Abstract
The liver is an important organ in the regulation of glucose and lipid metabolism. It is responsible for systemic energy homeostasis. When energy need exceeds the storage capacity in the liver, fatty acids are shunted into nonoxidative sphingolipid biosynthesis, which increases the level of cellular ceramides. Accumulation of ceramides alters substrate utilization from glucose to lipids, activates triglyceride storage, and results in the development of both insulin resistance and hepatosteatosis, increasing the likelihood of major metabolic diseases. Another sphingolipid metabolite, sphingosine 1-phosphate (S1P) is a bioactive signaling molecule that acts via S1P-specific G protein coupled receptors. It regulates many cellular and physiological events. Since an increase in plasma S1P is associated with obesity, it seems reasonable that recent studies have provided evidence that S1P is linked to lipid pathophysiology, including hepatosteatosis and fibrosis. Herein, we review recent findings on ceramides and S1P in obesity-mediated liver diseases and the therapeutic potential of these sphingolipid metabolites.
Keywords: ceramide, fibrosis, insulin resistance, obesity, sphingosine 1-phosphate, steatosis
INTRODUCTION
Overnutrition and consequent obesity is the major cause of metabolic dysfunction, a general term that includes insulin resistance, cardiometabolic diseases, and non-alcoholic fatty liver disease (NAFLD) (Barr et al., 2010; Hastie et al., 2010). Overeating inhibits catabolic metabolism while activating anabolic synthetic pathways and energy storage. Obese subjects have elevated free fatty acids (FFA) in the blood plasma, and these accumulate in tissues such as the liver that do not normally contain adipose tissue (Bradbury, 2006; Unger, 2003). Abnormal accumulation of FFAs in obese people promotes the synthesis of triglycerides (TGs), the preferential energy storage form in humans.
Patients with NAFLD do not consume a significant amount of alcohol, but do accumulate TGs as lipid droplets in their liver. The prevalence of NAFLD has increased 25% to 30% over the past 20 years (Bashiardes et al., 2016), and the prevalence of other metabolic diseases, for instance, type 2 diabetes, hyperlipidemia, and cardiovascular diseases, has also increased during this time (Younossi et al., 2016). NAFLD is an umbrella term for a broad range of liver diseases ranging in severity from simple steatosis to inflammatory steatohepatitis. Simple steatosis is the mildest form of NAFLD, and is characterized by TG deposition in liver and by the presence of lipid droplets in hepatocytes (Cohen et al., 2011; Sattar et al., 2014). The formation of lipid droplets in the liver results in part from imbalances in processes that increase fatty acid (FA) content, for example, synthesis and uptake, and processes that reduce it, such as export and oxidation (Postic and Girard, 2008). Non-alcoholic steatohepatitis (NASH) is more serious than simple steatosis and patients with this condition have hepatocyte injury and inflammation, which is usually accompanied by pericellular fibrosis. Adverse outcomes from NASH include cirrhosis, liver failure, and hepatocellular carcinoma (Lindenmeyer and McCullough, 2018; Rinella and Sanyal, 2016). Despite concerted efforts to elucidate the mechanism of NAFLD, it is not well understood. Likewise, despite attempts to identify therapeutic agents, no effective therapeutic agent has been approved for NASH.
When the energy needs of the body are met and no additional FFAs can be stored, excess FFAs are shunted into the nonoxidative sphingolipid synthesis pathway. Elevated sphingolipid metabolites, particularly ceramide and sphingosine 1-phosphate (S1P), are signaling molecules that indicate a cellular overnourished status. Upon reception of the signal, cells adapt to the oversupply of FA (Chaurasia et al., 2019; Rohrbach et al., 2017). In this review, we discuss the current understanding of the mechanism by which ceramide and S1P alter cellular metabolism and contribute to development of hepatosteatosis and fibrosis.
Sphingolipid metabolism as a sensor for FA surplus
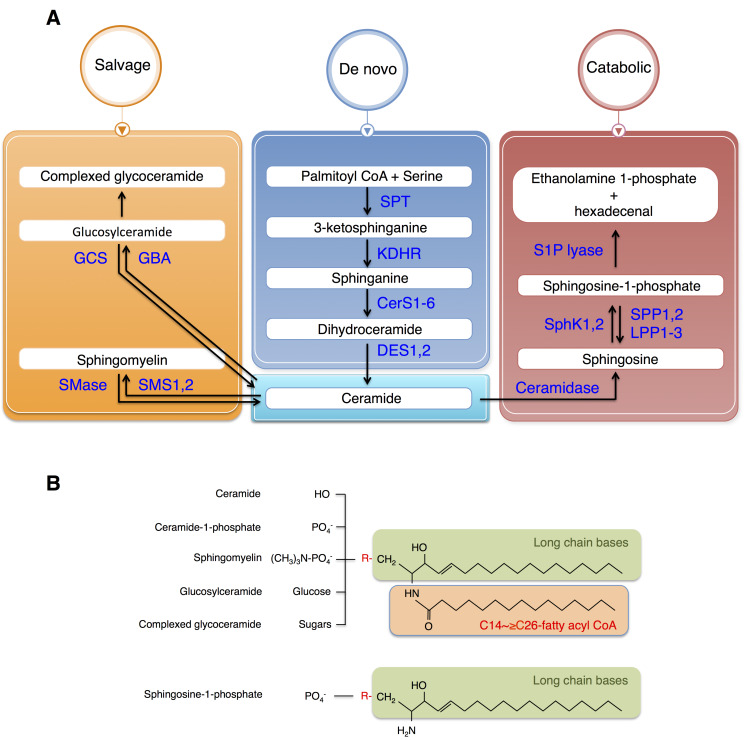
Sphingolipid metabolism is highly coordinated by a complex network of interconnected pathways, and not simply by the availability of FA substrate. The major biosynthetic site for sphingolipids is the endoplasmic reticulum (ER), where FA and amino acids are condensed to form ceramides (Fig. 1) (Merrill, 2002). The condensation of ceramide is a major branching point in the pathway; it may be used in the synthesis of S1P or converted to other complex sphingolipids including sphingomyelins and gangliosides. The enzymes converting ceramides into complex sphingolipids are localized in the Golgi apparatus. De novo synthesis of sphingolipids is initiated from condensation of serine and palmitoyl CoA by serine palmitoyltransferase (SPT) to produce 3-ketosphinganine, followed by a series of reactions involving the enzymes 3-ketosphinganine reductase, ceramide synthase (CerS), and dihydroceramide desaturase (DES) to produce ceramide. Dihydroceramides and ceramides are transported to the Golgi apparatus and used as substrates for the enzymes that synthesize complex sphingolipids. Specifically, these include sphingomyelin by sphingomyelin synthases, gangliosides by glucosylceramide synthase, and ceramide 1-phosphate by ceramide kinase.
Fig. 1. Sphingolipid synthesis pathways and structures.
(A) Ceramide is generated by a de novo synthetic pathway and further metabolized via a salvage pathway. Once synthesized, ceramide is converted to either glucosylceramide or sphingomyelin by adding glucose or phosphocholine, respectively. Ceramide is degraded via a catabolic pathway to sphingosine is phosphorylated by sphingosine kinase (SphK), which can be degraded by S1P lyase. (B) Ceramide is synthesized by adding fatty acyl CoA to the long chain bases (sphingosine or sphinganine) and further metabolized to ceramide 1-phosphate (by phosphorylation), sphingomyelin (by adding phosphocholine), glucosylceramide (by adding glucose), and complexed glycoceramide (by adding various sugars). Ceramide is also degraded to sphingosine, which can be phosphorylated to S1P. GCS, glucosylceramide synthase; GBA, glucocerebrosidase; SMase, sphingomyelinase; SMS, sphingomyelin synthase; SPT, serine palmitoyltransferase; KDHR, 3-keto-dehydrosphingosine reductase; CerS, ceramide synthase; DES, dihydroceramide desaturase; S1P lyase, sphingosine-1-phosphate lyase; SphK, sphingosine kinase; SPP, S1P-specific phosphatases; LPP, lipid phosphate phosphatase.
Another important route for ceramide metabolism is generation of S1P. In this pathway, ceramide is deacylated by ceramidases to produce sphingosine. Phosphorylation of sphingosine is catalyzed by sphingosine kinases (SphK1, 2) to generate S1P. Newly synthesized S1P is transported out of the cell by an ATP-binding cassette (ABC) transporters or by a member of the major facilitator superfamily member, spinster 2 (Spxlink) (Nishi et al., 2014; Takabe and Spiegel, 2014). After export, S1P binds to one of five S1P-specific G protein coupled receptors (S1PR1-5) and activates diverse cellular responses (Maceyka and Spiegel, 2014; Pyne and Pyne, 2010). Cellular S1P levels are tightly regulated by sphingosine levels, SphKs, and the enzymes that metabolize S1P, which include S1P lyase, two S1P-specific phosphatases (SPP1-2), and three phosphate phosphatase (LPP1-3) (Maceyka et al., 2012). S1P acts as both an extracellular and intracellular signal. These have different biological functions depending on the site of generation of the SphK involved (Schwalm et al., 2013).
Ceramide is a regulatory messenger for excess FFA
A number of researchers have suggested that ceramide synthesis can be activated by increased FFAs (Samad et al., 2006; Schilling et al., 2013); and it has been suggested that ceramide synthesis regulates uptake of FFA. Uptake and esterification of FFAs are important in initiating the production and action of ceramide and overexpression of acid ceramidase in the liver reduced not only C16- and C18-ceramides but also CD36, the FA transporter (Xia et al., 2015). Overexpression of acid ceramidase also downregulated the activity of the atypical protein kinase Cζ(PKCζ), which is activated by ceramide and stimulates lipid uptake. The finding that a ceramide analogue enhanced translocation of CD36 to the membrane via a PKCζ-dependent mechanism was interpreted as evidence that ceramide regulates FA uptake and esterification. Chaurasia et al. (2019) recently demonstrated that mice deficient in DES1 were protected from hepatic steatosis. Translocation of CD36 was stimulated by ceramide in cultured hepatocytes.
Since ceramide synthesis may reduce excess FFA, it may activate esterification of FFA into TGs. Indeed, sterol response element binding proteins (SREBPs), which are major regulators of TG and cholesterol synthesis, were activated by exogenous C16-ceramides and hepatic expression of Srebf1 and its downstream targets for FA biosynthesis including FAS and FA elongation such as Elovl6 (Jiang et al., 2015). The possible mechanism implicates atypical PKCs such as PKCλ and ζ which are ceramide effectors and inducers for hepatosteatosis and hypertriglyceridemia in mice (Chen et al., 2019; Taniguchi et al., 2006). In addition, the finding that ceramide analogues inhibited isoproterenol-stimulated phosphorylation of hormone-sensitive lipase (HSL) suggested that ceramide inhibits release of FAs from TGs (Turpin et al., 2014). Collectively, these data suggest that ceramide activates TG synthesis to relieve the FFA burden and prevents FA release from lipid droplets.
Although ceramide enhanced FA entry into cells, it inhibited the uptake of glucose (Summers et al., 1998; Wang et al., 1998). The primary effect of ceramide on glucose uptake appears to be to inhibit the insulin-responsive translocation of the GLUT4 glucose transporter to the plasma membrane, by blocking insulin-mediated phosphorylation of Akt, a serine/threonine kinase involved in insulin action, anabolic signaling, and cell survival (Hajduch et al., 2001; Summers et al., 1998; Wang et al., 1998). PKCζ activated by ceramide, phosphorylates Akt on a third inhibitory site in the enzyme’s PH domain, which reduces the kinase’s affinity for phosphoinositides and prevents its PI3 kinase-dependent activation (Powell et al., 2003). In addition, ceramide-activated protein phosphatase 2A (PP2A) enhances dephosphorylation of Akt (Zinda et al., 2001). The relative contribution of either PKCζ or PP2A pathway is dependent on cell type.
Collectively, based on these findings, we suggest that ceramide regulates lipid and glucose metabolism by modulating gene expression and signaling effectors. This mechanism is the adaptation process of the substrate oxidation to adjust to lipid-overload condition.
ROLES OF SPHINGOLIPIDS IN HEPATOSTEATOSIS AND FIBROSIS
Ceramides in hepatosteatosis
Most TGs contain the saturated C16-FA, palmitate. Palmitate is a critical factor in lipotoxicity and a metabolic syndrome. Palmitate treatment in vitro not only increases inflammation, apoptosis, ER stress, insulin resistance, but also promotes synthesis of C16-ceramide (Gao et al., 2012; Schilling et al., 2013; Watt et al., 2012). Myriocin, which inhibits de novo sphingolipid synthesis, protected subjects from diet-induced accumulation of TG in the liver and obesity (Kurek et al., 2013; Yang et al., 2009). This protection may have been due to the reduction of total ceramide and sphingosine (Yang et al., 2009). In animal models of NAFLD, total hepatic ceramides were not changed, but the length of their acyl chain changed from C24-ceramides to C16-ceramides (Turner et al., 2013; Turpin et al., 2014). Furthermore, mice lacking CerS5 and CerS6 were resistant to a high fat diet (HFD)-induced steatosis and obesity, suggesting that C16-ceramide formation by CerS5 and CerS6 is important in the development of both fatty liver and obesity (Gosejacob et al., 2016; Turpin et al., 2014). In contrast, CerS2 heterozygote (+/–) mice had more C16-ceramides than the wild type (WT); they also impaired FA oxidation and aggravated fatty liver after HFD (Raichur et al., 2014). PPAR-γ and its downstream target, CD36 expression, were downregulated and FA oxidation suppressed in livers from CerS6 knock-out (KO) mice compared to livers from the WT control, but both were increased in the liver from CerS2 heterozygous KO mice. Exogenous administration of C24-ceramide reduced PPAR-γ expression (Li et al., 2013; Park et al., 2014) and increased C16-ceramide also resulted in mitochondrial dysfunction, generation of reactive oxygen species (ROS) and caused ER stress (Kim et al., 2019; Zigdon et al., 2013). This is likely evidence that the balance between C16-ceramide and C24-ceramide is important in PPAR-γ signaling, mitochondrial function, ROS generation, FA oxidation, and ER stress. Recently, Chaurasia et al. (2019) deleted dihydroceramide desaturase-1 (DES1), which inserts a conserved double bond into the backbone of ceramides. DES1 deficient mice showed less hepatic ceramides and serum ceramide than the WT mice, resulting in improved insulin resistance and hepatic steatosis in leptin deficiency (ob/ob) mice.
Sphingomyelin (SM) is one of the most abundant sphingolipids and it can serve as a reservoir to regulate the amount of ceramide by sphingomyelinase (SMase). There are three SMases that hydrolyze sphingomyelin to ceramide and phosphocholine. Expression of acid sphingomyelinase (aSMase) is increased in NAFLD (Longato et al., 2012; Moles et al., 2010) and aSMase deficiency protects from not only diet-induced accumulation of TGs and ER stress, but also alcohol-induced steatohepatitis (Deevska et al., 2009; Fucho et al., 2014). Furthermore, aSMase inhibition by amitriptyline prevented HFD-induced steatosis, inflammation, and fibrosis (Fucho et al., 2014). aSMase is overexpressed in the liver of NASH patients; aSMase regulates autophagy and lysosomal membrane permeabilization (Fucho et al., 2014). Although neutral SMase2 (nSMase2) is the main nSMase in the SMase family and it can be activated by inflammatory cytokines or glutathione (GSH) depletion (Rutkute et al., 2007a; 2007b), there are few studies of this in NAFLD. nSMase2 deficiency mice have a dwarf phenotype, with joint deformation, shortened long bones, and lower levels of growth hormone than the WT, so it seems clear that nSMase2 is important in bone formation (Stoffel et al., 2007). The nSMase2 inhibitor, GW4869, reduced inflammation and atherosclerosis formation in ApoE–/– mice and increased insulin resistance in C2C12 cells (Lallemand et al., 2018; Verma et al., 2014). Recently, GW4869 is emerging as important exosome secretion regulator, which can modulate inflammation through the NF-kB pathway and insulin sensitivity (Gao et al., 2016; Ying et al., 2017). Thus, nSMase2 may have an indirect role in the development of NAFLD.
Most SM is generated by sphingomyelin synthase 1 (SMS1). Therefore, it is not surprising that SMS1-null mice exhibited moderate neonatal lethality and severe pancreatic dysfunction (Yano et al., 2011). This precludes the use of these mice in obesity experiments. Instead, mice deficient in sphingomyelin synthase (SMS 2), which are more sensitive to insulin and diet-induced obesity than WT mice (Mitsutake et al., 2011), have less PPAR-γ and its downstream target CD36, and have smaller lipid droplets. Overexpression of SMS2 in the liver had the opposite effect: it stimulated FA uptake, resulting hepatic steatosis (Li et al., 2013). Although there is a consensus that ceramide levels correlate positively with insulin resistance and development of fatty liver, SMS2 liver-specific transgenic mice had less ceramide and were more susceptible to diet-induced fatty liver formation than WT mice, while mice deficient in SMS2 had more ceramide than the WT mice and were more susceptible to diet-induced fatty liver formation. Since SMS2 is located in plasma membrane and a novel regulator of a plasma membrane microdomain, SMS2 appears to regulate FA uptake via Caveolin1 and CD36, which are also located in the microdomain (Mitsutake et al., 2011). Inhibition of glucosylceramide synthase (GCS), another enzyme that converts ceramide to glucosylceramide, also protected against HFD-induced fatty liver. These mice also had less SREBP-1c and its downstream targets, FA synthase (FAS), and stearoyl-CoA desaturase-1 (SCD-1) than mice in which GCS had not been inhibited, but PPAR-α and PPAR-γ were unaffected (Zhao et al., 2009). The exact mechanism of how GCS inhibition affects the development of fatty liver and insulin resistance is unclear, but the reduced ganglioside GM3, which plays an important role in insulin resistance (Tagami et al., 2002; Yamashita et al., 2003), could increase insulin sensitivity.
Sphingosine 1-phosphate in steatosis and obesity
Accumulating evidence is consistent with the proposal that S1P levels correlate with the insulin resistance and occurrence of hepatic steatosis. Recently, evidence suggested to workers that SphKs and S1P are major players in lipid accumulation in liver (steatosis) and lipid storage in adipose tissue (obesity) (Sanyal and Pacana, 2015). A lipidomic study using a model for NAFLD in which obesity was induced in mice with a HFD provided evidence that increasing sphingolipid resulted in sphingosine and S1P levels in the liver that were comparable to those in mice supplies with a normal chow diet. It may be that the HFD-induced de novo sphingolipid synthesis is associated with NAFLD progression (Sanyal and Pacana, 2015).
The role of sphingosine kinase 1 (SphK1) in NAFLD is controversial. SphK1 expression increased in hepatic steatosis and SphK1-deficient mice are protected against hepatic steatosis. S1P generated from SphK1 action regulates PPAR-γ through the S1PR-Akt-mTOR signaling pathway (Chen et al., 2016a), However, SphK1-null mice had increased insulin resistance, attributed to reduced insulin production resulting from increased lipotoxicity-induced β-cell death (Qi et al., 2013). Moreover, SphK1 is involved in inflammatory cytokine production in a NF-kB pathway-dependent manner (Geng et al., 2015). SphK1 overexpression induced with the adenovirus AdSphK1 resulted in lower basal glucose level and improved glucose tolerance as well as fatty liver in diabetic mice compared to mice with normal expression of SphK1 (Ma et al., 2007). In contrast, though, in another study mRNA of SphK1 and SphK activity decreased in HFD-fed mice liver compared to mice fed normal diets (Kowalski et al., 2015) and SphK1 overexpression by adeno-associated virus (AAV)-SphK1 had reduced hepatic triglyceride in mice fed low-fat diets, but did not affect steatosis or insulin resistance in mice fed HFD (Kowalski et al., 2015). Although SphK2 expression does not increase during the development of fatty liver (Chen et al., 2016a), overexpression of SphK2 achieved with an adenovirus increased S1P, but reduced sphinganine; sphingosine; C16- and C18-ceramides; and C16- and C18-sphingomyelins. Hepatic triglyceride and cholesterol levels were reduced and FA oxidation was activated upon SphK2 overexpression (Lee et al., 2015) compared to mice normally expressing SphK2. Additional evidence provide support for this notion. SphK2 KO mice fed a HFD developed hepatosteatosis, while those fed a normal diet did not (Nagahashi et al., 2015). Therefore, it may be that elevated S1P in the nucleus, via the action of SphK2, is linked to reduced fat accumulation in liver, and that SphK2 is a key regulator of FA oxidation and TG metabolism.
The fingolimod FTY720 is a therapeutic drug for multiple sclerosis and the autoimmune disease. The drug acts as a functional antagonist of S1PR1 to induce S1PR1 degradation (Brinkmann et al., 2010). In mice fed a high-calorie diet to induce NASH, FTY720 administration reduced body and liver weight, and these effects were accompanied by decreasing hepatocyte ballooning, hepatic inflammation, and fibrosis in liver compared to mice fed a normal diet (Mauer et al., 2017). When mice fed a western diet supplemented with sweet water, the administration of FTY720 alleviated hepatosteatosis, and this was accompanied by decreasing hepatic inflammation and sphingolipid species—specifically, ceramide, dihydroceramide, S1P, and dihydro-S1P (Rohrbach et al., 2019). In contrast to mice deficient in S1PR1, the mice lacking S1PR2 rapidly developed fatty livers on a HFD (Nagahashi et al., 2015). From this evidence, it may be that the S1P receptor isotypes contribute differently to the development of fatty livers. The effects on fatty liver and insulin resistance caused by manipulation of sphingolipid levels are summarized in Table 1.
Table 1.
The effects of sphingolipids changes on fatty liver and insulin resistance
| Pathway | Chemical treated or KO mice | Insulin resistance | Fatty liver | Phenotype | Reference |
|---|---|---|---|---|---|
| De novo ceramide biosynthesis | Myriocin | Improved | Improved | Weight gain after HFD ↓ Serum ceramide ↓ Insulin signaling in liver and muscle ↑ Energy expenditure ↑ (UCP3 ↑, SOCS-3 ↓ in adipose tissue) |
(Holland et al., 2007; Kurek et al., 2013; Ussher et al., 2010; Yang et al., 2009) |
| DES1 KO mice | Improved | Improved | Weight gain in ob/ob mice ↓ Hepatic C16, C18, C20, C22, C24-Cer ↓ Serum C16, C18, C20, C22, C24-Cer ↓ White adipose tissue mass ↓ |
(Chaurasia et al., 2019) | |
| CerS6 KO mice | Improved | Improved | Weight gain after HFD ↓ Hepatic p-Akt, p-GSK3 ↑ Energy expenditure ↑ β-oxidation capacity in brown adipose tissue ↑ Hepatic β-oxidation ↑ PPAR-γ, CD36 ↓ |
(Turpin et al., 2014) | |
| CerS5 KO mice | Improved | Improved | Weight gain after HFD ↓ | (Gosejacob et al., 2016) | |
| CerS2 heterozygote (+/–) mice | Aggravated | Aggravated | Serum cholesterol ↑ γImpaired lipid oxidation γImparted electron transport chain activity | (Raichur et al., 2014) | |
| Salvage pathway | nSMase2 KO mice | No study | No study | Dwarfism phenotype Joint deformation Short statured long bones Growth hormone ↓ |
(Stoffel et al., 2007) |
| aSMase KO mice | Improved | Improved | Hepatic stellate cells proliferation ↓ Liver fibrosis after bile duct ligation ↓ ER stress ↓ |
(Fucho et al., 2014) | |
| aSMase inhibition (amitrypsine, imipramine, desipramine) | Improved | Improved | Inflammation ↓ Serum ceramide ↓ C16-Cer, C24-Cer ↓ p-Akt, p-p70S6K ↑ p-p38, p-JNK ↓ |
(Fucho et al., 2014; Jin et al., 2013; Liangpunsakul et al., 2012) | |
| GCS inhibition (Genz-123346, Genz-112638, AMP-DNM) | Improved | Improved | FAS, SCD-1, ACC1 ↓ p-Insulin receptor-β, p-mTOR ↑ GM3 ↓ |
(Aerts et al., 2007; Zhao et al., 2007; 2009) | |
| Sphingomyelin pathway | SMS1 KO mice | No study | No study | Mitochondrial dysfunction Impaired insulin secretion |
(Yano et al., 2011) |
| SMS2 KO mice | Improved | Improved | Weight gain after HFD ↓ PPAR-γ, CD36 ↓ |
(Mitsutake et al., 2011) | |
| SMS2 transgenic mice | No study | Aggravated | CD36 ↑ | (Li et al., 2013) | |
| Catabolic pathway | SphK1 null mice | Aggravated | Improved | Hepatic triglyceride, cholesterol ↓ Hepatic S1P ↓ PPAR-γ, CD36, UCP2, Cidea ↓ Pancreatic β-cell mass in HFD-fed ↓ Insulin production in HFD-fed ↓ |
(Chen et al., 2016a; Qi et al., 2013) |
| SphK1 overexpression by AdSphK1 | Improved | Improved | Hepatic triglyceride ↓ p-Akt, p-GSK3 ↑ |
(Ma et al., 2007) | |
| SphK2 overexpression by AdSphK2 | Improved | Improved | p-Akt ↑ C16, C18, C24:1-Cer ↓ Sphingosine, sphinganine ↓ PPAR-α, CPT1, ACOX1 ↑ |
(Lee et al., 2015) | |
| FTY720 (S1PR1 antagonist) | Improved | Improved | Macrophage infiltration ↓ Ly6-C (monocyte-derived macrophages marker), CCR2 (C-C chemokine receptor type 2) ↓ α-smooth muscle actin ↓ C16-, C24:1-ceramide ↓ Fatty acid synthase ↓ S1P, dihydro-S1P ↓ |
(Mauer et al., 2017; Rohrbach et al., 2019) | |
| S1PR2 deficiency mice | No study | Aggravated | H3K9 acetylation ↓ H4K5 acetylation ↓ H2BK12 acetylation ↓ |
(Nagahashi et al., 2015) |
Implication of ceramides and S1P in liver fibrosis
Liver fibrosis is a chronic liver disease that results from excess production of extracellular matrix proteins (Bissell, 1998), as a result of multiple injuries, functional wound healing, or chronic liver disease. It can lead to cirrhosis or liver cancer (Kisseleva et al., 2012; Kitatani et al., 2015). During fibrosis, hepatocytes undergo apoptosis and activate hepatic stellate cells by activating Kupffer cells, which release proinflammatory cytokines (Higuchi and Gores, 2003; Pessayre et al., 2002). Activated hepatic stellate cells secrete extracellular matrix to fill the space called the Disse space, and they proliferate and replace dead hepatocytes with fibrous scar tissue; this is fibrosis (Bataller and Brenner, 2005; Schuppan and Afdhal, 2008; Shea and Tager, 2012). Thus, drugs that block or inhibit hepatic stellate cell activation may be effective in preventing fibrosis (Rippe and Brenner, 2004).
Ceramide and liver fibrosis
As ceramide accumulates, cells undergo apoptosis and trigger the fibrotic events. Fibrosis is a defense mechanism to protect the tissue from lysing cells. In mice on which fibrosis was induced with carbon tetrachloride treatment, the total amount of ceramide was increased in both plasma and liver (Ichi et al., 2007), and mice lacking acid SMase had lower ceramide levels than mice with this enzyme (Mari et al., 2008). Acid sphingomyelinase activates hepatic stellate cells, which promote fibrogenesis through promoting migration of the cells and extracellular matrix secretion (Moles et al., 2010). Ceramide regulates the expression of collagen genes, important components of the extracellular matrix, via a mechanism of “regulated intra membrane proteolysis”. Cyclic AMP response element 3 like 1 (CREB3L1) is a transcriptional factor, which regulate collagen synthesis. CREB3L1 is cleaved by two Golgi-localized protease, site-1 and site-2 proteases (S1P/S2P), leading to enter the nucleus where it binds Smad4 and then upregulates transcription of genes for assembly of collagen-containing extracellular matrix (Chen et al., 2016b). Ceramides alter the orientation of TM4SF20, a protein blocking the access of S1P/S2P to CREB3L1, and activate fibrogenic processes (Chen et al., 2016b; Denard et al., 2012). In a recent study, administration of myriocin significantly reduced ceramide levels and reduced liver inflammation and fibrosis (Jiang et al., 2019). Thus, ceramide is believed to be important in regulating apoptosis of hepatocytes. It is also a potential, major target for the treatment of NASH and fibrosis.
S1P and liver fibrosis
S1P regulates the expression of various extracellular matrices during liver fibrosis (Li et al., 2009a). S1P also activates the proliferation and migration of hepatic stellate cells in vitro and increases the expression of extracellular matrix proteins, such as α-smooth muscle actin and collagen I and III (Al Fadel et al., 2016; Friedman, 2008; Gonzalez-Fernandez et al., 2017). In liver fibrosis studies, the levels of S1P were elevated consequent to increased hepatic SphK1 expression compared to the control tissue; this was observed both in fibrous liver tissue from mouse and human patients (King et al., 2017; Li et al., 2011). The expression level of Spxlink mRNA, which encodes a transporter of S1P, was elevated in fibrotic human liver compared to normal liver, indicating increased export of S1P and its binding to specific receptors, leading to fibrosis and inflammation (Sato et al., 2016). In a recent report, workers analyzed 95 patients with end-stage liver disease and observed that patients with low concentrations of plasma S1P had a poor prognosis (Becker et al., 2017). It seems, then, that S1P has a complex role in the development of advanced liver fibrosis and cirrhosis.
S1PRs and liver fibrosis
Two S1P receptors, S1PR1 and S1PR3, are considered to be the two major S1PRs that are important in liver fibrosis. Their levels were also elevated in cholestasis-induced liver fibrosis and in human fibrotic samples, whereas the S1PR2 levels were decreased, compared to the appropriate normal controls (Li et al., 2011; Xiu et al., 2015). Antagonists of S1PR1 and S1PR3 blocked upregulation of Ang1 and alleviated fibrosis in the damaged liver, whereas the S1PR2 antagonist had no effect in angiogenesis (Yang et al., 2013). Silencing of S1PR3 diminished not only the ability of bone marrow-derived cells to migrate to the liver but also their transdifferentiation into myofibroblast-like cells (Li et al., 2009a). Recently, human embryonic lethal abnormal visual protein (HuR) was induced during liver fibrosis via S1P and increased expression of S1PR3. HuR, an mRNA binding protein, affects the vitality of bone marrow-derived cells and further stabilize their mRNA (Chang et al., 2017). On the other hand, a KO mutant of S1PR2 in animal models of liver fibrosis protects mice from the development of fibrosis (Ikeda et al., 2009), and the expression of S1PR2 was reduced in the liver analysis from the patients with liver fibrosis (Li et al., 2011). We suggest that further studies are needed to identify the roles of S1P and S1PRs in the fibrosis process induced by various cells. Major sphingolipids and the mechanism for development of fatty liver and fibrosis are summarized in Fig. 2.
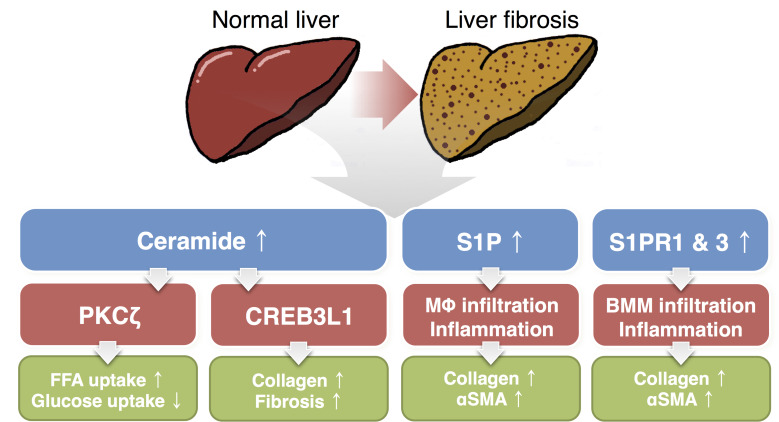
Fig. 2. The roles of ceramide and S1P in liver fibrosis.
During liver fibrosis, ceramide and S1P levels are elevated. Ceramide promotes PKCζ activation, which induces CD36-mediated fatty acid uptake (Xia et al., 2015) and disturbs glucose uptake (Powell et al., 2003). Ceramide also stimulates CREB3L1 cleavage, which activates fibrogenic processes (Chen et al., 2016b; Denard et al., 2012). S1P induces Kupffer cell infiltration, which increases expressions of collagen and α-smooth muscle actin (Al Fadel et al., 2016; Friedman, 2008; Gonzalez-Fernandez et al., 2017). S1PR1 and S1PR3 is also involved in bone marrow-derived macrophage/monocytes migration to the liver (Li et al., 2011; Xiu et al., 2015). PKCζ, protein kinase C zeta; FFA, free fatty acids; CREB3L1, cAMP responsive element binding protein3 like 1; S1P, sphingosine 1-phosphate; MΦ, macrophage; αSMA, α-smooth muscle actin; S1PR, S1P receptor; BMM, bone marrow-derived macrophage/monocytes.
Therapeutic targets in the treatment and prevention of liver fibrosis
Tracking and altering the signaling pathway of S1P may aid in the treatment of liver fibrosis. For example, the discovery of fingolimod, also known as FTY720 and a modulator of S1PR1, is promising. This drug has been approved by the U.S. Food and Drug Administration (FDA) and is the first oral drug that effectively treats recurrent multiple sclerosis. The development of inhibitors of S1P signaling and approaches that target enzymes in the sphingolipid pathway are novel fields in the search for efficient antifibrotic drugs (Dyckman, 2017; Park and Im, 2017). The major candidate drugs that mediate antifibrotic activity through the regulation of S1P or its receptors reported so far are summarized in Table 2.
Table 2.
The candidate drugs/agents of antifibrotic activity
| Action | Drug/agent | Reference |
|---|---|---|
| Sphingosine kinase inhibitor | PF543 (SphK inhibitor) | (Gonzalez-Fernandez et al., 2017) |
| SKI-II (SphK inhibitor, non-selective) | (Yang et al., 2013) | |
| N,N-dimethylsphingosine (DMS, SphK inhibitor) | (Brunati et al., 2008; Wang et al., 2017b; Xiu et al., 2015) | |
| S1P receptor agonist/antagonist | FTY720 (S1PR1 and S1PR3 agonist) | (Brunati et al., 2008; King et al., 2017; Kong et al., 2014) |
| VPC23019 (S1PR1 and S1PR3 antagonist) | (Brunati et al., 2008; Yang et al., 2012; 2013) | |
| SEW2871 (S1PR1 agonist) | (Ding et al., 2016) | |
| W146 (S1PR1 antagonist) | (King et al., 2017; Liu et al., 2011; Yang et al., 2012; 2013) | |
| JTE-013 (S1PR2 antagonist) | (Kageyama et al., 2012; Wang et al., 2017a; Xu et al., 2016; Yang et al., 2015) | |
| Suramin (S1PR3 antagonist) | (Li et al., 2009a; 2009b) | |
| KRP203 (FTY720 analog) | (Kaneko et al., 2006; Khattar et al., 2013) | |
| CAY-10444 (S1PR3 antagonist) | (Yang et al., 2015) | |
| VPC24191 (S1PR1 and S1PR3 antagonist) | (Al Fadel et al., 2016) | |
| Other inhibitor | Pertussis toxin (PTX; G-protein-coupled receptor signaling inhibitor) | (Brunati et al., 2008; Gonzalez-Fernandez et al., 2017; Yang et al., 2015) |
| Melatonin (melatonin receptors agonist) |
CONCLUSION
Ceramides are the nutrient sensors that alleviate the FFA oversupply, inhibit glucose utilization, and activate deposition of TGs in the liver. Acting independently of ceramides, S1P activates cellular signaling via S1PR binding and has important roles in hepatic pathologies. While pharmacological intervention of sphingolipid biosynthesis for NAFLD is promising, more detailed understanding of the pathways is needed. It is expected that the second generation of therapeutics for liver diseases can be pursued in light of these metabolic pathways.
ACKNOWLEDGMENTS
This research was supported by the Gachon University research fund of 2019 (GCU-2019-0705) and the Bio & Medical Technology Development Program through the National Research Foundation of Korea (NRF), funded by the Korean government (MSIP) (2018M3A9F3020970) to T.S.P. W.J.P. is supported by National Research Foundation of Korea grants funded by the Korean Government (Ministry of Education, Science and Technology) (NRF-2016R1D1A1B04930619).
Footnotes
AUTHOR CONTRIBUTIONS
W.J.P. and T.S.P. contributed to the conception and design of the manuscript. J.H.S., G.T.K., W.J.P., and T.S.P. wrote the manuscript. J.H.S. and G.T.K. summarized tables and W.J.P. designed figures. W.J.P. and T.S.P. reviewed and edited the final manuscript.
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to disclose.
REFERENCES
- Aerts J.M., Ottenhoff R., Powlson A.S., Grefhorst A., van Eijk M., Dubbelhuis P.F., Aten J., Kuipers F., Serlie M.J., Wennekes T., et al. Pharmacological inhibition of glucosylceramide synthase enhances insulin sensitivity. Diabetes. 2007;56:1341–1349. doi: 10.2337/db06-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Fadel F., Fayyaz S., Japtok L., Kleuser B. Involvement of sphingosine 1-phosphate in palmitate-induced non-alcoholic fatty liver disease. Cell. Physiol. Biochem. 2016;40:1637–1645. doi: 10.1159/000453213. [DOI] [PubMed] [Google Scholar]
- Barr E.L., Cameron A.J., Balkau B., Zimmet P.Z., Welborn T.A., Tonkin A.M., Shaw J.E. HOMA insulin sensitivity index and the risk of all-cause mortality and cardiovascular disease events in the general population: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab) study. Diabetologia. 2010;53:79–88. doi: 10.1007/s00125-009-1588-0. [DOI] [PubMed] [Google Scholar]
- Bashiardes S., Shapiro H., Rozin S., Shibolet O., Elinav E. Non-alcoholic fatty liver and the gut microbiota. Mol. Metab. 2016;5:782–794. doi: 10.1016/j.molmet.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataller R., Brenner D.A. Liver fibrosis. J. Clin. Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S., Kinny-Koster B., Bartels M., Scholz M., Seehofer D., Berg T., Engelmann C., Thiery J., Ceglarek U., Kaiser T. Low sphingosine-1-phosphate plasma levels are predictive for increased mortality in patients with liver cirrhosis. PLoS One. 2017;12:e0174424. doi: 10.1371/journal.pone.0174424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell D.M. Hepatic fibrosis as wound repair: a progress report. J. Gastroenterol. 1998;33:295–302. doi: 10.1007/s005350050087. [DOI] [PubMed] [Google Scholar]
- Bradbury M.W. Lipid metabolism and liver inflammation. I. Hepatic fatty acid uptake: possible role in steatosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G194–G198. doi: 10.1152/ajpgi.00413.2005. [DOI] [PubMed] [Google Scholar]
- Brinkmann V., Billich A., Baumruker T., Heining P., Schmouder R., Francis G., Aradhye S., Burtin P. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discov. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- Brunati A.M., Tibaldi E., Carraro A., Gringeri E., D'Amico F., Jr, Toninello A., Massimino M.L., Pagano M.A., Nalesso G., Cillo U. Cross-talk between PDGF and S1P signalling elucidates the inhibitory effect and potential antifibrotic action of the immunomodulator FTY720 in activated HSC-cultures. Biochim. Biophys. Acta. 2008;1783:347–359. doi: 10.1016/j.bbamcr.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Chang N., Ge J., Xiu L., Zhao Z., Duan X., Tian L., Xie J., Yang L., Li L. HuR mediates motility of human bone marrow-derived mesenchymal stem cells triggered by sphingosine 1-phosphate in liver fibrosis. J. Mol. Med. 2017;95:69–82. doi: 10.1007/s00109-016-1460-x. [DOI] [PubMed] [Google Scholar]
- Chaurasia B., Tippetts T.S., Mayoral Monibas R., Liu J., Li Y., Wang L., Wilkerson J.L., Sweeney C.R., Pereira R.F., et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science. 2019;365:386–392. doi: 10.1126/science.aav3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Wang W., Qi Y., Kaczorowski D., McCaughan G.W., Gamble J.R., Don A.S., Gao X., Vadas M.A., Xia P. Deletion of sphingosine kinase 1 ameliorates hepatic steatosis in diet-induced obese mice: role of PPARγ. Biochim. Biophys. Acta. 2016a;1861:138–147. doi: 10.1016/j.bbalip.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Chen Q., Denard B., Lee C.E., Han S., Ye J.S., Ye J. Inverting the topology of a transmembrane protein by regulating the translocation of the first transmembrane helix. Mol. Cell. 2016b;63:567–578. doi: 10.1016/j.molcel.2016.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.C., Lee R.A., Tsai S.L., Kanamaluru D., Gray N.E., Yiv N., Cheang R.T., Tan J.H., Lee J.Y., Fitch M.D., et al. An ANGPTL4-ceramide-protein kinase Czeta axis mediates chronic glucocorticoid exposure-induced hepatic steatosis and hypertriglyceridemia in mice. J. Biol. Chem. 2019;294:9213–9224. doi: 10.1074/jbc.RA118.006259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J.C., Horton J.D., Hobbs H.H. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deevska G.M., Rozenova K.A., Giltiay N.V., Chambers M.A., White J., Boyanovsky B.B., Wei J., Daugherty A., Smart E.J., Reid M.B., et al. Acid sphingomyelinase deficiency prevents diet-induced hepatic triacylglycerol accumulation and hyperglycemia in mice. J. Biol. Chem. 2009;284:8359–8368. doi: 10.1074/jbc.M807800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denard B., Lee C., Ye J. Doxorubicin blocks proliferation of cancer cells through proteolytic activation of CREB3L1. Elife. 2012;1:e00090. doi: 10.7554/eLife.00090.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B.S., Liu C.H., Sun Y., Chen Y., Swendeman S.L., Jung B., Chavez D., Cao Z., Christoffersen C., Nielsen L.B., et al. HDL activation of endothelial sphingosine-1-phosphate receptor-1 (S1P1) promotes regeneration and suppresses fibrosis in the liver. JCI Insight. 2016;1:e87058. doi: 10.1172/jci.insight.87058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyckman A.J. Modulators of sphingosine-1-phosphate pathway biology: recent advances of sphingosine-1-phosphate receptor 1 (S1P1) agonists and future perspectives. J. Med. Chem. 2017;60:5267–5289. doi: 10.1021/acs.jmedchem.6b01575. [DOI] [PubMed] [Google Scholar]
- Friedman S.L. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucho R., Martínez L., Baulies A., Torres S., Tarrats N., Fernández A., Ribas V., Astudillo A.M., Balsinde J., Garcia-Rovés P., et al. ASMase regulates autophagy and lysosomal membrane permeabilization and its inhibition prevents early stage non-alcoholic steatohepatitis. J. Hepatol. 2014;61:1126–1134. doi: 10.1016/j.jhep.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D., Pararasa C., Dunston C.R., Bailey C.J., Griffiths H.R. Palmitate promotes monocyte atherogenicity via de novo ceramide synthesis. Free Radic. Biol. Med. 2012;53:796–806. doi: 10.1016/j.freeradbiomed.2012.05.026. [DOI] [PubMed] [Google Scholar]
- Gao W., Liu H., Yuan J., Wu C., Huang D., Ma Y., Zhu J., Ma L., Guo J., Shi H., et al. Exosomes derived from mature dendritic cells increase endothelial inflammation and atherosclerosis via membrane TNF-α mediated NF-κB pathway. J. Cell. Mol. Med. 2016;20:2318–2327. doi: 10.1111/jcmm.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng T., Sutter A., Harland M.D., Law B.A., Ross J.S., Lewin D., Palanisamy A., Russo S.B., Chavin K.D., Cowart L.A. SphK1 mediates hepatic inflammation in a mouse model of NASH induced by high saturated fat feeding and initiates proinflammatory signaling in hepatocytes. J. Lipid Res. 2015;56:2359–2371. doi: 10.1194/jlr.M063511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Fernandez B., Sanchez D.I., Crespo I., San-Miguel B., Alvarez M., Tunon M.J., Gonzalez-Gallego J. Inhibition of the SphK1/S1P signaling pathway by melatonin in mice with liver fibrosis and human hepatic stellate cells. BioFactors. 2017;43:272–282. doi: 10.1002/biof.1342. [DOI] [PubMed] [Google Scholar]
- Gosejacob D., Jäger P.S., Vom Dorp K., Frejno M., Carstensen A.C., Köhnke M., Degen J., Dörmann P., Hoch M. Ceramide synthase 5 is essential to maintain C16:0-ceramide pools and contributes to the development of diet-induced obesity. J. Biol. Chem. 2016;291:6989–7003. doi: 10.1074/jbc.M115.691212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajduch E., Balendran A., Batty I.H., Litherland G.J., Blair A.S., Downes C.P., Hundal H.S. Ceramide impairs the insulin-dependent membrane recruitment of protein kinase B leading to a loss in downstream signalling in L6 skeletal muscle cells. Diabetologia. 2001;44:173–183. doi: 10.1007/s001250051596. [DOI] [PubMed] [Google Scholar]
- Hastie C.E., Padmanabhan S., Slack R., Pell A.C., Oldroyd K.G., Flapan A.D., Jennings K.P., Irving J., Eteiba H., Dominiczak A.F., et al. Obesity paradox in a cohort of 4880 consecutive patients undergoing percutaneous coronary intervention. Eur. Heart J. 2010;31:222–226. doi: 10.1093/eurheartj/ehp317. [DOI] [PubMed] [Google Scholar]
- Higuchi H., Gores G.J. Mechanisms of liver injury: an overview. Curr. Mol. Med. 2003;3:483–490. doi: 10.2174/1566524033479528. [DOI] [PubMed] [Google Scholar]
- Holland W.L., Brozinick J.T., Wang L.P., Hawkins E.D., Sargent K.M., Liu Y., Narra K., Hoehn K.L., Knotts T.A., Siesky A., et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Ichi I., Nakahara K., Fujii K., Iida C., Miyashita Y., Kojo S. Increase of ceramide in the liver and plasma after carbon tetrachloride intoxication in the rat. J. Nutr. Sci. Vitaminol. 2007;53:53–56. doi: 10.3177/jnsv.53.53. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Watanabe N., Ishii I., Shimosawa T., Kume Y., Tomiya T., Inoue Y., Nishikawa T., Ohtomo N., Tanoue Y., et al. Sphingosine 1-phosphate regulates regeneration and fibrosis after liver injury via sphingosine 1-phosphate receptor 2. J. Lipid Res. 2009;50:556–564. doi: 10.1194/jlr.M800496-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C., Xie C., Li F., Zhang L., Nichols R.G., Krausz K.W., Cai J., Qi Y., Fang Z.Z., Takahashi S., et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J. Clin. Invest. 2015;125:386–402. doi: 10.1172/JCI76738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M., Li C., Liu Q., Wang A., Lei M. Inhibiting ceramide synthesis attenuates hepatic steatosis and fibrosis in rats with non-alcoholic fatty liver disease. Front. Endocrinol. 2019;10:665. doi: 10.3389/fendo.2019.00665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J., Zhang X., Lu Z., Perry D.M., Li Y., Russo S.B., Cowart L.A., Hannun Y.A., Huang Y. Acid sphingomyelinase plays a key role in palmitic acid-amplified inflammatory signaling triggered by lipopolysaccharide at low concentrations in macrophages. Am. J. Physiol. Endocrinol. Metab. 2013;305:E853–E867. doi: 10.1152/ajpendo.00251.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama Y., Ikeda H., Watanabe N., Nagamine M., Kusumoto Y., Yashiro M., Satoh Y., Shimosawa T., Shinozaki K., Tomiya T., et al. Antagonism of sphingosine 1-phosphate receptor 2 causes a selective reduction of portal vein pressure in bile duct-ligated rodents. Hepatology. 2012;56:1427–1438. doi: 10.1002/hep.25780. [DOI] [PubMed] [Google Scholar]
- Kaneko T., Murakami T., Kawana H., Takahashi M., Yasue T., Kobayashi E. Sphingosine-1-phosphate receptor agonists suppress concanavalin A-induced hepatic injury in mice. Biochem. Biophys. Res. Commun. 2006;345:85–92. doi: 10.1016/j.bbrc.2006.04.067. [DOI] [PubMed] [Google Scholar]
- Khattar M., Deng R., Kahan B.D., Schroder P.M., Phan T., Rutzky L.P., Stepkowski S.M. Novel sphingosine-1-phosphate receptor modulator KRP203 combined with locally delivered regulatory T cells induces permanent acceptance of pancreatic islet allografts. Transplantation. 2013;95:919–927. doi: 10.1097/TP.0b013e3182842396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.R., Lee E.J., Shin K.O., Kim M.H., Pewzner-Jung Y., Lee Y.M., Park J.W., Futerman A.H., Park W.J. Hepatic triglyceride accumulation via endoplasmic reticulum stress-induced SREBP-1 activation is regulated by ceramide synthases. Exp. Mol. Med. 2019;51:1–16. doi: 10.1038/s12276-019-0340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A., Houlihan D.D., Kavanagh D., Haldar D., Luu N., Owen A., Suresh S., Than N.N., Reynolds G., Penny J., et al. Sphingosine-1-phosphate prevents egress of hematopoietic stem cells from liver to reduce fibrosis. Gastroenterology. 2017;153:233–248.:e16. doi: 10.1053/j.gastro.2017.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisseleva T., Cong M., Paik Y., Scholten D., Jiang C., Benner C., Iwaisako K., Moore-Morris T., Scott B., Tsukamoto H., et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc. Natl. Acad. Sci. U. S. A. 2012;109:9448–9453. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitatani K., Taniguchi M., Okazaki T. Role of sphingolipids and metabolizing enzymes in hematological malignancies. Mol. Cells. 2015;38:482–495. doi: 10.14348/molcells.2015.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y., Wang H., Wang S., Tang N. FTY720, a sphingosine-1 phosphate receptor modulator, improves liver fibrosis in a mouse model by impairing the motility of bone marrow-derived mesenchymal stem cells. Inflammation. 2014;37:1326–1336. doi: 10.1007/s10753-014-9877-2. [DOI] [PubMed] [Google Scholar]
- Kowalski G.M., Kloehn J., Burch M.L., Selathurai A., Hamley S., Bayol S.A.M., Lamon S., Watt M.J., Lee-Young R.S., McConville M.J., et al. Overexpression of sphingosine kinase 1 in liver reduces triglyceride content in mice fed a low but not high-fat diet. Biochim. Biophys. Acta. 2015;1851:210–219. doi: 10.1016/j.bbalip.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Kurek K., Piotrowska D.M., Wiesiołek P., Lukaszuk B., Chabowski A., Górski J., Żendzian-Piotrowska M. Inhibition of ceramide de novo synthesis reduces liver lipid accumulation in rats with nonalcoholic fatty liver disease. Liver Int. 2013;34:1074–1083. doi: 10.1111/liv.12331. [DOI] [PubMed] [Google Scholar]
- Lallemand T., Rouahi M., Swiader A., Grazide M.H., Geoffre N., Alayrac P., Recazens E., Coste A., Salvayre R., Nègre-Salvayre A., et al. nSMase2 (type 2-neutral sphingomyelinase) deficiency or inhibition by GW4869 reduces inflammation and atherosclerosis in Apoe-/- mice. Arterioscler. Thromb. Vasc. Biol. 2018;38:1479–1492. doi: 10.1161/ATVBAHA.118.311208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.Y., Hong I.K., Kim B.R., Shim S.M., Lee J.S., Lee H.Y., Choi C.S., Kim B.K., Park T.S. Activation of sphingosine kinase 2 by endoplasmic reticulum stress ameliorates hepatic steatosis and insulin resistance in mice. Hepatology. 2015;62:135–146. doi: 10.1002/hep.27804. [DOI] [PubMed] [Google Scholar]
- Li C., Jiang X., Yang L., Liu X., Yue S., Li L. Involvement of sphingosine 1-phosphate (SIP)/S1P3 signaling in cholestasis-induced liver fibrosis. Am. J. Pathol. 2009a;175:1464–1472. doi: 10.2353/ajpath.2009.090037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Kong Y., Wang H., Wang S., Yu H., Liu X., Yang L., Jiang X., Li L., Li L. Homing of bone marrow mesenchymal stem cells mediated by sphingosine 1-phosphate contributes to liver fibrosis. J. Hepatol. 2009b;50:1174–1183. doi: 10.1016/j.jhep.2009.01.028. [DOI] [PubMed] [Google Scholar]
- Li C., Zheng S., You H., Liu X., Lin M., Yang L., Li L. Sphingosine 1-phosphate (S1P)/S1P receptors are involved in human liver fibrosis by action on hepatic myofibroblasts motility. J. Hepatol. 2011;54:1205–1213. doi: 10.1016/j.jhep.2010.08.028. [DOI] [PubMed] [Google Scholar]
- Li Y., Dong J., Ding T., Kuo M.S., Cao G., Jiang X.C., Li Z. Sphingomyelin synthase 2 activity and liver steatosis: an effect of ceramide-mediated peroxisome proliferator-activated receptor γ2 suppression. Arterioscler. Thromb. Vasc. Biol. 2013;33:1513–1520. doi: 10.1161/ATVBAHA.113.301498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liangpunsakul S., Rahmini Y., Ross R.A., Zhao Z., Xu Y., Crabb D.W. Imipramine blocks ethanol-induced ASMase activation, ceramide generation, and PP2A activation, and ameliorates hepatic steatosis in ethanol-fed mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;302:G515–G523. doi: 10.1152/ajpgi.00455.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenmeyer C.C., McCullough A.J. The natural history of nonalcoholic fatty liver disease-an evolving view. Clin. Liver Dis. 2018;22:11–21. doi: 10.1016/j.cld.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Yue S., Li C., Yang L., You H., Li L. Essential roles of sphingosine 1-phosphate receptor types 1 and 3 in human hepatic stellate cells motility and activation. J. Cell. Physiol. 2011;226:2370–2377. doi: 10.1002/jcp.22572. [DOI] [PubMed] [Google Scholar]
- Longato L., Tong M., Wands J.R., de la Monte S.M. High fat diet induced hepatic steatosis and insulin resistance: role of dysregulated ceramide metabolism. Hepatol. Res. 2012;42:412–427. doi: 10.1111/j.1872-034X.2011.00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M.M., Chen J.L., Wang G.G., Wang H., Lu Y., Li J.F., Yi J., Yuan Y.J., Zhang Q.W., Mi J., et al. Sphingosine kinase 1 participates in insulin signalling and regulates glucose metabolism and homeostasis in KK/Ay diabetic mice. Diabetologia. 2007;50:891–900. doi: 10.1007/s00125-006-0589-5. [DOI] [PubMed] [Google Scholar]
- Maceyka M., Harikumar K.B., Milstien S., Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012;22:50–60. doi: 10.1016/j.tcb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maceyka M., Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. 2014;510:58–67. doi: 10.1038/nature13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari M., Colell A., Morales A., Caballero F., Moles A., Fernandez A., Terrones O., Basanez G., Antonsson B., Garcia-Ruiz C., et al. Mechanism of mitochondrial glutathione-dependent hepatocellular susceptibility to TNF despite NF-kappaB activation. Gastroenterology. 2008;134:1507–1520. doi: 10.1053/j.gastro.2008.01.073. [DOI] [PubMed] [Google Scholar]
- Mauer A.S., Hirsova P., Maiers J.L., Shah V.H., Malhi H. Inhibition of sphingosine 1-phosphate signaling ameliorates murine nonalcoholic steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2017;312:G300–G313. doi: 10.1152/ajpgi.00222.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill A.H. De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J. Biol. Chem. 2002;277:25843–25846. doi: 10.1074/jbc.R200009200. [DOI] [PubMed] [Google Scholar]
- Mitsutake S., Zama K., Yokota H., Yoshida T., Tanaka M., Mitsui M., Ikawa M., Okabe M., Tanaka Y., Yamashita T., et al. Dynamic modification of sphingomyelin in lipid microdomains controls development of obesity, fatty liver, and type 2 diabetes. J. Biol. Chem. 2011;286:28544–28555. doi: 10.1074/jbc.M111.255646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles A., Tarrats N., Morales A., Domínguez M., Bataller R., Caballería J., Garcia-Ruiz C., Fernandez-Checa J.C., Mari M. Acidic sphingomyelinase controls hepatic stellate cell activation and in vivo liver fibrogenesis. Am. J. Pathol. 2010;177:1214–1224. doi: 10.2353/ajpath.2010.091257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahashi M., Takabe K., Liu R., Peng K., Wang X., Wang Y., Hait N.C., Wang X., Allegood J.C., Yamada A., et al. Conjugated bile acid-activated S1P receptor 2 is a key regulator of sphingosine kinase 2 and hepatic gene expression. Hepatology. 2015;61:1216–1226. doi: 10.1002/hep.27592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi T., Kobayashi N., Hisano Y., Kawahara A., Yamaguchi A. Molecular and physiological functions of sphingosine 1-phosphate transporters. Biochim. Biophys. Acta. 2014;1841:759–765. doi: 10.1016/j.bbalip.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Park S.J., Im D.S. Sphingosine 1-phosphate receptor modulators and drug discovery. Biomol. Ther. 2017;25:80–90. doi: 10.4062/biomolther.2016.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park W.J., Park J.W., Merrill A.H., Storch J., Pewzner-Jung Y., Futerman A.H. Hepatic fatty acid uptake is regulated by the sphingolipid acyl chain length. Biochim. Biophys. Acta. 2014;1841:1754–1766. doi: 10.1016/j.bbalip.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessayre D., Mansouri A., Fromenty B. Nonalcoholic steatosis and steatohepatitis. V. Mitochondrial dysfunction in steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;282:G193–G199. doi: 10.1152/ajpgi.00426.2001. [DOI] [PubMed] [Google Scholar]
- Postic C., Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J. Clin. Invest. 2008;118:829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell D.J., Hajduch E., Kular G., Hundal H.S. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCzeta-dependent mechanism. Mol. Cell. Biol. 2003;23:7794–7808. doi: 10.1128/MCB.23.21.7794-7808.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne N.J., Pyne S. Sphingosine 1-phosphate and cancer. Nat. Rev. Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- Qi Y., Chen J., Lay A., Don A., Vadas M., Xia P. Loss of sphingosine kinase 1 predisposes to the onset of diabetes via promoting pancreatic β-cell death in diet-induced obese mice. FASEB J. 2013;27:4294–4304. doi: 10.1096/fj.13-230052. [DOI] [PubMed] [Google Scholar]
- Raichur S., Wang S.T., Chan P.W., Li Y., Ching J., Chaurasia B., Dogra S., Öhman M.K., Takeda K., Sugii S., et al. CerS2 haploinsufficiency inhibits β-Oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 2014;20:687–695. doi: 10.1016/j.cmet.2014.09.015. [DOI] [PubMed] [Google Scholar]
- Rinella M.E., Sanyal A.J. Management of NAFLD: a stage-based approach. Nat. Rev. Gastroenterol. Hepatol. 2016;13:196–205. doi: 10.1038/nrgastro.2016.3. [DOI] [PubMed] [Google Scholar]
- Rippe R.A., Brenner D.A. From quiescence to activation: gene regulation in hepatic stellate cells. Gastroenterology. 2004;127:1260–1262. doi: 10.1053/j.gastro.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Rohrbach T., Maceyka M., Spiegel S. Sphingosine kinase and sphingosine-1-phosphate in liver pathobiology. Crit. Rev. Biochem. Mol. Biol. 2017;52:543–553. doi: 10.1080/10409238.2017.1337706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbach T.D., Asgharpour A., Maczis M.A., Montefusco D., Cowart L.A., Bedossa P., Sanyal A.J., Spiegel S. FTY720/fingolimod decreases hepatic steatosis and expression of fatty acid synthase in diet-induced nonalcoholic fatty liver disease in mice. J. Lipid Res. 2019;60:1311–1322. doi: 10.1194/jlr.M093799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkute K., Asmis R.H., Nikolova-Karakashian M.N. Regulation of neutral sphingomyelinase-2 by GSH: a new insight to the role of oxidative stress in aging-associated inflammation. J. Lipid Res. 2007a;48:2443–2452. doi: 10.1194/jlr.M700227-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkute K., Karakashian A.A., Giltiay N.V., Dobierzewska A., Nikolova-Karakashian M.N. Aging in rat causes hepatic hyperresposiveness to interleukin-1beta which is mediated by neutral sphingomyelinase-2. Hepatology. 2007b;46:1166–1176. doi: 10.1002/hep.21777. [DOI] [PubMed] [Google Scholar]
- Samad F., Hester K.D., Yang G., Hannun Y.A., Bielawski J. Altered adipose and plasma sphingolipid metabolism in obesity: a potential mechanism for cardiovascular and metabolic risk. Diabetes. 2006;55:2579–2587. doi: 10.2337/db06-0330. [DOI] [PubMed] [Google Scholar]
- Sanyal A.J., Pacana T. A lipidomic readout of disease progression in a diet-induced mouse model of nonalcoholic fatty liver disease. Trans. Am. Clin. Climatol. Assoc. 2015;126:271–288. [PMC free article] [PubMed] [Google Scholar]
- Sato M., Ikeda H., Uranbileg B., Kurano M., Saigusa D., Aoki J., Maki H., Kudo H., Hasegawa K., Kokudo N., et al. Sphingosine kinase-1, S1P transporter spinster homolog 2 and S1P2 mRNA expressions are increased in liver with advanced fibrosis in human. Sci. Rep. 2016;6:32119. doi: 10.1038/srep32119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar N., Forrest E., Preiss D. Non-alcoholic fatty liver disease. BMJ. 2014;349:g4596. doi: 10.1136/bmj.g4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling J.D., Machkovech H.M., He L., Sidhu R., Fujiwara H., Weber K., Ory D.S., Schaffer J.E. Palmitate and lipopolysaccharide trigger synergistic ceramide production in primary macrophages. J. Biol. Chem. 2013;288:2923–2932. doi: 10.1074/jbc.M112.419978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuppan D., Afdhal N.H. Liver cirrhosis. Lancet. 2008;371:838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalm S., Pfeilschifter J., Huwiler A. Sphingosine-1-phosphate: a Janus-faced mediator of fibrotic diseases. Biochim. Biophys. Acta. 2013;1831:239–250. doi: 10.1016/j.bbalip.2012.07.022. [DOI] [PubMed] [Google Scholar]
- Shea B.S., Tager A.M. Sphingolipid regulation of tissue fibrosis. Open Rheumatol. J. 2012;6:123–129. doi: 10.2174/1874312901206010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel W., Jenke B., Holz B., Binczek E., Günter R.H., Knifka J., Koebke J., Niehoff A. Neutral sphingomyelinase (SMPD3) deficiency causes a novel form of chondrodysplasia and dwarfism that is rescued by Col2A1-driven smpd3 transgene expression. Am. J. Pathol. 2007;171:153–161. doi: 10.2353/ajpath.2007.061285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers S.A., Garza L.A., Zhou H., Birnbaum M.J. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol. