Abstract
Tertiary lymphoid structures (TLS) are ectopic lymphoid formations that form within nonlymphoid tissue. They share structural and functional characteristics with secondary lymphoid structures such as lymph nodes and can contain B-cell follicles and germinal centers surrounded by a T-cell region. TLS have been described in several types of cancers and are usually associated with positive patient outcomes. However, TLS differ vastly in cellular composition and location within tissue types. In this review, we discuss factors confounding the interpretation of the evidence for a prognostic role for TLS in cancer and frame these factors in the context of translation to regular clinical use.
Keywords: tertiary lymphoid structure, prognosis, cancer, tissue
Subject terms: Prognostic markers, Tumour immunology
Introduction
Multiple reviews and research articles have discussed the composition and possible origin of tertiary lymphoid structures (TLS) in health and disease. A correlation has been found between high densities of TLS and prolonged patient survival in many cancers. As this correlation has been reviewed extensively elsewhere,1–4 the scope of this review is to consider the practical use and limitations of TLS as part of a prognostic armamentarium for human cancer.
Tertiary lymphoid structures
TLS are discrete, structured organizations of infiltrating immune cells.5 Unlike primary or secondary lymphoid structures or organs, TLS are not formed prenatally and are not present under normal conditions, although lymphocyte-rich nodules can be found in the absence of disease. Instead, TLS appear to be induced in chronically inflamed locations, such as persistent pathogen infection, autoimmune disorders, allograft rejection, and cancer.3,6
TLS are very similar to lymph nodes in both structure and development, and the organization and integrity of TLS are supported by stromal cells7. Well-developed TLS contain B-cell follicles with actively replicating B-cell germinal centers (GCs) surrounded by a T-cell region. Interspersed throughout the TLS are high endothelial venules (HEV) and dendritic cell-lysosomal associated membrane protein (DC-LAMP)+ dendritic cells (DC). However, TLS are not encapsulated and occur within various nonlymphoid tissues, such as epithelial tissues and stroma.6 Thus, data suggest that there is a continuum of lymphoid tissues: from the preprogrammed and well organized secondary lymphoid organs (SLOs), to the nonencapsulated, nonlymphoid tissues such as Peyer’s patches and pre-existing lymphoid follicles in the colon, and to the plastic and transient TLS, which can develop after birth under infectious or cancerous conditions. In contrast to SLOs, which are well defined and refer to specific structures such as lymph nodes, TLS refer to structures with varying organization. TLS can be simple lymphocyte aggregates or more organized structures (Fig. 1, Table 1). We refer to TLS throughout this review as any sort of lymphoid aggregate with similarities to SLO present in a nonlymphoid structure.
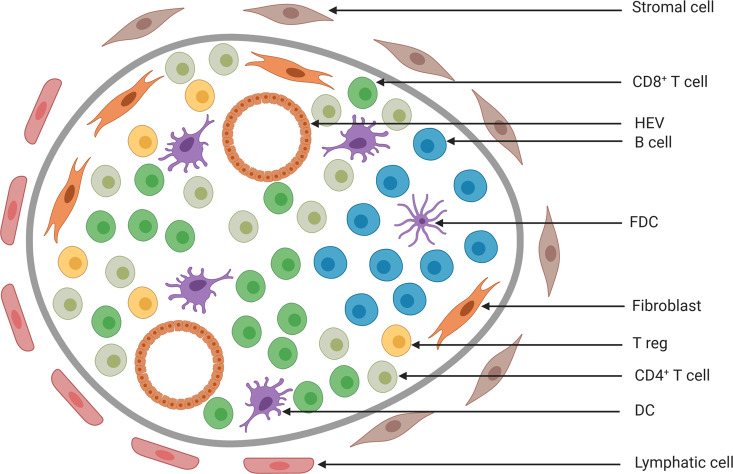
Fig. 1.
Model of the composition of a tertiary lymphoid structure. Cells are segregated into T- and B-cell zones. Mature dendritic cells (DCs) are present within the T-cell zone. High endothelial venules (HEVs) allow the extravasation of immune cells from the blood, such as DCs, T and B cells, and T regulatory cells (Tregs). B cells can cluster and form B-cell follicles with actively replicating B-cell germinal centers around follicular dendritic cells (FDCs). Compared to lymph nodes, tertiary lymphoid structures are not embedded in a collagen-rich fibrous capsule
Table 1.
Composition of TLSs and detection of markers
| Structure/cell | Corresponding marker | |
|---|---|---|
| Most common TLS content |
High endothelial venules Dendritic cells B cells T cells |
No specific marker, MECA-79 DC-LAMP CD20 CD3 |
| Possible components of TLSs |
Germinal centers Proliferating B cells T follicular helper cells |
Ki67 Ki67 CD4, CXCR5, CD40L, IL21, IL6 |
| Other markers associated with TLSs |
Chemokines Cytotoxic T cells T regulatory cells Endothelial cells Follicular DCs, mature B cells |
CCL-2,3,4,5,8,18,19,21 CXCL-9,10,11,13 CD8 FOXP3 CD31 CD23 |
Prognostic value of TLS
In autoimmune disorders, the presence of TLS is usually associated with an increased negative prognostic outcome, such as in rheumatoid arthritis, Sjögren’s syndrome, and Hashimoto’s thyroiditis, where increased frequencies of TLS are associated with increased disease severity.8 Tumor-associated TLS, however, are often associated with a good prognostic outcome in most cancers, including breast, colorectal (CRC), and lung cancer.3 Negative prognostic associations of TLS with cancer may be a result of compositionally different TLS.
There is extensive data on the prognostic value of TLS in multiple cancers; these have been recently reviewed and summarized previously9–11 and therefore will not be covered here. The mechanisms of antitumor immunity provided by TLS may be due to individual components, including effector cytokines and directly cytotoxic molecules produced by T cells, antitumor antibodies produced by B cells and increased presentation of tumor antigens by local dendritic cells. Antitumor mechanisms of TLS have been recently reviewed by Sautes-Fridman et al.11.
Given the similar structural and compositional nature of TLS to lymph nodes, their proximity to inflammatory locations, and their generally contrasting prognostic outcome associations with autoimmune diseases and cancer, it is likely that TLS play a direct role in influencing the immune response. Several studies have demonstrated the use of TLS as a prognostic indicator for cancer. These studies have been summarized and reviewed elsewhere;1–4 however, there is evidence that the ability to use TLS prognostically is confounded by the tissue of origin, tumor subtype, tumor location, and tumor stage. The heterogeneity of the composition of TLS, the variability in tumor-infiltrating lymphocytes, and the means used to quantify TLS in cancer further confound their use in the clinic. Studies observing TLS, lymphoid aggregates, or SLOs, within or associated with tumors, have used hematoxylin and eosin (H&E) staining for the visual quantification of cell aggregates, potentially underestimating the true number of TLS.12 However, more sophisticated analyses, observing the location and composition of TLS, have identified clues to the potential ontogeny and function of TLS in cancer. While recent papers have shown some clinical benefits associated with the presence of TLS, it is important to consider immunological and practical limitations to the use of TLS as a prognostic tool in the clinic.
Methods for evaluating TLS in cancer
The defined composition of TLS, while including some core components, can vary. Generally, a TLS contains HEVs, DC-LAMP+ cells, CD20+ B cells and CD3+ T cells but may or may not include an active GC, proliferating B cells or T follicular helper (TFH) cells.3,13 Furthermore, some analyses also consider one or all of the following as essential or complementary markers of a TLS: CD4, CD8, CD31, CD23, CD163, and FOXP3.3 The nonimmune components include fibroblasts14 and stromal cells7. Recent studies using multiparametric tools have highlighted the “double counting” that can occur when studying single molecules in a complex environment15 (Table 2). How then should one define a TLS? It is likely that this definition will need to be modified for different cancers.
Table 2.
Methods to detect and quantify TLS
| Method of detecting/characterizing TLS | Features of method |
|---|---|
| Hematoxylin and eosin (H&E) staining27 | Most basic histological analysis. Highly defined TLS with GCs can be confidently identified with this method, but distal sections (of the TLS structure) without clear B/T-cell zone borders or poorly formed TLS can be indistinguishable from other non-TLS lymphocytic aggregates. |
| Hematoxylin + 3,3′-diaminobenzidine (H-DAB) staining, H&E-DAB, H-DAB + alkaline phosphatase (AP) staining1,4,12,18,22,28,30–33,36–45,48,49,51 |
Single or double marker (H-DAB + AP) staining with a morphological stain increases confidence in accurately classifying lymphocytic aggregates as TLS. The drawback is that only 1–2 markers can be assessed per section; phenotypic composition of TLS with sequential sections using a variety of markers can be inferred but not quantified, and this approach introduces slice-to-slice variability. Assessment of the developmental/maturational status of TLS depends heavily on marker selection; at a minimum, a B-cell marker is required for a 1-marker panel, followed by a T-cell marker for a 2-marker panel. |
| Fluorescence immunohistochemistry (f-IHC)14,23,25 |
The ability to use 3–4 markers within a single slice facilitates basic cell phenotyping, which is a significant step up from H-DAB methods. However, several panels are still needed if maturational and organizational structures are to be identified; an example of this would be for the first panel to identify T and B cells as well as the CD4 status of the former, while the second panel would look for the presence of Ki67+ cells, lymphatics and FDCs. |
| Multiplex IHC19,34 |
These methods are able to simultaneously identify 5+ markers within the same tissue section. As a result, a variety of phenotypes, as well as the TLS maturation/organizational status, can be identified without being hampered by section-to-section variability. |
| Multiplex IHC + histocytometry16 |
The addition of a histocytometric analysis approach allows accurate phenotypic characterization of cells using all the available markers, resulting in a large number of phenotypes that can be identified with high specificity. Phenotypes can be quantified and advanced approaches can also derive statistical analyses from spatial relationships between phenotypes of interest. Analysis can be automated (after machine learning) allowing for high throughput. |
| RNA seq5,17,46,47 |
Identifying the presence of TLS within a whole-tissue RNA digest is a relatively simple method, provided that a reference signature dataset is available and is ideal for high-throughput analysis. However, this method is subject to any limitations and bias inherent in the reference dataset. Establishing a reference signature dataset is a significant undertaking; requiring access to large numbers of samples both in order to generate and validate a signature. |
| Laser capture microdissection (LCM) + RNA seq23 |
LCM allows specific targeting of TLS tissue, removing contaminating non-TLS RNA in the downstream analysis. This is the best RNA analysis approach of TLS short of spatial transcriptomics; however, this approach is driven by a minimum RNA quantity requirement (and therefore minimum region of interest size for dissection), which may restrict the specificity of the area selection. The laser capture-dissection step is the most time-consuming step of the procedure, and requires an uncommon piece of equipment. |
| Flow cytometry14 |
Flow cytometry requires a single-cell suspension of tissue, which results in a homogenous population from various tissue structures. Determining the presence of TLS with flow cytometry is difficult, as immune cells from non-TLS regions are mixed with ones from TLS. However, under certain conditions: careful tissue collection (e.g., ensuring if possible that no lymph nodes are present in the tissue sample) and use of markers to identify cell populations mainly present in lymphoid follicles can detect for the presence of TLS within a sample. Relative proportions of key cells (e.g., Ki67+ B cells) can also be assessed with this method. |
| Spatial transcriptomics21 |
Regions of interest on tissue section are selected based on H&E or with a limited selection of protein markers and are transcriptionally analyzed for a large selection of RNA markers or proteins. Histocytometric approaches depend on the method employed, with some methods such as GeoMx chosen due to the requirement of 20–200 cells for minimum RNA/protein content per comparative sample to be analyzed; therefore, histocytometric approaches are not feasible. Currently, there is only one other commercially available system (Visium from 10X Genomics). Both require significant operator expertize across several aspects of the procedure. |
The quantification of DC-LAMP+ cells within CD3+ cell aggregates has been proposed as the most consistent and reliable method to quantify TLS across multiple cancers and with simple methodology.3 Immunohistochemical (via H&E) assays are typically used to quantify TLS. Multiplexed imaging, such as that performed by Yamaguchi et al.16 highlights the diversity of TLS. Mirroring the complexity as well as devising a simple and effective way of evaluating TLS may be difficult. However, several studies have reported the use of straightforward tools, such as H&E, to provide meaningful clinical data. Shen et al.17 showed that a single region tumor sample was sufficient when stained with H&E to quantify TLS in hepatocellular carcinoma (HCC), and Pffanstiel et al.18 used the mean distance between TLS and the tumor to study prognosis in bladder cancer.
TLS gene expression signatures, which encompass genes from multiple cell types, have also been used.19,20 The latter includes chemokine receptor genes based on evidence linking a defined set of chemokine receptors and their ligands in the development and maintenance of TLS in tumors.8,21–23 Specifically, a 12-chemokine signature previously associated with the presence of TLS in CRC identified the presence of TLS in a cohort of over 14,000 solid tumors of various origins. The signature genes were of the CC chemokines CCL-2, -3, -4, -5, -8, -18, -19, -21 and the CXCL chemokines CXCL-9, -10, -11, -13. Furthermore, this signature was associated with an overall good prognostic outcome.5
The list of markers that can be used to define a TLS is exhaustive (reviewed in Engelhard et al.24), and decisions made on which markers to choose may depend on the available technology. The increased use of multiplex immunohistochemistry is likely to generate data proving the importance of specific cell subsets or proteins within a TLS.15 Despite the varied tools and definitions of TLS and the composition of TLS, there is significant evidence of a role for these structures in promoting antitumor immune responses.
