Abstract
Aldehyde is one of most synthetically versatile functional groups and can participate in numerous chemical transformations. While a variety of simple aromatic aldehydes are commercially available, those with a more complex substitution pattern are often difficult to obtain. Benzylic oxygenation of methylarenes is a highly attractive method for aldehyde synthesis as the starting materials are easy to obtain and handle. However, regioselective oxidation of functionalized methylarenes, especially those that contain heterocyclic moieties, to aromatic aldehydes remains a significant challenge. Here we show an efficient electrochemical method that achieves site-selective electrooxidation of methyl benzoheterocycles to aromatic acetals without using chemical oxidants or transition-metal catalysts. The acetals can be converted to the corresponding aldehydes through hydrolysis in one-pot or in a separate step. The synthetic utility of our method is highlighted by its application to the efficient preparation of the antihypertensive drug telmisartan.
Subject terms: Chemical synthesis, Electrochemistry, Synthetic chemistry methodology
Benzylic oxygenation of methylarenes is a direct but challenging method for aldehyde synthesis from simple starting materials. Here, the authors show an electrochemical, site-selective method for the oxidation of methyl benzoheterocycles to aromatic acetals without using chemical oxidants or transition metal catalysts.
Introduction
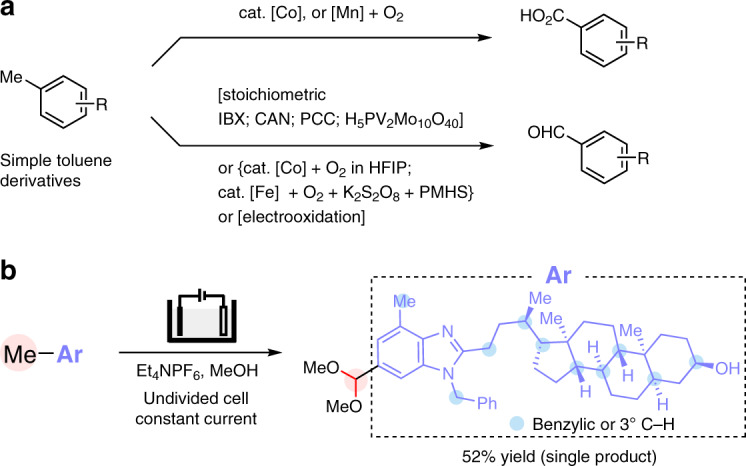
Benzylic oxygenation of alkylarenes provides crucial access to many industrial chemicals, such as terephthalic acid, phenol, and acetone, on a multimillion-ton scale1. Aldehyde is one of the most versatile synthetic handles and can be converted to numerous functionalities. As a result, aromatic aldehydes have been widely used in the manufacture of fine chemicals, nutraceuticals, performance materials, and pharmaceuticals. The oxygenation of methylarenes is a straightforward and attractive strategy for the preparation of aromatic aldehydes, especially considering that the starting materials are widely available and easy to handle. However, partial oxidation of methylarenes to aldehydes remains a largely unsolved challenge due to the strong propensity of product overoxidation under aerobic conditions (Fig. 1a)2,3, and unsatisfactory chemo- and regioselectivity with substrates bearing multiple oxidizable C–H bonds and/or functionalities4. Despite these difficulties, oxygenation of simple methylarenes to aldehydes has been achieved using stoichiometric chemical oxidants such as o-iodoxybenzoic acid (IBX)5, ceric ammonium nitrate (CAN)6, pyridinium chlorochromate7 or polyoxometalate H5PV2Mo10O408. Transition metal-catalyzed aerobic oxidation using hexafluoro-2-propanol as solvent9 or by adding polymethylhydrosiloxane as reagents to avoid overoxidation10 have also been reported (Fig. 1a). As an alternative to chemical oxidation, electrooxidation eliminates the use of stoichiometric chemical oxidants and is attracting increasing interests11–26. Notably, electrooxidation of electron-rich toluene derivatives to substituted benzaldehydes has been applied in the industrial production of p-anisaldehyde and 3,4,5-trimethoxybenzaldehyde27–29. Despite these accomplishments, the conversion of structurally complex methylarenes, including medicinally relevant benzoheterocycles in particular, has remained synthetically elusive because of selectivity issues and catalyst inhibition by the coordinating heteroatoms30.
