.pone.0176004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niaré K, et al. In vivo efficacy and parasite clearance of artesunate + sulfadoxine-pyrimethamine versus artemether-lumefantrine in Mali. Am. J. Trop. Med. Hyg. 2016;94:634–639. doi: 10.4269/ajtmh.15-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dorkenoo AM, et al. Therapeutic efficacy trial of artemisinin-based combination therapy for the treatment of uncomplicated malaria and investigation of mutations in k13 propeller domain in Togo, 2012–2013. Malar. J. 2016;15:1–9. doi: 10.1186/s12936-016-1381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Talundzic E, et al. Molecular Epidemiology of Plasmodium falciparum kelch13 Mutations in Senegal Determined by Using Targeted Amplicon Deep Sequencing. Antimicrob. Agents Chemother. 2017;61:e02116–16. doi: 10.1128/AAC.02116-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Velavan TP, Nderu D, Agbenyega T, Ntoumi F, Kremsner PG. An alternative dogma on reduced artemisinin susceptibility: A new shadow from east to west. Proc. Natl. Acad. Sci. 2019;116:12611–12612. doi: 10.1073/pnas.1907142116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma AI, Demas AR, Hartl DL, Wirth DF. Polymorphisms of pfcoronin in natural populations: Implications for functional significance. Proc. Natl. Acad. Sci. USA. 2019;116:12613–12614. doi: 10.1073/pnas.1907920116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henrici RC, Sutherland CJ. Alternative pathway to reduced artemisinin susceptibility in Plasmodium falciparum. Proc. Natl. Acad. Sci. USA. 2018;115:12556–12558. doi: 10.1073/pnas.1818287115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Programme National de Lutte contre le Paludisme (PNLP). Bulletin épidémiologique annuel du paludisme au Sénégal. (2018).
- 41.WHO. Management of severe malaria. (World Health Organization, 2012).
- 42.WHO. Malaria microscopy quality assurance manual. (WHO, 2015).
- 43.Daniels R, et al. A general SNP-based molecular barcode for Plasmodium falciparum identification and tracking. Malar. J. 2008;7:223. doi: 10.1186/1475-2875-7-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daniels RF, et al. Modeling malaria genomics reveals transmission decline and rebound in Senegal. Proc. Natl. Acad. Sci. USA. 2015;112:7067–7072. doi: 10.1073/pnas.1505691112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fulakeza J, et al. Comparison of msp genotyping and a 24 SNP molecular assay for differentiating Plasmodium falciparum recrudescence from reinfection. Malar. J. 2019;18:84. doi: 10.1186/s12936-019-2695-0. [DOI] [PMC free article] [PubMed] [Google Scholar]