Abstract
Oncolytic viruses (OVs) represent a promising new class of cancer therapeutics and cause antitumor effects by two major mechanisms: (1) directly killing cancer cells in a process known as oncolysis, or (2) initiating a powerful antitumor immune response. Interestingly, energy metabolism, within either cancer cells or immune cells, plays a pivotal role in defining the outcome of OV-mediated antitumor effects. Following therapeutic administration, OVs must hijack host cell metabolic pathways to acquire building blocks such as nucleotides, lipids, and amino acids for the process of replication that is necessary for oncolysis. Additionally, OV-stimulated antitumor immune responses are highly dependent on the metabolic state within the tumor microenvironment. Thus, metabolic reprogramming strategies bear the potential to enhance the efficacy of both OV-mediated oncolysis and antitumor immune responses.
Keywords: oncolytic virus, cancer metabolism, metabolic reprogramming, glycolysis, oxidative phosphorylation, TCA cycle, pyruvate metabolism, immunometabolism
Graphical Abstract
Oncolytic viruses (OVs) represent a new class of cancer immunotherapies. In this mini-review, Kennedy et al. critically assess how therapeutic reprogramming of cellular metabolism, targeting either cancer or immune cells, can be used to enhance the efficacy of OV-based therapies in clinics.
Oncolytic Viruses
Oncolytic viruses (OVs) cause anticancer effects through two main mechanisms: (1) by directly killing cancer cells (oncolysis), and (2) by promoting antitumor immunity that can attack cancer cells at local as well as metastatic sites.1 In 2005, the potential of OVs was finally realized when the first OV-based therapy, Oncorine (H101), was approved by the Chinese State Food and Drug Administration (SFDA) for clinical use against nasopharyngeal carcinoma.2 The first US Food and Drug Administration (FDA)-approved OV, a modified herpes simplex virus (HSV), talimogene laherparepvec (T-Vec), was approved in 2015 for clinical use against metastatic melanoma.3 These historic approvals amplified the interest in OV research and promoted greater focus on the strategies to improve OV-based therapies in clinical settings. Thus far, it has become apparent that, to achieve optimal antitumor outcomes, OVs will need to be combined with interventions that synergistically enhance OV-based anticancer mechanisms. Not surprisingly, there are 96 clinical trials worldwide testing OVs against a wide range of cancers (https://clinicaltrials.gov/). Indeed, most of these clinical trials employ some type of combination intervention to achieve optimum anticancer benefits. Recent discoveries now suggest that cell metabolism within the tumor microenvironment (TME) (i.e., of either cancer or immune cells) can be strategically exploited to formulate efficacious, novel OV-based combination therapies.4, 5, 6, 7
Cancer Metabolism
Energy metabolism is essential for bioenergy production and synthesis of biological building blocks in all cells. However, energy metabolism in cancers is different from that of normal cells.8, 9, 10, 11, 12 Glucose, the major energy source for all cells, is consumed and broken down through glycolysis to pyruvate by both normal and cancer cells, albeit at much higher rates by cancer cells. In normal cells, pyruvate enters the tricarboxylic acid (TCA) cycle where it is oxidized through a series of reactions to release stored energy. Conversely, cancer cells undergo a process known as the “Warburg effect” or aerobic glycolysis, where glucose is used as a precursor for macromolecule synthesis in addition to being broken down to pyruvate. The pyruvate that is produced is typically reduced to lactic acid, generating nicotinamide adenine dinucleotide (NAD)+, which is required for continued glycolysis. This diversion is largely regulated by a cancer-induced block at pyruvate dehydrogenase (PDH).13 To compensate for the loss of glucose flux into the TCA cycle, cancers often supplement their TCA cycle with the breakdown of glutamine through a process called glutaminolysis,14 as well as increased flux into acetyl coenzyme A (CoA) from fatty acid oxidation.15 Such metabolic rewiring establishes a pro-growth state suitable to sustain proliferation by cancer cells. Interestingly, the reliance of cancer cells on the “Warburg effect” makes them susceptible to certain metabolic inhibitors. Some examples of this phenomenon include: an increased sensitivity of cancer cells to inhibition of the glycolytic enzyme hexokinase with 2-deoxy-glucose, activation of PDH with dichloroacetate (DCA), or inhibition of glutaminolysis16 with compound 968.17 Whether such cancer-specific metabolic vulnerabilities can be exploited to promote the antitumor efficacy of OVs has remained mostly speculative thus far.
Cancer Metabolism and Oncolytic Viral Infection
Similar to cells, viruses require macromolecules such as amino acids, nucleic acids, and lipids for replication. Because most viruses do not intrinsically encode enzymes required for synthesis of these biomolecules, they often hijack host cell metabolic pathways to meet their needs for replication.5 Below we will describe the relationship between OVs and specific cancer metabolic pathways. Also, we will discuss how metabolic reprogramming through pharmacological or genetic means has been used to alter antitumor effects of OVs (summarized in Figure 1).