Fig. 1. Methylarene oxidation.
a Examples of reported oxidation of relatively simple toluene derivatives to benzoic acids or benzaldehydes. b The present study focuses on site-selective electrooxidation of methyl benzoheterocycles to aromatic aldehydes.
We have been interested in electrochemical synthesis of heterocycles23,31,32 and recently reported intramolecular dehydrogenative cyclization reactions for the preparation of several types of benzoheterocycles33–37. Alternatively, we envision the synthesis of functionalized benzoheterocycles by modification of alkyl side chains of existing benzoheterocyclic scaffolds. Herein we report a generally applicable electrochemical strategy capable of oxidizing various methyl benzoheterocycles to aromatic acetals in a site-selective manner (Fig. 1b). These side chain oxidation reactions allow access to various functionalized benzoheterocycles difficult to obtain directly through cyclization processes.
Results
Reaction optimization
We began our study by first optimizing the electrooxidation of benzimidazole 1 bearing Me groups at positions 4 and 6 (Table 1). The best results were achieved in an undivided cell with refluxing methanol as solvent, Et4NPF6 (0.5 equiv) as electrolyte, a Pt plate cathode, and a reticulated vitreous carbon anode. Under these conditions, compound 1 reacted site-selectively at C6-Me group to give dimethyl acetal 2 in 72% yield (entry 1) without overoxidation to orthoester or unwanted oxidation of other potentially labile substituents such as C4-Me, N–Bn, or 3° C–H on the cyclohexyl moiety. Lowering the reaction temperature to RT dramatically decreased the yield of 2 to 25% (entry 2). Furthermore, moderate reduction in reaction efficiency was observed when the electrolysis of 1 was performed at a different current (entries 3 and 4), under air (entry 5), with a decreased amount of Et4NPF6 (entry 6), or with another electrolyte such as n-Bu4NPF6 (entry 8) or Et4NOTs (entry 9). Et4NBF4 (entry 7) was, however, equally effective as a supporting electrolyte. Product formation was also diminished with the use of a Pt (entry 10) or graphite anode (entry 11).
Table 1.
Optimization of reaction conditionsa.
| Entry | Deviation from standard conditions | Yield (%)b |
|---|---|---|
| 1 | None | 72c |
| 2 | Reaction at RT | 25 |
| 3 | Reaction at 8 mA | 61 |
| 4 | Reaction at 12 mA | 66 |
| 5 | Reaction under air | 50 |
| 6 | Et4NPF6 (0.2 equiv) | 63 |
| 7 | Et4NBF4 instead of Et4NPF6 | 72 |
| 8 | n-Bu4NPF6 instead of Et4NPF6 | 64 |
| 9 | Et4NOTs instead of Et4NPF6 | 66 |
| 10 | Pt plate (1 cm × 1 cm) as anode | 20 (69)d |
| 11 | Graphite plate (1 cm × 1 cm) as anode | 56 |
Bn benzyl
aReaction conditions: RVC anode, Pt plate cathode, 1 (0.2 mmol), MeOH (9 mL), Et4NPF6 (0.1 mmol), 10 mA, 2.3 h (4.3 F mol–1).
bDetermined by 1H NMR analysis using 1,3,5-trimethoxybenzene as the internal standard.
cIsolated yield.
dUnreacted 1 in bracket.