Composition of TLS
The prognostic value of TLS has been especially well studied in colorectal cancer.4,25,26 Yamaguchi et al.16 used multiplex staining to characterize the cellular composition of TLS and determine the effect of cell types within TLS on disease recurrence. The densities of CD4+ T cells and macrophages were both associated with a higher rate of relapse in patients. The authors defined five types of TLS (GC-rich, B-cell rich, follicular DC rich, Th (CD4) rich, and CTL (CD8/B/Th rich) as a means to quantify TLS composition. In contrast, Posch et al. defined three types of TLS based on the composition of the aggregates, including the activation state of the cells within each TLS. These types of TLS reflected different stages of maturation: early TLS (dense aggregates with nondifferentiated FDCs); primary follicle-like TLS (B-cell clusters with FDC networks but without GCs); and secondary follicle-like TLS (with GCs). They found that a high proportion of mature TLS gave a better prognostic association with outcome than total TLS, highlighting the importance of the TLS composition.25 In support of this hypothesis, Kim et al.27 studied a cohort of lung cancer patients and stratified them based on the presence or absence of TLS, low or high TLS, and the presence or absence of GC in TLS. These tiered approaches can be more easily classified, such as with IHC as done in this study, which may make clinical translation more feasible.
B cells are increasingly being studied as mediators of antitumor immunity.19,20 Given the presence of B cells in almost all TLS, more research is now focused on the impact of the B-cell component of TLS. Seow et al.28 used multiplex imaging in patients with triple negative breast cancer to identify TLS and showed that tumors with a high frequency of plasma cells had higher numbers of TLS, supporting a functional role for B cells in prognosis. Zhu et al.29 showed that the presence of B cells in TLS supported greater T-cell clonality in patients with non-small cell lung carcinoma. The B-cell follicles have also been associated with antigen-specific antibody responses in transplantation (reviewed in Koenig et al.30). In a study of patients with esophagogastric adenocarcinoma,31 B cells organized into TLS and produced tumor-specific antibodies. Together, these data support a functional role for TLS-associated B cells in preventing tumor growth.
Although most research has examined T cells and B cells in TLS, many other rare immune cell populations are present and can impact patient survival. For example, NKp44+ innate lymphoid cells identified in early-stage CRC patients had high expression of TLS formation genes, such as those encoding LTα, LTβ, and TNF, and a decrease in these ILCs was associated with a decrease in TLS density.32
The composition of a TLS, much like frequency, may also depend on the type of cancer (reviewed in Colbeck et al.2). A study of TLS in rectal cancer found that while the overall infiltrate of CD3+ T cells was associated with improved survival, CD3+ CD83+ lymphoid nodules were ubiquitously present, thus limiting their use in predicting patient outcome.33 Similarly, the high number of lymphoid aggregates in desmoplastic melanoma was actually a high number of TLS. In contrast to melanoma, where TLS remain predominantly in the peritumoral areas, TLS in desmoplastic melanoma were found both around and within the tumor.34
Number and density of TLSs and effect on patient outcome
Historically, an increased density of DC-LAMP+ dendritic cells was used to associate TLS with outcome,35,36 but more recent studies have shown a similar prognostic significance for CD20+ aggregates (B-cell rich; refs. 37–40) and CD3+ aggregates (T-cell rich33,41). However, using a single parameter to quantify cells within a TLS is likely to generate results different from those obtained by counting the overall number or density of aggregates using H&E.42
One difficulty in understanding the impact of TLS density is a lack of consensus on what constitutes “high” or “low” density. The number of TLS per slide or tumor can be subcategorized. For example, in a study of breast cancer, the TLS were counted within 5 mm from the infiltrative tumor border: negative, low to moderate (1–4) or high (5+).13 Alternatively, high versus low can be divided as above or below the median for the specific cohort, as for other immune quantification.43 Many research papers clearly define their quantification method, such as work by Kim et al.27, which evaluated TLS as a percentage of the circumference of the tumor front. Pffanstiel et al.18 showed that high numbers of TLS within a close tumor distance correlated with an inflamed phenotype and favorable outcome for bladder cancer patients. However, without a consensus in methodology, it will be difficult to standardize the use of TLS density as a clinical marker.
Across multiple human cancers, a higher frequency of TLS has been associated with prolonged patient survival. This finding has been reviewed and summarized extensively elsewhere.2,3,9 Despite an overall positive association, there have been negative associations of TLS density with patient outcome, often dependent on the type or stage of cancer. A possible reason for the difference in outcome data is a lack of agreement over what constitutes a TLS and how the quantification is performed in the laboratory.
Location of TLS associated with tumor and its effect on patient outcome
Recent studies have highlighted the importance of the immune infiltrate in improving patient survival, and this is most well documented and validated in CRC.44 The CD3+ T cell and CD8+ T cell infiltrate is now being used alongside other traditional staging tools. The location of these cells, such as at the invasive margin, rather than the center of the tumor, is an important positive predictor of outcome. Similarly, the location of the TLS is likely to be important in determining their efficacy in predicting outcome, and it has been postulated that the location of the TLS at the tumor site reflects its function in the tumor immune response.24 However, the location of TLS in relation to prognostic significance can be difficult to assess.
A study showing the prognostic significance of TLS in breast cancer quantified the density of peritumoral TLS as 5 mm from the infiltrative border – adjacent TLS were at this limit, and distal TLS were defined as having normal breast tissue between the TLS and the tumor. A high frequency of adjacent TLS was associated with worse disease-free survival for patients; a low frequency was associated with a lower tumor burden.13 However, those patients with high densities of both adjacent and distal TLS had the worst outcome. Similarly, Posch et al.,25 in a cohort of CRC patients, defined the peritumoral area as 7 mm from the invasive margin – this would encompass multiple tissue regions, and Di Caro et al.41 quantified TLS in CRC at the invasive margin but did not define an invasive margin, also noting the presence of TLS in stroma. Our own studies with CRC (unpublished) have identified TLS in broad regions in peritumoral tissue up to 7 mm from the invasive edge, including the mucosa, submucosa, myenteric nerve layer, and mesentery (where lymph nodes were also found). We also found that the morphology of TLS can differ significantly within the same tumor biopsy sample depending on the tissue; for example, mucosal TLS tended to be squished and slender or teardrop-shaped, whereas submucosal and mesenteric TLS appeared very large and quite round. Finally, the individual tissues within the immunohistochemistry sections used to quantify TLS can vary according to the plane of the section.
Collectively, these data suggest that the predictive effect of TLS may be due not only to the number and composition but also to the function and activity of the cells inside, which in turn is affected or controlled by the site of such a localized immune reaction. In support of this hypothesis, a recent publication showed that patients with more tumor-infiltrating TLS had a higher frequency of melanomas with a tumor-infiltrating CD8+ T cell pattern than those with fewer tumor-infiltrating TLS. These data suggest that TLS form an essential part of the infiltrating antitumor immune response.20 Gobert et al.45 showed that Tregs in infiltrating primary breast cancer were not associated with tumor growth, but Tregs within TLS had an activated phenotype. Finally, Calderaro et al.46 showed that the number of TLS in healthy liver tissue of people with HCC had no association with recurrence. However, patients with a high frequency of intratumoral TLS had a lower risk of early recurrence after surgery compared with patients with a low frequency of intratumoral TLS.
Finally, there can be significant differences in the effect of TLS in primary versus metastatic tumors in a range of cancers. CRC lung metastases were associated with well-developed TLS, whereas renal cell carcinoma lung metastases were associated with poorly developed TLS;47 in a study of melanoma patients, TLS developed in metastases but not in the primary tumor.48 Similarly, in a study of breast cancer patients, tumor-infiltrating lymphocytes were present in almost all metastasized organs, but TLS were present only in the lungs and liver.49
Almost all studies describe peritumoral TLS rather than intratumoral TLS, but it is often difficult to determine the exact location. Standardization of location within cancer subtypes will be essential to properly quantify the TLS and accurately predict outcomes.
The potential use of TLS in clinical practice
Widespread clinical uptake of new findings requires robust, reproducible evidence of the reliability of those findings. Patient management plans are multidisciplinary and guideline-driven.
For example, international best practice requires every patient with a new diagnosis of malignancy to be discussed at a multidisciplinary meeting comprising surgeons, pathologists, oncologists, and radiologists.50 Despite this multidisciplinary approach, the clinicians managing patient care cannot be expected to keep abreast of all new prognostic and treatment developments as they arise. To address this issue and facilitate fair, equitable, and evidence-based distribution of patient treatment in a resource-limited environment, national guidelines are developed and used.
The studies discussed in this review demonstrate that TLS are likely to be clinically important in patient prognosis. Better understanding of the sequence of immunological events that contribute to patient outcomes will assist with identifying which patients may require more aggressive management and identifying specific patients likely to benefit from existing immunotherapies. For example, two recent studies showed that the density of TLS was higher in melanoma patients responsive to immunotherapy than in those who were nonresponsive.19,20 Table 3 identifies what steps need to be taken to facilitate clinical uptake of TLS research.
Table 3.
How may TLS be of use in the clinical environment?
| TLS feature | Clinical application | Barrier to clinical uptake |
|---|---|---|
| High TLS numbers are associated with a positive patient prognosis in many cancers. | A more accurate prognosis will assist with better patient selection for treatments. |
There is mixed evidence for prognostic ability based on the frequency of TLS alone. There is no evidence yet that TLS provide prognostic information over and above that provided by existing validated tools. |
| Subtypes of TLS features are associated with differing patient outcomes. | Better characterization of TLS subtype features may assist with patient selection for existing immunotherapies and developing new therapies. | Multiplex characterization of TLS is an ongoing research area. |
Above all, uptake of TLS in the clinical field is only rational if TLS data confer additional prognostic accuracy relative to existing clinical tools. Landmark TLS research will require a review of existing TLS definitions and markers, with a large cohort multiplex analysis of each marker. Large cohort analyses may reveal one or two markers that are reliable prognostic tools that confer additional prognostic information. If so, robust marker validation and interrater reliability validation may lead to clinical uptake of TLS quantification as a prognostic tool. Identifying one or two key TLS markers is intrinsic to facilitating clinical uptake. Single assays are more clinically accessible than multiplex IHC, imaging, and analysis.
Conclusion
Despite developing evidence that TLS are intrinsic to the immune response in cancer, the clinical application of this information is far from standard practice. A new prognostic tool must be robust, reliable, and well described, and there needs to be high interrater reliability. All health systems function with financial constraints; consequently, the ideal new tool will use low-cost reagents, and imaging techniques will use existing laboratory equipment. Ongoing research into the ontogeny and function of TLS in association with patient data in large cohorts will provide the information needed to transform TLS from an interesting immunological phenomenon to a reliable clinical tool.
Acknowledgements
L.M.E. was supported by the Maurice Wilkins Centre for Biodiscovery, New Zealand. J.L.R. is supported by a Health Research Council Clinical Fellowship. We thank Hamish Angus, Ginny Niemi and Justin Tirados for critical review of the manuscript.
Author contributions
L.M.E. and R.A.K. conceived the scope and content; all authors contributed to writing the manuscript.
Competing interests
The authors declare no competing interests.
References
- 1.Bergomas F, et al. Tertiary intratumor lymphoid tissue in colo-rectal cancer. Cancers. 2011;4:1–10. doi: 10.3390/cancers4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colbeck EJ, Ager A, Gallimore A, Jones GW. Tertiary lymphoid structures in cancer: drivers of antitumor immunity, immunosuppression, or bystander sentinels in disease? Front. Immunol. 2017;8:1830. doi: 10.3389/fimmu.2017.01830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dieu-Nosjean MC, Goc J, Giraldo NA, Sautes-Fridman C, Fridman WH. Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 2014;35:571–580. doi: 10.1016/j.it.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Trajkovski G, et al. Tertiary lymphoid structures in colorectal cancers and their prognostic value. Open Access Maced. J. Med. Sci. 2018;6:1824–1828. doi: 10.3889/oamjms.2018.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Messina JL, et al. 12-Chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy? Sci. Rep. 2012;2:765. doi: 10.1038/srep00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pimenta EM, Barnes BJ. Role of tertiary lymphoid structures (TLS) in anti-tumor immunity: potential tumor-induced cytokines/chemokines that regulate TLS formation in epithelial-derived cancers. Cancers. 2014;6:969–997. doi: 10.3390/cancers6020969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denton AE, Carr EJ, Magiera LP, Watts AJB, Fearon DT. Embryonic FAP(+) lymphoid tissue organizer cells generate the reticular network of adult lymph nodes. J. Exp. Med. 2019;216:2242–2252. doi: 10.1084/jem.20181705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carragher DM, Rangel-Moreno J, Randall TD. Ectopic lymphoid tissues and local immunity. Semin. Immunol. 2008;20:26–42. doi: 10.1016/j.smim.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sautes-Fridman C, et al. Tertiary lymphoid structures in cancers: prognostic value, regulation, and manipulation for therapeutic intervention. Front. Immunol. 2016;7:407. doi: 10.3389/fimmu.2016.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin L, Hu X, Zhang H, Hu H. Tertiary lymphoid organs in cancer immunology: mechanisms and the new strategy for immunotherapy. Front. Immunol. 2019;10:1398. doi: 10.3389/fimmu.2019.01398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sautes-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer. 2019;19:307–325. doi: 10.1038/s41568-019-0144-6. [DOI] [PubMed] [Google Scholar]
- 12.Buisseret L, et al. Reliability of tumor-infiltrating lymphocyte and tertiary lymphoid structure assessment in human breast cancer. Mod. Pathol. 2017;30:1204–1212. doi: 10.1038/modpathol.2017.43. [DOI] [PubMed] [Google Scholar]
- 13.Sofopoulos M, et al. The prognostic significance of peritumoral tertiary lymphoid structures in breast cancer. Cancer Immunol. Immunother. 2019;68:1733–1745. doi: 10.1007/s00262-019-02407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nayar S, et al. Immunofibroblasts are pivotal drivers of tertiary lymphoid structure formation and local pathology. Proc. Natl Acad. Sci. USA. 2019;116:13490–13497. doi: 10.1073/pnas.1905301116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leman JK, Sandford SK, Rhodes JL, Kemp RA. Multiparametric analysis of colorectal cancer immune responses. World J. Gastroenterol. 2018;24:2995–3005. doi: 10.3748/wjg.v24.i27.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamaguchi K, et al. Helper T cell-dominant tertiary lymphoid structures are associated with disease relapse of advanced colorectal cancer. Oncoimmunology. 2020;9:1724763. doi: 10.1080/2162402X.2020.1724763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen YC, et al. Reliability of a single-region sample to evaluate tumor immune microenvironment in hepatocellular carcinoma. J. Hepatol. 2020;72:489–497. doi: 10.1016/j.jhep.2019.09.032. [DOI] [PubMed] [Google Scholar]
- 18.Pfannstiel, C. et al. BRIDGE Consortium, Germany. The tumor immune microenvironment drives a prognostic relevance that correlates with bladder cancer subtypes. Cancer Immunol. Res.7, 923–938 (2019). [DOI] [PubMed]
- 19.Helmink BA, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577:549–555. doi: 10.1038/s41586-019-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cabrita R, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577:561–565. doi: 10.1038/s41586-019-1914-8. [DOI] [PubMed] [Google Scholar]
- 21.Coelho FM, et al. Naive B-cell trafficking is shaped by local chemokine availability and LFA-1-independent stromal interactions. Blood. 2013;121:4101–4109. doi: 10.1182/blood-2012-10-465336. [DOI] [PubMed] [Google Scholar]
- 22.Luther SA, et al. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J. Immunol. 2002;169:424–433. doi: 10.4049/jimmunol.169.1.424. [DOI] [PubMed] [Google Scholar]
- 23.de Chaisemartin L, et al. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res. 2011;71:6391–6399. doi: 10.1158/0008-5472.CAN-11-0952. [DOI] [PubMed] [Google Scholar]
- 24.Engelhard VH, et al. Immune cell infiltration and tertiary lymphoid structures as determinants of antitumor immunity. J. Immunol. 2018;200:432–442. doi: 10.4049/jimmunol.1701269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Posch F, et al. Maturation of tertiary lymphoid structures and recurrence of stage II and III colorectal cancer. Oncoimmunology. 2018;7:e1378844. doi: 10.1080/2162402X.2017.1378844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schweiger T, et al. Tumor-infiltrating lymphocyte subsets and tertiary lymphoid structures in pulmonary metastases from colorectal cancer. Clin. Exp. Metastasis. 2016;33:727–739. doi: 10.1007/s10585-016-9813-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim A, et al. The prognostic significance of tumor-infiltrating lymphocytes assessment with hematoxylin and eosin sections in resected primary lung adenocarcinoma. PLoS ONE. 2019;14:e0224430. doi: 10.1371/journal.pone.0224430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seow DYB, et al. Tertiary lymphoid structures and associated plasma cells play an important role in the biology of triple-negative breast cancers. Breast Cancer Res. Treat. 2020;180:369–377. doi: 10.1007/s10549-020-05548-y. [DOI] [PubMed] [Google Scholar]
- 29.Zhu W, et al. A high density of tertiary lymphoid structure B cells in lung tumors is associated with increased CD4(+) T cell receptor repertoire clonality. Oncoimmunology. 2015;4:e1051922. doi: 10.1080/2162402X.2015.1051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koenig A, Thaunat O. Lymphoid neogenesis and tertiary lymphoid organs in transplanted organs. Front. Immunol. 2016;7:646. doi: 10.3389/fimmu.2016.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlosser HA, et al. B cells in esophago-gastric adenocarcinoma are highly differentiated, organize in tertiary lymphoid structures and produce tumor-specific antibodies. Oncoimmunology. 2019;8:e1512458. doi: 10.1080/2162402X.2018.1512458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikeda, A. et al. Human NKp44+ group 3 innate lymphoid cells associate with tumor-associated tertiary lymphoid structures in colorectal cancer. Cancer Immunol. Res.10.1158/2326-6066.CIR-19-0775 (2020). [DOI] [PubMed]
- 33.McMullen TP, Lai R, Dabbagh L, Wallace TM, de Gara CJ. Survival in rectal cancer is predicted by T cell infiltration of tumour-associated lymphoid nodules. Clin. Exp. Immunol. 2010;161:81–88. doi: 10.1111/j.1365-2249.2010.04147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stowman AM, et al. Lymphoid aggregates in desmoplastic melanoma have features of tertiary lymphoid structures. Melanoma Res. 2018;28:237–245. doi: 10.1097/CMR.0000000000000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ladanyi A, et al. Density of DC-LAMP(+) mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol. Immunother. 2007;56:1459–1469. doi: 10.1007/s00262-007-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dieu-Nosjean MC, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J. Clin. Oncol. 2008;26:4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 37.Germain C, et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am. J. Respir. Crit. Care Med. 2014;189:832–844. doi: 10.1164/rccm.201309-1611OC. [DOI] [PubMed] [Google Scholar]
- 38.Meshcheryakova A, et al. B cells and ectopic follicular structures: novel players in anti-tumor programming with prognostic power for patients with metastatic colorectal cancer. PLoS ONE. 2014;9:e99008. doi: 10.1371/journal.pone.0099008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hennequin A, et al. Tumor infiltration by Tbet+ effector T cells and CD20+ B cells is associated with survival in gastric cancer patients. Oncoimmunology. 2016;5:e1054598. doi: 10.1080/2162402X.2015.1054598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wirsing AM, Rikardsen OG, Steigen SE, Uhlin-Hansen L, Hadler-Olsen E. Characterisation and prognostic value of tertiary lymphoid structures in oral squamous cell carcinoma. BMC Clin. Pathol. 2014;14:38. doi: 10.1186/1472-6890-14-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Caro G, et al. Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. Clin. Cancer Res. 2014;20:2147–2158. doi: 10.1158/1078-0432.CCR-13-2590. [DOI] [PubMed] [Google Scholar]
- 42.Hiraoka N, et al. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br. J. Cancer. 2015;112:1782–1790. doi: 10.1038/bjc.2015.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward-Hartstonge KA, et al. Inclusion of BLIMP-1(+) effector regulatory T cells improves the Immunoscore in a cohort of New Zealand colorectal cancer patients: a pilot study. Cancer Immunol. Immunother. 2017;66:515–522. doi: 10.1007/s00262-016-1951-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pages F, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391:2128–2139. doi: 10.1016/S0140-6736(18)30789-X. [DOI] [PubMed] [Google Scholar]
- 45.Gobert M, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009;69:2000–2009. doi: 10.1158/0008-5472.CAN-08-2360. [DOI] [PubMed] [Google Scholar]
- 46.Calderaro J, et al. Intra-tumoral tertiary lymphoid structures are associated with a low risk of early recurrence of hepatocellular carcinoma. J. Hepatol. 2019;70:58–65. doi: 10.1016/j.jhep.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 47.Remark R, et al. Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: influence of tumor origin. Clin. Cancer Res. 2013;19:4079–4091. doi: 10.1158/1078-0432.CCR-12-3847. [DOI] [PubMed] [Google Scholar]
- 48.Cipponi A, et al. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res. 2012;72:3997–4007. doi: 10.1158/0008-5472.CAN-12-1377. [DOI] [PubMed] [Google Scholar]
- 49.Lee M, et al. Presence of tertiary lymphoid structures determines the level of tumor-infiltrating lymphocytes in primary breast cancer and metastasis. Mod. Pathol. 2019;32:70–80. doi: 10.1038/s41379-018-0113-8. [DOI] [PubMed] [Google Scholar]
- 50.Basta YL, Bolle S, Fockens P, Tytgat K. The value of multidisciplinary team meetings for patients with gastrointestinal malignancies: a systematic review. Ann. Surg. Oncol. 2017;24:2669–2678. doi: 10.1245/s10434-017-5833-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ladanyi A, et al. Ectopic lymphoid structures in primary cutaneous melanoma. Pathol. Oncol. Res. 2014;20:981–985. doi: 10.1007/s12253-014-9784-8. [DOI] [PubMed] [Google Scholar]