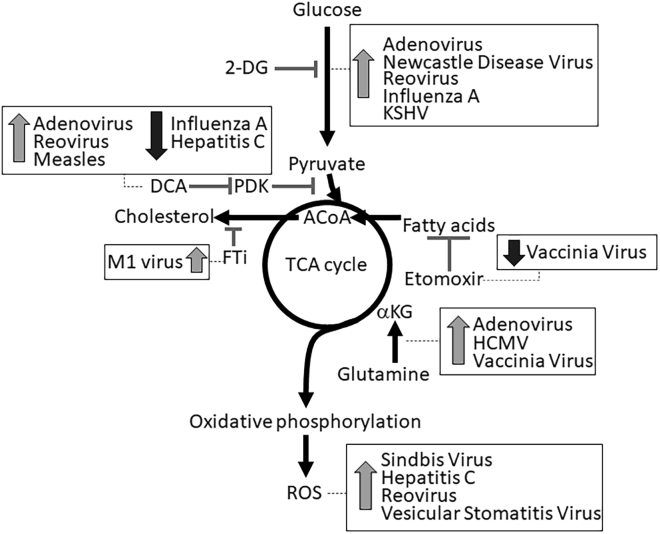
Figure 1.
Metabolic Reprogramming Strategies to Enhance Efficacy of OV Therapies
There are several targets in central energy metabolism that have the potential to alter the replication and oncolytic capacity of OVs. Dotted lines point to viruses that can be affected through targeting the respective metabolic pathway. Upward-facing arrows show viruses that were enhanced by the indicated metabolic inhibitor, while downward-facing arrows are used to identify viruses that were negatively influenced by the respective metabolic inhibitor. The following metabolic pathways are highlighted: glycolysis, pyruvate metabolism, TCA cycle, fatty acid β-oxidation, cholesterol synthesis, glutaminolysis, and oxidative phosphorylation. Bold lines represent the direction of metabolic flux in cancer cells, and inhibitory lines indicate targets of metabolic inhibitors. 2-DG, 2-deoxy-glucose; DCA, dichloroacetate; PDK, pyruvate dehydrogenase kinase; ACoA, acetyl coenzyme A; αKG, α ketoglutarate; FTi, inhibitor of farnesyl transferase; ROS, reactive oxygen species.
Glycolysis
Virus infection in both normal and cancer cells is typically associated with an upregulation of glucose uptake and glycolysis.18, 19, 20, 21, 22, 23, 24, 25 For example, Mayaro virus and oncolytic HSV-1 activate the glycolytic enzyme 6-phosphofructo-1-kinase (PFK-1),26,27 oncolytic human cytomegalovirus (HCMV) and human immunodeficiency virus type 1 increase glucose transporter expression,28,29 and hepatitis C virus enhances hexokinase activity.30 Despite this virus-induced increased glycolysis, inhibition of glycolysis with inhibitors such as 2-deoxy-glucose enhances oncolysis by many viruses, including adenovirus, Newcastle disease virus, Kaposi’s sarcoma-associated herpesvirus (KSHV), influenza, and reovirus.4,6,31, 32, 33 Interestingly, anti-viral defenses through type I interferon require glycolysis to fight viral infection through an unclear mechanism.34,35 These findings suggest that infection-induced glycolytic upregulation is an anti-viral mechanism. Taken together, these studies show that blocking glycolysis can be a strategy to increase the susceptibility of cancer cells to OVs.
Pyruvate Metabolism
PDH catalyzes pyruvate oxidation fueling the TCA cycle. Inhibition by phosphorylation of PDH is a common occurrence in cancers and contributes to the production of lactic acid. Infection of several OVs, including vaccinia virus, hepatitis C virus, influenza A (H1N1) virus, and reovirus, inhibit PDH activity through increased expression of inhibitory PDH kinases (PDKs).4,25,36,37 Similar to increased glycolysis, inhibition of PDH appears to be anti-viral in action, as the activation of PDH, through DCA-induced inhibition of PDKs, results in enhanced oncolysis by adenovirus, measles, and reovirus.4,38,39 These observations suggest that an increased flux of pyruvate into the TCA cycle supports augmented oncolysis by OVs. Alternatively, in the absence of the cancer context, pharmacological or genetic inhibition of PDKs decreases the replication of influenza and hepatitis C virus, indicating possible context-dependent as well as virus-specific effects for PDH during host-virus interactions.25,36
TCA Cycle
The TCA cycle supports cellular anabolic pathways that lead to the generation of amino acids, nucleic acids, and lipids. Viruses depend on these essential building blocks for replication, and therefore have evolved to hijack cellular anabolic pathways. For example, citrate-derived fatty acids and oxaloacetate-derived nucleic acids have been shown to be incorporated into viral progeny in several OVs such as influenza,40 HCMV,20,41, 42, 43 M1 virus,7 and vaccinia virus.44 Therefore, it is hypothesized that an enhanced TCA cycle flux creates a state that is conducive for viral replication. Indeed, increased metabolic flux through glutamine carboxylation into alpha ketoglutarate enhances oncolytic properties of adenovirus and HCMV.6,41 Similarly, the inhibition of fatty acid β-oxidation and flux into TCA cycle with etomoxir or trimetazidine reduces vaccinia replication.45 Here, it is noteworthy that the activity of the RIG-I-MDA5-MAVS complex, a major anti-virus defense system that is directly bound to mitochondrial membranes, is suppressed by increasing metabolic flux into the TCA cycle, resulting in an environment that favors virus replication.39 These observations further support the notion that the enhancement of flux into the TCA cycle is beneficial for OV replication and subsequent oncolysis.