Evaluation of substrate scope
With the optimized reaction conditions defined, we set out to explore the scope of the electrooxidation of methylarenes. Notably, the site-selectivity was not significantly affected by introducing a phenyl (3), bromo (4), or cyano group (5) at C5, or by varying the substituent on N1 (6–9) or C2 (11–29) of the C4,C6-dimethylated benzimidazole substrate (Fig. 2). However, the installation of a carbamoyl group on N1 resulted in a slight decrease of regioselectivity (10). The method showed broad compatibility with common functional groups or moieties such as alkyl bromide (7), alkyne (8), alkene (9), ester (15, 16), alcohol (17, 18), ketone (19), aldehyde (20), Boc-protected amine (21–23), ketal (24), azido (25), and aromatic heterocycles (12, 13, 26, and 27). Molecular fragments derived from natural products dehydroabietic acid (28) and lithocholic acid (29) were equally well tolerated. On the other hand, site-selective oxidation of the C6-Me group in C5,C6-dimethylated benzimidazoles bearing an aryl substituent at C2 (30–32) could also be achieved. The replacement of the aryl group with a cyclohexyl, however, resulted in a moderate site-selectivity (33). This reduction in site-selectivity for the 2-cyclohexyl substrate was probably caused by the increased reactivity of the corresponding radical cation compared with the 2-aryl counterparts.
Fig. 2. Scope of site-selective electrooxidation of benzimidazoles.
Reaction conditions: heterocycle (0.2 mmol), MeOH (0.022 M), reflux, 2.2–4.5 h. All yields are isolated yields. Regioisomers were not observed unless otherwise mentioned.
Benzoxazoles (34–37) and benzothiazoles (38, 40–42) with multiple open benzylic positions were all found to undergo site-selective oxidation at the C6-Me group (Fig. 3). The site-selectivity was maintained even for substrates bearing an ethyl (37) or isopropyl group (41) at C5 that contained secondary or tertiary benzylic C–H bonds. Notably, the oxidation of 5,6,7-trimethyl benzothiazole proceeded site-selectively as intended despite the high steric hindrance of its C6-Me substituent. However, the resultant product mixture comprised mono-methoxylated 42 as the main product with a minor amount of acetal 43, because the steric environment was detrimental to the second C–H cleavage38. Meanwhile, oxidation of a C4,C7-dimethylated benzothiazole with a methylated phenyl group on C2 occurred preferentially on the C4-Me (44). The electrooxidation method was successfully extended to many other benzoheterocycles, including 2-benzimidazolidinone (45, 46), 2-benzoxazolone (47, 48), 2-oxindole (49), 3,4-dihydro-1H-quinolin-2-one (50), and quinoxalinone (51). Once again, probably due to the steric hinderance, monomethoxylated product 46′ could be obtained selectively with good yield when the electrolysis was stopped at 4.1 F mol−1. The electrochemical method was not limited to benzoheterocycles as demonstrated by the site-selective oxidation of methylated alkoxybenzenes (52–54). The relatively electron-deficient 3,4-dimethylphenylboronic acid (55), however, decomposed into intractable material and did not afforded any aldehyde product. The above results clearly suggested that the site-selectivity for the electrochemical benzylic oxidation reaction are not controlled by steric effects or bond dissociate energies (BDEs) of the C–H bonds.
Fig. 3. Electrooxidation of various methyl benzoheterocycles.
Reaction conditions: methylarene (0.2 mmol), MeOH (0.022 M), reflux, 2.2–5.3 h. All yields are isolated yields. Regioisomers were not observed unless otherwise mentioned. aElectrolysis was followed by hydrolysis with aqueous HCl (2 N).
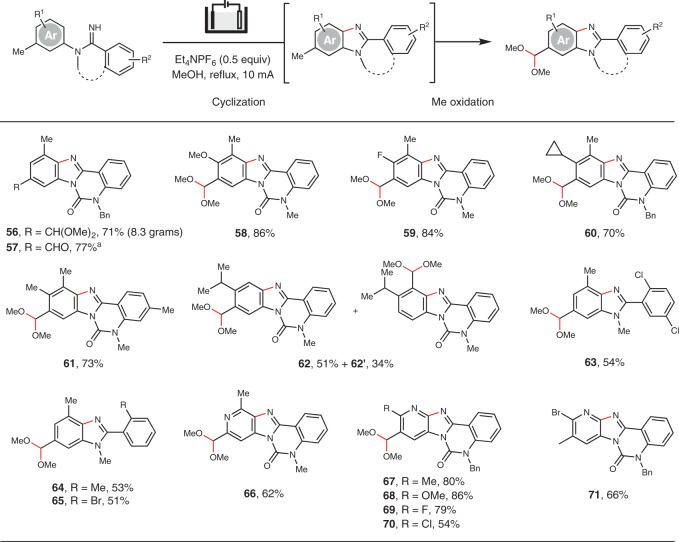
The Me oxidation reaction could be coupled with amidine cyclization that we previously described to construct functionalized benzimidazoles (56–65) and imidazopyridines (66–70) (Fig. 4)33. The reaction of an amidine containing a 3,4-disubstituted phenyl ring afforded two products 62 and 62′ because of unselective cyclization. The benzylic oxidation was, however, selective for both regioisomers. Compound 71 did not undergo further Me oxidation because its oxidation potential exceeded the decomposition potential of MeOH solvent. The tandem cyclization/Me oxidation process provided access to benzimidazoles with substitution patterns difficult for the existing methods33,39.
Fig. 4. Electrochemical cyclization/benzylic oxidation of amidines.
General reaction conditions: amidine (0.2 mmol), MeOH (0.022 M), reflux, 3.5–12.5 h. All yields are isolated yields. Regioisomers were not observed unless otherwise mentioned. aElectrolysis was followed by one-pot hydrolysis with HCl (2 N).
Synthesis of telmisartan
The synthetic utility of our electrooxidation reaction was demonstrated through the construction of the antihypertensive drug telmisartan (Fig. 5a). We first prepared benzimidazole 75 from a commercially available aniline 72 in four steps. Subsequently, site-selective electrooxidation of 75 afforded dimethyl acetal 76 in 46% yield on a decagram scale. In contrast, the oxidation of 75 by a stoichiometric amount of chemical oxidant such as CAN6 or IBX5, in the presence of a Co catalyst under aerobic conditions9, or iron catalyzed oxidation10 with K2S2O8 afforded 80 in <10% yield despite the success of these methods with toluene derivatives (Fig. 5b). Compound 76 was then converted to telmisartan by treating with aqueous HCl to hydrolyze its acetal group to aldehyde and remove its tBu, followed by condensation with o-phenylenediamine 78. Notably, the starting material 72 employed in this synthetic route are much less expensive than ester 79 used in a previously published 8-step method40.
Fig. 5. Synthesis of telmisartan.
a Our synthetic route employing site-selective benzylic electrooxidation as a key step. b Oxidation of 75 under reported conditions for benzylic oxidation. aMe ester was recovered instead of the original tBu ester 75 because of transesterification. NHPI N-Hydroxyphthalimide, Fe(II)Pc iron(II) phthalocyanine.
Mechanistic investigation
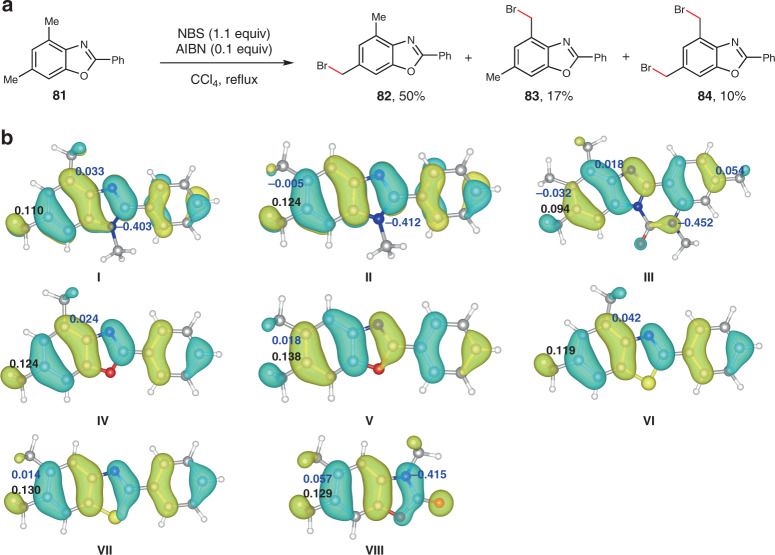
The reaction regioselectivity that we observed in this study suggested that the mechanism likely involved single electron transfer oxidation of the benzene nucleus to a radical cation, followed by benzylic C–H cleavage28. This hypothesis is further supported by the finding that bromination of benzoxazole 81 with NBS, known to proceed through hydrogen atom transfer, afforded a regioisomeric mixture of 82 (50%) and 83 (17%) along with dibrominated 84 (10%) (Fig. 6a). Density functional theory calculations were also performed to provide a plausible rationale for the origin of the observed site-selectivity (Fig. 6b). We first analyzed the distributions of the lowest unoccupied molecular orbitals (LUMO) of the radical cations derived from benzimidazoles (I–III), benzoxazoles (IV, V), benzothiazoles (VI, VII), and 2-benzoxazolone (VIII) that bear multiple Me groups. As shown in Fig. 6b, the LUMOs are delocalized throughout the carbon skeletons of the benzoheterocycles with the distributions on C6 atoms being higher than other carbon atoms attached with a Me group. Furthermore, the natural population analysis shows that the charges of C6 are also more positive than those of other atoms bearing a Me group, indicating deprotonation of the C6-Me groups is preferred41.
Fig. 6. Investigation on the site-selectivity of the electrooxidation of methylarenes.
a Radical bromination of 81 with NBS. b Computed LUMOs and NPA charges of radical cations derived from various methylated benzoheterocycles. N, C, O, S, and H atoms are colored in blue, gray, red, yellow and white, respectively. The LUMOs are visualized by light blue and yellow isosurfaces. The NPA charges of the atoms bearing a Me group are indicated by black (C6) and blue numbers. NBS N-bromosuccinimide, AIBN azobisisobutyronitrile.
In summary, we have shown that electrooxidation of methyl benzoheterocycles occurs in a site-selective manner to afford a wide range of structurally diverse aromatic acetals. The site-selectivity is governed by the innate electronic properties of the benzo ring instead of BDEs of the C(sp3)–H bonds. The benzylic oxidation takes place efficiently in a simple undivided cell and employs traceless electric current as the reagents without need for stoichiometric chemical oxidants. These features render the reactions scalable and attractive for industrial scale applications.
Methods
Representative procedure for the electrooxidation of methylarenes
A 10 mL three-necked round-bottomed flask was charged with 1 (0.20 mmol, 1.0 equiv) and Et4NPF6 (0.10 mmol, 0.5 equiv). The flask was then equipped with a condenser, a reticulated vitreous carbon (100 PPI, 1.2 cm × 1.0 cm × 0.8 cm) anode and a platinum plate (1.0 cm × 1.0 cm) cathode, and flushed with argon. MeOH (9.0 mL) was then added. The electrolysis was carried out at 80 °C (oil bath temperature) using a constant current of 10 mA until complete consumption of the substrate (2.3 h, 4.3 F mol–1). The reaction mixture was cooled to RT and concentrated under reduced pressure. The residue was chromatographed through silica gel eluting with ethyl acetate/hexanes containing 1% triethylamine to give the desired product 2 in 72% yield as a white solid. All new compounds were fully characterized (See the Supplementary methods).
Supplementary information
Acknowledgements
The authors acknowledge the financial support of this research from MOST (2016YFA0204100), NSFC (No. 21672178, 21971213), and Fundamental Research Funds for the Central Universities.
Author contributions
P.Xiong and H.B.Z. contributed equally to this work. P.Xiong, H.B.Z., L.H.J., H.L., P.Xu, Z.J.L., and Z.J.W. performed the experiments and analyzed the data, X.T.F. and J.C. conducted the theoretical studies. H.C.X. designed and directed the project and wrote the manuscript.
Data availability
The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number 1964756. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. The data supporting the findings of this study are available within the article and its Supplementary Information files. Any further relevant data are available from the authors on request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks David Cardoso and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jun Cheng, Email: chengjun@xmu.edu.cn.
Hai-Chao Xu, Email: haichao.xu@xmu.edu.cn.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-16519-8.
References
- 1.Sterckx H, Morel B, Maes BUW. Catalytic aerobic oxidation of C(sp3)−H bonds. Angew. Chem. Int. Ed. 2019;58:7946–7970. doi: 10.1002/anie.201804946. [DOI] [PubMed] [Google Scholar]
- 2.Ishii Y, Sakaguchi S, Iwahama T. Innovation of hydrocarbon oxidation with molecular oxygen and related reactions. Adv. Synth. Catal. 2001;343:393–427. doi: 10.1002/1615-4169(200107)343:5<393::AID-ADSC393>3.0.CO;2-K. [DOI] [Google Scholar]
- 3.Yoshino Y, Hayashi Y, Iwahama T, Sakaguchi S, Ishii Y. Catalytic oxidation of alkylbenzenes with molecular oxygen under normal pressure and temperature by N-hydroxyphthalimide combined with Co(OAc)2. J. Org. Chem. 1997;62:6810–6813. doi: 10.1021/jo9708147. [DOI] [Google Scholar]
- 4.White MC, Zhao J. Aliphatic C–H oxidations for late-stage functionalization. J. Am. Chem. Soc. 2018;140:13988–14009. doi: 10.1021/jacs.8b05195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicolaou KC, Baran PS, Zhong YL. Selective oxidation at carbon adjacent to aromatic systems with IBX. J. Am. Chem. Soc. 2001;123:3183–3185. doi: 10.1021/ja011338b. [DOI] [PubMed] [Google Scholar]
- 6.Fehr C, Chaptal-Gradoz N, Galindo J. Synthesis of (−)-vulcanolide by enantioselective protonation. Chem. Eur. J. 2002;8:853–858. doi: 10.1002/1521-3765(20020215)8:4<853::AID-CHEM853>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Hosseinzadeh R, Tajbakhsh M, Vahedi H. Selective oxidation of methylarenes with pyridinium chlorochromate. Synlett. 2005;2005:2769–2770. doi: 10.1055/s-2005-917118. [DOI] [Google Scholar]
- 8.Sarma BB, Efremenko I, Neumann R. Oxygenation of methylarenes to benzaldehyde derivatives by a polyoxometalate mediated electron transfer–oxygen transfer reaction in aqueous sulfuric acid. J. Am. Chem. Soc. 2015;137:5916–5922. doi: 10.1021/jacs.5b01745. [DOI] [PubMed] [Google Scholar]
- 9.Gaster E, Kozuch S, Pappo D. Selective aerobic oxidation of methylarenes to benzaldehydes catalyzed by N-hydroxyphthalimide and cobalt(II) acetate in hexafluoropropan-2-ol. Angew. Chem. Int. Ed. 2017;56:5912–5915. doi: 10.1002/anie.201702511. [DOI] [PubMed] [Google Scholar]
- 10.Hu P, et al. Bio-inspired iron-catalyzed oxidation of alkylarenes enables late-stage oxidation of complex methylarenes to arylaldehydes. Nat. Commun. 2019;10:2425. doi: 10.1038/s41467-019-10414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan M, Kawamata Y, Baran PS. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 2017;117:13230–13319. doi: 10.1021/acs.chemrev.7b00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waldvogel SR, Lips S, Selt M, Riehl B, Kampf CJ. Electrochemical arylation reaction. Chem. Rev. 2018;118:6706–6765. doi: 10.1021/acs.chemrev.8b00233. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida J-i, Shimizu A, Hayashi R. Electrogenerated cationic reactive intermediates: the pool method and further advances. Chem. Rev. 2018;118:4702–4730. doi: 10.1021/acs.chemrev.7b00475. [DOI] [PubMed] [Google Scholar]
- 14.Sauermann N, Meyer TH, Qiu Y, Ackermann L. Electrocatalytic C–H activation. ACS Catal. 2018;8:7086–7103. doi: 10.1021/acscatal.8b01682. [DOI] [Google Scholar]
- 15.Yang QL, Fang P, Mei TS. Recent advances in organic electrochemical C—H functionalization. Chin. J. Chem. 2018;36:338–352. doi: 10.1002/cjoc.201700740. [DOI] [Google Scholar]
- 16.Kärkäs MD. Electrochemical strategies for C–H functionalization and C–N bond formation. Chem. Soc. Rev. 2018;47:5786–5865. doi: 10.1039/C7CS00619E. [DOI] [PubMed] [Google Scholar]
- 17.Feng R, Smith JA, Moeller KD. Anodic cyclization reactions and the mechanistic strategies that enable optimization. Acc. Chem. Res. 2017;50:2346–2352. doi: 10.1021/acs.accounts.7b00287. [DOI] [PubMed] [Google Scholar]
- 18.Ye Z, Zhang F. Recent advances in constructing nitrogen-containing heterocycles via electrochemical dehydrogenation. Chin. J. Chem. 2019;37:513–528. doi: 10.1002/cjoc.201900049. [DOI] [Google Scholar]
- 19.Francke R, Little RD. Redox catalysis in organic electrosynthesis: basic principles and recent developments. Chem. Soc. Rev. 2014;43:2492–2521. doi: 10.1039/c3cs60464k. [DOI] [PubMed] [Google Scholar]
- 20.Yuan Y, Lei A. Electrochemical oxidative cross-coupling with hydrogen evolution reactions. Acc. Chem. Res. 2019;52:3309–3324. doi: 10.1021/acs.accounts.9b00512. [DOI] [PubMed] [Google Scholar]
- 21.Jiang Y, Xu K, Zeng C. Use of electrochemistry in the synthesis of heterocyclic structures. Chem. Rev. 2018;118:4485–4540. doi: 10.1021/acs.chemrev.7b00271. [DOI] [PubMed] [Google Scholar]
- 22.Nutting JE, Rafiee M, Stahl SS. Tetramethylpiperidine N-oxyl (TEMPO), phthalimide N-oxyl (PINO), and related N-oxyl species: electrochemical properties and their use in electrocatalytic reactions. Chem. Rev. 2018;118:4834–4885. doi: 10.1021/acs.chemrev.7b00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong P, Xu HC. Chemistry with electrochemically generated N-centered radicals. Acc. Chem. Res. 2019;52:3339–3350. doi: 10.1021/acs.accounts.9b00472. [DOI] [PubMed] [Google Scholar]
- 24.Sauer GS, Lin S. An electrocatalytic approach to the radical difunctionalization of alkenes. ACS Catal. 2018;8:5175–5187. doi: 10.1021/acscatal.8b01069. [DOI] [Google Scholar]
- 25.Das A, Nutting JE, Stahl SS. Electrochemical C–H oxygenation and alcohol dehydrogenation involving Fe-oxo species using water as the oxygen source. Chem. Sci. 2019;10:7542–7548. doi: 10.1039/C9SC02609F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horn EJ, et al. Scalable and sustainable electrochemical allylic C-H oxidation. Nature. 2016;533:77–81. doi: 10.1038/nature17431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cardoso DSP, Sljukic B, Santos DMF, Sequeira CAC. Organic electrosynthesis: from laboratorial practice to industrial applications. Org. Process Res. Dev. 2017;21:1213–1226. doi: 10.1021/acs.oprd.7b00004. [DOI] [Google Scholar]
- 28.Wendt H, Bitterlich S. Anodic synthesis of benzaldehydes-i. voltammetry of the anodic-oxidation of toluenes in nonaqueous solutions. Electrochim. Acta. 1992;37:1951–1958. doi: 10.1016/0013-4686(92)87108-C. [DOI] [Google Scholar]
- 29.Zhu YH, et al. A promising electro-oxidation of methyl-substituted aromatic compounds to aldehydes in aqueous imidazole ionic liquid solutions. J. Electroanal. Chem. 2015;751:105–110. doi: 10.1016/j.jelechem.2015.05.034. [DOI] [Google Scholar]
- 30.Lumb JP. Stopping aerobic oxidation in its tracks: chemoselective synthesis of benzaldehydes from methylarenes. Angew. Chem. Int. Ed. 2017;56:9276–9277. doi: 10.1002/anie.201704160. [DOI] [PubMed] [Google Scholar]
- 31.Cai C-Y, Shu X-M, Xu H-C. Practical and stereoselective electrocatalytic 1,2-diamination of alkenes. Nat. Commun. 2019;10:4953. doi: 10.1038/s41467-019-13024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai C-Y, Xu H-C. Dehydrogenative reagent-free annulation of alkenes with diols for the synthesis of saturated O-heterocycles. Nat. Commun. 2018;9:3551. doi: 10.1038/s41467-018-06020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao H-B, et al. Amidinyl radical formation through anodic N−H bond cleavage and its application in aromatic C−H bond functionalization. Angew. Chem. Int. Ed. 2017;56:587–590. doi: 10.1002/anie.201610715. [DOI] [PubMed] [Google Scholar]
- 34.Xu F, Long H, Song J, Xu H-C. De novo synthesis of highly functionalized benzimidazolones and benzoxazolones through an electrochemical dehydrogenative cyclization cascade. Angew. Chem. Int. Ed. 2019;58:9017–9021. doi: 10.1002/anie.201904931. [DOI] [PubMed] [Google Scholar]
- 35.Hou Z-W, Yan H, Song J-S, Xu H-C. Electrochemical synthesis of (Aza)indolines via dehydrogenative [3+2] annulation: application to total synthesis of (±)-hinckdentine A. Chin. J. Chem. 2018;36:909–915. doi: 10.1002/cjoc.201800301. [DOI] [Google Scholar]
- 36.Wu Z-J, Li S-R, Long H, Xu H-C. Electrochemical dehydrogenative cyclization of 1,3-dicarbonyl compounds. Chem. Commun. 2018;54:4601–4604. doi: 10.1039/C8CC02472C. [DOI] [PubMed] [Google Scholar]
- 37.Qian, X.-Y., Li, S.-Q., Song, J. & Xu, H.-C. TEMPO-catalyzed electrochemical C–H thiolation: synthesis of benzothiazoles and thiazolopyridines from thioamides. ACS Catal., 2730–2734 (2017).
- 38.Baciocchi E, Bietti M, Lanzalunga O. Mechanistic aspects of β-bond-cleavage reactions of aromatic radical cations. Acc. Chem. Res. 2000;33:243–251. doi: 10.1021/ar980014y. [DOI] [PubMed] [Google Scholar]
- 39.Brasche G, Buchwald SL. C-H functionalization/C-N bond formation: copper-catalyzed synthesis of benzimidazoles from amidines. Angew. Chem. Int. Ed. 2008;47:1932–1934. doi: 10.1002/anie.200705420. [DOI] [PubMed] [Google Scholar]
- 40.Ries UJ, et al. 6-Substituted benzimidazoles as new nonpeptide angiotensin II receptor antagonists: synthesis, biological activity, and structure-activity relationships. J. Med. Chem. 1993;36:4040–4051. doi: 10.1021/jm00077a007. [DOI] [PubMed] [Google Scholar]
- 41.Margrey KA, McManus JB, Bonazzi S, Zecri F, Nicewicz DA. Predictive model for site-selective aryl and heteroaryl C–H functionalization via organic photoredox catalysis. J. Am. Chem. Soc. 2017;139:11288–11299. doi: 10.1021/jacs.7b06715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number 1964756. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. The data supporting the findings of this study are available within the article and its Supplementary Information files. Any further relevant data are available from the authors on request.