Oxidative Phosphorylation
Oxidative phosphorylation, a process in which ATP is produced as a result of the transfer of electrons from NADH or FADH2 to oxygen in the mitochondria, has been linked to infection and replication of several viruses. For example, high rates of oxidative phosphorylation are required for the replication of oncolytic HCMV and Sindbis virus,43,46, whereas inhibition of oxidative phosphorylation or inherently low rates of oxidative phosphorylation impedes KSHV, influenza, and herpes replication.33,47,48 Furthermore, reactive oxygen species (ROS), a byproduct of oxidative phosphorylation, also plays a role in viral replication and oncolysis. High levels of ROS enhance virus-induced cytotoxicity of reovirus,4 Sindbis virus,46 and hepatitis C virus.49 Furthermore, activation of NRF2, a ROS-activated transcription factor, enhances oncolysis by vesicular stomatitis virus.50 Conversely, the prevention of ROS generation with antioxidants augments oncolytic effects of adenovirus in glioma.51 In line with increased metabolic flux into the TCA cycle, these findings suggest that high rates of oxidative phosphorylation and ROS production are typically associated with enhanced oncolysis.
Immunometabolism in the Context of Cancer and OV Therapies
In addition to OV-mediated oncolysis, the other major consideration for OV-based therapies is the OV-induced potent antitumor immune response. As the latest advances in immunometabolism have captured, metabolism shapes both quantitative and qualitative aspects of immune responses.52 Thus, the effect of metabolic reprogramming on the antitumor immune response should be considered while strategically designing an optimal OV therapy. Within an immunosuppressive TME, each component of an antitumor immune response has a unique metabolic requirement (summarized in Figure 2).52 Specifically, CD8+ cytotoxic T cells require a substantial glycolytic flux, ROS generation from mitochondria, and glutaminolysis.53, 54, 55 Alternatively, CD8+ memory T cells rely on a high metabolic flux (most notably from fatty acid oxidation) into mitochondria.56,57 Thus, CD8+ T cell function can be modified through the use of metabolic reprogramming agents. For example, inhibition of glycolysis by 2-deoxy-glucose drives a memory like phenotype in CD8+ T cells and can be therapeutically used during ex vivo expansion of CD8+ T cells to enhance in vivo antitumor functions.58 Additionally, CD4+ T helper cells preferentially use glycolysis over oxidative phosphorylation, whereas immunosuppressive CD4+Foxp3+ regulatory T cells (Tregs) use oxidative phosphorylation over glycolysis.59,60 Indeed, forcing metabolic flux into mitochondria, by PDK inhibition with DCA, drives the formation of Tregs.61 Pro-inflammatory (M1) macrophages show breaks at isocitrate dehydrogenase and succinate dehydrogenase within the TCA cycle, which result in inflammatory gene expression and nitric oxide production. Conversely, anti-inflammatory (M2) macrophages rely on an unbroken TCA cycle.62 Experimentally, M1 macrophage differentiation can be driven by mimicking the break in the TCA cycle through the inhibition of glutaminolysis using the glutaminase 1 inhibitor BPTES (bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide).63 These findings show the importance of considering the consequences of metabolic reprogramming strategies on immune cells.
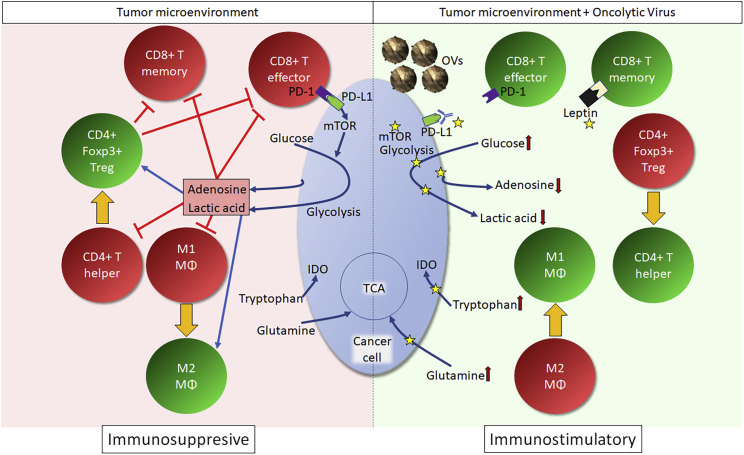
Figure 2.
Potential Metabolic Targets to Enhance OV-Driven Antitumor Immune Response
Left panel: An immunosuppressive metabolic milieu within TME. This non-conducive metabolic profile within TME alters the function and differentiation of infiltrating immune cells (e.g., inhibition of CD8+ T effector and memory cells, promotion of immunosuppressive M2 macrophages [MΦ] and CD4+ Tregs). Furthermore, immune checkpoints (e.g., PD-1, PD-L1) exaggerate the Warburg effect in cancers through promotion of mTOR-driven glycolysis. Right panel: The proposed metabolic reprogramming strategies to enhance OV-induced antitumor immune response. For this, the metabolic inhibitors targeting cancer-specific metabolic aberrations (e.g., inhibitors of mTOR, glycolysis, indoleamine 2,3-dioxygenase [IDO, tryptophan catabolism enzyme], glutaminolysis, or adenosine synthesis) can be used to correct TME-associated negative effects on immune cells. Additionally, the inhibition of checkpoint molecules can also be used to cause metabolic reprogramming of TME. Another option, as reported by Rivadeneira et al.,64 would be to use OVs to induce the expression of proteins, such as leptin (shown in black), to influence immune cell metabolism. The ultimate goal of such metabolic reprogramming strategies will be to potentiate OV-induced antitumor immunity. Yellow arrows indicate differentiation; green cells represent functionally competent cells; red cells represent dysfunctional cells; yellow stars indicate potential targets for inhibition; small red arrows indicate desired metabolic levels for optimal antitumor immune response. Treg, regulatory T cell; PD-1, programmed cell death protein 1; PD-L1, programmed cell death-ligand 1; mTOR, mammalian target of rapamycin; IDO, indoleamine 2,3-dioxygenase; TCA, tricarboxylic acid cycle; OVs, oncolytic viruses.
Metabolically aberrant TME is non-conducive for the initiation and sustenance of competent antitumor immune responses, aiding the immunological subversion of tumor cells. Cancer cells outcompete immune cells for essential metabolites, such as glucose, tryptophan, and glutamine, and produce immunosuppressive metabolites, including lactic acid (through aerobic glycolysis), kynurenine (through IDO [indoleamine 2,3-dioxygenase]-mediated breakdown of tryptophan), and adenosine (through CD73/CD39/CD38-mediated breakdown of extracellular ATP or NAD+). Such metabolic aberrations in TME establish an immunosuppressive environment that favors macrophage polarization into pro-tumor M2 phenotype, CD8+ T cell exhaustion, and Treg development.65, 66, 67, 68, 69 Additionally, immune checkpoints (i.e., PD-L1 [programmed cell death-ligand 1]/PD-1 [programmed cell death protein 1]) exaggerate the metabolic disparity between cancer and immune cells by further promoting the Warburg effect in cancers through mTOR (mammalian target of rapamycin) signaling.66 However, an engineered manipulation of cancer metabolism can be used to enhance antitumor immune response. For example, the inhibition of glycolysis to lower lactate production in cancer cells, using either knockdown of glycolytic genes or immune checkpoint inhibitors, promotes antitumor CD8+ T effector cell response.55,66 Thus, strategic targeting of cancer metabolism can enhance the antitumor ability of infiltrating immune cells.
The implications for metabolic reprogramming on OV-induced antitumor immunity remain largely unknown but are being acknowledged. Indeed, a recent study by Rivadeneira et al.64 showed that engineered oncolytic vaccinia virus expressing the adipokine, leptin, in cancer cells results in a functionally competent antitumor CD8+ T cell response. Here, OV-expressed leptin induces the memory phenotype in infiltrating CD8+ T cells through stimulation of mitochondrial activity.64 With such ability to accommodate metabolism-regulating genes, OVs can be harnessed to correct the suppressive metabolic milieu, ultimately allowing for the development of desired antitumor immune reactivities.
Conclusion
Based on the evidence thus far, it is clear that cell metabolism is pivotal in regulating the efficacy of OV-based cancer therapies. OVs often prefer a specific, yet definable, intracellular metabolic environment, which can be engineered through metabolic reprogramming. Furthermore, the OV-induced antitumor response can also be optimized through strategic metabolic reprogramming of either cancer or immune cells. Hence, targeted metabolic reprogramming can be used to improve OV-induced oncolysis as well as antitumor immunity, and thus can be harnessed to formulate efficacious OV-based cancer therapies.
Conflicts of Interest
The authors declare no competing interests.
References
- 1.Lee P., Gujar S. Potentiating prostate cancer immunotherapy with oncolytic viruses. Nat. Rev. Urol. 2018;15:235–250. doi: 10.1038/nrurol.2018.10. [DOI] [PubMed] [Google Scholar]
- 2.Liang M. Oncorine, the world first oncolytic virus medicine and its update in China. Curr. Cancer Drug Targets. 2018;18:171–176. doi: 10.2174/1568009618666171129221503. [DOI] [PubMed] [Google Scholar]
- 3.Conry R.M., Westbrook B., McKee S., Norwood T.G. Talimogene laherparepvec: first in class oncolytic virotherapy. Hum. Vaccin. Immunother. 2018;14:839–846. doi: 10.1080/21645515.2017.1412896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennedy B.E., Murphy J.P., Clements D.R., Konda P., Holay N., Kim Y., Pathak G.P., Giacomantonio M.A., Hiani Y.E., Gujar S. Inhibition of pyruvate dehydrogenase kinase enhances the antitumor efficacy of oncolytic reovirus. Cancer Res. 2019;79:3824–3836. doi: 10.1158/0008-5472.CAN-18-2414. [DOI] [PubMed] [Google Scholar]
- 5.Thaker S.K., Ch’ng J., Christofk H.R. Viral hijacking of cellular metabolism. BMC Biol. 2019;17:59. doi: 10.1186/s12915-019-0678-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dyer A., Schoeps B., Frost S., Jakeman P., Scott E.M., Freedman J., Jacobus E.J., Seymour L.W. Antagonism of glycolysis and reductive carboxylation of glutamine potentiates activity of oncolytic adenoviruses in cancer cells. Cancer Res. 2019;79:331–345. doi: 10.1158/0008-5472.CAN-18-1326. [DOI] [PubMed] [Google Scholar]
- 7.Liang J., Guo L., Li K., Xiao X., Zhu W., Zheng X., Hu J., Zhang H., Cai J., Yu Y. Inhibition of the mevalonate pathway enhances cancer cell oncolysis mediated by M1 virus. Nat. Commun. 2018;9:1524. doi: 10.1038/s41467-018-03913-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porporato P.E., Filigheddu N., Pedro J.M.B., Kroemer G., Galluzzi L. Mitochondrial metabolism and cancer. Cell Res. 2018;28:265–280. doi: 10.1038/cr.2017.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Claus C., Liebert U.G. A renewed focus on the interplay between viruses and mitochondrial metabolism. Arch. Virol. 2014;159:1267–1277. doi: 10.1007/s00705-013-1841-1. [DOI] [PubMed] [Google Scholar]
- 10.Weinberg S.E., Chandel N.S. Targeting mitochondria metabolism for cancer therapy. Nat. Chem. Biol. 2015;11:9–15. doi: 10.1038/nchembio.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vyas S., Zaganjor E., Haigis M.C. Mitochondria and cancer. Cell. 2016;166:555–566. doi: 10.1016/j.cell.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jitschin R., Hofmann A.D., Bruns H., Giessl A., Bricks J., Berger J., Saul D., Eckart M.J., Mackensen A., Mougiakakos D. Mitochondrial metabolism contributes to oxidative stress and reveals therapeutic targets in chronic lymphocytic leukemia. Blood. 2014;123:2663–2672. doi: 10.1182/blood-2013-10-532200. [DOI] [PubMed] [Google Scholar]
- 13.Kim J.W., Tchernyshyov I., Semenza G.L., Dang C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 14.DeBerardinis R.J., Mancuso A., Daikhin E., Nissim I., Yudkoff M., Wehrli S., Thompson C.B. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carracedo A., Cantley L.C., Pandolfi P.P. Cancer metabolism: fatty acid oxidation in the limelight. Nat. Rev. Cancer. 2013;13:227–232. doi: 10.1038/nrc3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin L., Alesi G.N., Kang S. Glutaminolysis as a target for cancer therapy. Oncogene. 2016;35:3619–3625. doi: 10.1038/onc.2015.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J.B., Erickson J.W., Fuji R., Ramachandran S., Gao P., Dinavahi R., Wilson K.F., Ambrosio A.L., Dias S.M., Dang C.V., Cerione R.A. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell. 2010;18:207–219. doi: 10.1016/j.ccr.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh V.N., Singh M., August J.T., Horecker B.L. Alterations in glucose metabolism in chick-embryo cells transformed by Rous sarcoma virus: intracellular levels of glycolytic intermediates. Proc. Natl. Acad. Sci. USA. 1974;71:4129–4132. doi: 10.1073/pnas.71.10.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green M., Henle G., Deinhardt F. Respiration and glycolysis of human cells grown in tissue culture. Virology. 1958;5:206–219. doi: 10.1016/0042-6822(58)90019-9. [DOI] [PubMed] [Google Scholar]
- 20.Munger J., Bajad S.U., Coller H.A., Shenk T., Rabinowitz J.D. Dynamics of the cellular metabolome during human cytomegalovirus infection. PLoS Pathog. 2006;2:e132. doi: 10.1371/journal.ppat.0020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landini M.P. Early enhanced glucose uptake in human cytomegalovirus-infected cells. J. Gen. Virol. 1984;65:1229–1232. doi: 10.1099/0022-1317-65-7-1229. [DOI] [PubMed] [Google Scholar]
- 22.Klemperer H. Glucose breakdown in chick embryo cells infected with influenza virus. Virology. 1961;13:68–77. doi: 10.1016/0042-6822(61)90033-2. [DOI] [PubMed] [Google Scholar]
- 23.Levy H.B., Baron S. The effect of animal viruses on host cell metabolism. II. Effect of poliomyelitis virus on glycolysis and uptake of glycine by monkey kidney tissue cultures. J. Infect. Dis. 1957;100:109–118. doi: 10.1093/infdis/100.2.109. [DOI] [PubMed] [Google Scholar]
- 24.Bardell D., Essex M. Glycolysis during early infection of feline and human cells with feline leukemia virus. Infect. Immun. 1974;9:824–827. doi: 10.1128/iai.9.5.824-827.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung G.S., Jeon J.H., Choi Y.K., Jang S.Y., Park S.Y., Kim S.W., Byun J.K., Kim M.K., Lee S., Shin E.C. Pyruvate dehydrogenase kinase regulates hepatitis C virus replication. Sci. Rep. 2016;6:30846. doi: 10.1038/srep30846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Bacha T., Menezes M.M., Azevedo e Silva M.C., Sola-Penna M., Da Poian A.T. Mayaro virus infection alters glucose metabolism in cultured cells through activation of the enzyme 6-phosphofructo 1-kinase. Mol. Cell. Biochem. 2004;266:191–198. doi: 10.1023/b:mcbi.0000049154.17866.00. [DOI] [PubMed] [Google Scholar]
- 27.Abrantes J.L., Alves C.M., Costa J., Almeida F.C., Sola-Penna M., Fontes C.F., Souza T.M. Herpes simplex type 1 activates glycolysis through engagement of the enzyme 6-phosphofructo-1-kinase (PFK-1) Biochim. Biophys. Acta. 2012;1822:1198–1206. doi: 10.1016/j.bbadis.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Yu Y., Maguire T.G., Alwine J.C. Human cytomegalovirus activates glucose transporter 4 expression to increase glucose uptake during infection. J. Virol. 2011;85:1573–1580. doi: 10.1128/JVI.01967-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sorbara L.R., Maldarelli F., Chamoun G., Schilling B., Chokekijcahi S., Staudt L., Mitsuya H., Simpson I.A., Zeichner S.L. Human immunodeficiency virus type 1 infection of H9 cells induces increased glucose transporter expression. J. Virol. 1996;70:7275–7279. doi: 10.1128/jvi.70.10.7275-7279.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramière C., Rodriguez J., Enache L.S., Lotteau V., André P., Diaz O. Activity of hexokinase is increased by its interaction with hepatitis C virus protein NS5A. J. Virol. 2014;88:3246–3254. doi: 10.1128/JVI.02862-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Shammari A.M., Abdullah A.H., Allami Z.M., Yaseen N.Y. 2-Deoxyglucose and Newcastle disease virus synergize to kill breast cancer cells by inhibition of glycolysis pathway through glyceraldehyde3-phosphate downregulation. Front. Mol. Biosci. 2019;6:90. doi: 10.3389/fmolb.2019.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smallwood H.S., Duan S., Morfouace M., Rezinciuc S., Shulkin B.L., Shelat A., Zink E.E., Milasta S., Bajracharya R., Oluwaseum A.J. Targeting metabolic reprogramming by influenza infection for therapeutic intervention. Cell Rep. 2017;19:1640–1653. doi: 10.1016/j.celrep.2017.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delgado T., Carroll P.A., Punjabi A.S., Margineantu D., Hockenbery D.M., Lagunoff M. Induction of the Warburg effect by Kaposi’s sarcoma herpesvirus is required for the maintenance of latently infected endothelial cells. Proc. Natl. Acad. Sci. USA. 2010;107:10696–10701. doi: 10.1073/pnas.1004882107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burke J.D., Platanias L.C., Fish E.N. Beta interferon regulation of glucose metabolism is PI3K/Akt dependent and important for antiviral activity against coxsackievirus B3. J. Virol. 2014;88:3485–3495. doi: 10.1128/JVI.02649-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang H., Shi H., Sun M., Wang Y., Meng Q., Guo P., Cao Y., Chen J., Gao X., Li E., Liu J. PFKFB3-driven macrophage glycolytic metabolism is a crucial component of innate antiviral defense. J. Immunol. 2016;197:2880–2890. doi: 10.4049/jimmunol.1600474. [DOI] [PubMed] [Google Scholar]
- 36.Yamane K., Indalao I.L., Chida J., Yamamoto Y., Hanawa M., Kido H. Diisopropylamine dichloroacetate, a novel pyruvate dehydrogenase kinase 4 inhibitor, as a potential therapeutic agent for metabolic disorders and multiorgan failure in severe influenza. PLoS ONE. 2014;9:e98032. doi: 10.1371/journal.pone.0098032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazzon M., Peters N.E., Loenarz C., Krysztofinska E.M., Ember S.W., Ferguson B.J., Smith G.L. A mechanism for induction of a hypoxic response by vaccinia virus. Proc. Natl. Acad. Sci. USA. 2013;110:12444–12449. doi: 10.1073/pnas.1302140110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao L., Li X., Niu N., Qian J., Xie G., Wang Y. Dichloroacetate (DCA) enhances tumor cell death in combination with oncolytic adenovirus armed with MDA-7/IL-24. Mol. Cell. Biochem. 2010;340:31–40. doi: 10.1007/s11010-010-0397-6. [DOI] [PubMed] [Google Scholar]
- 39.Li C., Meng G., Su L., Chen A., Xia M., Xu C., Yu D., Jiang A., Wei J. Dichloroacetate blocks aerobic glycolytic adaptation to attenuated measles virus and promotes viral replication leading to enhanced oncolysis in glioblastoma. Oncotarget. 2015;6:1544–1555. doi: 10.18632/oncotarget.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ackermann W.W. The relation of the Krebs cycle to viral synthesis. II. The effect of sodium fluoroacetate on the propagation of influenza virus in mice. J. Exp. Med. 1951;93:635–642. doi: 10.1084/jem.93.6.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chambers J.W., Maguire T.G., Alwine J.C. Glutamine metabolism is essential for human cytomegalovirus infection. J. Virol. 2010;84:1867–1873. doi: 10.1128/JVI.02123-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vastag L., Koyuncu E., Grady S.L., Shenk T.E., Rabinowitz J.D. Divergent effects of human cytomegalovirus and herpes simplex virus-1 on cellular metabolism. PLoS Pathog. 2011;7:e1002124. doi: 10.1371/journal.ppat.1002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaarbø M., Ager-Wick E., Osenbroch P.O., Kilander A., Skinnes R., Müller F., Eide L. Human cytomegalovirus infection increases mitochondrial biogenesis. Mitochondrion. 2011;11:935–945. doi: 10.1016/j.mito.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 44.Fontaine K.A., Camarda R., Lagunoff M. Vaccinia virus requires glutamine but not glucose for efficient replication. J. Virol. 2014;88:4366–4374. doi: 10.1128/JVI.03134-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greseth M.D., Traktman P. De novo fatty acid biosynthesis contributes significantly to establishment of a bioenergetically favorable environment for vaccinia virus infection. PLoS Pathog. 2014;10:e1004021. doi: 10.1371/journal.ppat.1004021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silva da Costa L., Pereira da Silva A.P., Da Poian A.T., El-Bacha T. Mitochondrial bioenergetic alterations in mouse neuroblastoma cells infected with Sindbis virus: implications to viral replication and neuronal death. PLoS ONE. 2012;7:e33871. doi: 10.1371/journal.pone.0033871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ackermann W.W., Johnson R.B. Some energy relations in a host-virus system. J. Exp. Med. 1953;97:315–322. doi: 10.1084/jem.97.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ackermann W.W., Klernschmidt E. Concerning the relation of the Krebs cycle to virus propagation. J. Biol. Chem. 1951;189:421–428. [PubMed] [Google Scholar]
- 49.Piccoli C., Quarato G., Ripoli M., D’Aprile A., Scrima R., Cela O., Boffoli D., Moradpour D., Capitanio N. HCV infection induces mitochondrial bioenergetic unbalance: causes and effects. Biochim. Biophys. Acta. 2009;1787:539–546. doi: 10.1016/j.bbabio.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 50.Olagnier D., Lababidi R.R., Hadj S.B., Sze A., Liu Y., Naidu S.D., Ferrari M., Jiang Y., Chiang C., Beljanski V. Activation of Nrf2 signaling augments vesicular stomatitis virus oncolysis via autophagy-driven suppression of antiviral immunity. Mol. Ther. 2017;25:1900–1916. doi: 10.1016/j.ymthe.2017.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim C.K., Ahmed A.U., Auffinger B., Ulasov I.V., Tobias A.L., Moon K.S., Lesniak M.S. N-acetylcysteine amide augments the therapeutic effect of neural stem cell-based antiglioma oncolytic virotherapy. Mol. Ther. 2013;21:2063–2073. doi: 10.1038/mt.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Neill L.A., Kishton R.J., Rathmell J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016;16:553–565. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Angajala A., Lim S., Phillips J.B., Kim J.H., Yates C., You Z., Tan M. Diverse roles of mitochondria in immune responses: novel insights into immuno-metabolism. Front. Immunol. 2018;9:1605. doi: 10.3389/fimmu.2018.01605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sena L.A., Li S., Jairaman A., Prakriya M., Ezponda T., Hildeman D.A., Wang C.R., Schumacker P.T., Licht J.D., Perlman H. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013;38:225–236. doi: 10.1016/j.immuni.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cascone T., McKenzie J.A., Mbofung R.M., Punt S., Wang Z., Xu C., Williams L.J., Wang Z., Bristow C.A., Carugo A. Increased tumor glycolysis characterizes immune resistance to adoptive T cell therapy. Cell Metab. 2018;27:977–987.e4. doi: 10.1016/j.cmet.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buck M.D., O’Sullivan D., Pearce E.L. T cell metabolism drives immunity. J. Exp. Med. 2015;212:1345–1360. doi: 10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van der Windt G.J., Everts B., Chang C.H., Curtis J.D., Freitas T.C., Amiel E., Pearce E.J., Pearce E.L. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sukumar M., Liu J., Ji Y., Subramanian M., Crompton J.G., Yu Z., Roychoudhuri R., Palmer D.C., Muranski P., Karoly E.D. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J. Clin. Invest. 2013;123:4479–4488. doi: 10.1172/JCI69589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coe D.J., Kishore M., Marelli-Berg F. Metabolic regulation of regulatory T cell development and function. Front. Immunol. 2014;5:590. doi: 10.3389/fimmu.2014.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Battaglia M., Stabilini A., Roncarolo M.G. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 61.Gerriets V.A., Kishton R.J., Nichols A.G., Macintyre A.N., Inoue M., Ilkayeva O., Winter P.S., Liu X., Priyadharshini B., Slawinska M.E. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J. Clin. Invest. 2015;125:194–207. doi: 10.1172/JCI76012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Neill L.A. A broken Krebs cycle in macrophages. Immunity. 2015;42:393–394. doi: 10.1016/j.immuni.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 63.Liu P.S., Wang H., Li X., Chao T., Teav T., Christen S., Di Conza G., Cheng W.C., Chou C.H., Vavakova M. α-Ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol. 2017;18:985–994. doi: 10.1038/ni.3796. [DOI] [PubMed] [Google Scholar]
- 64.Rivadeneira D.B., DePeaux K., Wang Y., Kulkarni A., Tabib T., Menk A.V., Sampath P., Lafyatis R., Ferris R.L., Sarkar S.N. Oncolytic viruses engineered to enforce leptin expression reprogram tumor-infiltrating T cell metabolism and promote tumor clearance. Immunity. 2019;51:548–560.e4. doi: 10.1016/j.immuni.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Comito G., Iscaro A., Bacci M., Morandi A., Ippolito L., Parri M., Montagnani I., Raspollini M.R., Serni S., Simeoni L. Lactate modulates CD4+ T-cell polarization and induces an immunosuppressive environment, which sustains prostate carcinoma progression via TLR8/miR21 axis. Oncogene. 2019;38:3681–3695. doi: 10.1038/s41388-019-0688-7. [DOI] [PubMed] [Google Scholar]
- 66.Chang C.H., Qiu J., O’Sullivan D., Buck M.D., Noguchi T., Curtis J.D., Chen Q., Gindin M., Gubin M.M., van der Windt G.J. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yin Z., Bai L., Li W., Zeng T., Tian H., Cui J. Targeting T cell metabolism in the tumor microenvironment: an anti-cancer therapeutic strategy. J. Exp. Clin. Cancer Res. 2019;38:403. doi: 10.1186/s13046-019-1409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Munn D.H., Mellor A.L. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. 2016;37:193–207. doi: 10.1016/j.it.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leone R.D., Emens L.A. Targeting adenosine for cancer immunotherapy. J. Immunother. Cancer. 2018;6:57. doi: 10.1186/s40425-018-0360-8. [DOI] [PMC free article] [PubMed] [Google Scholar]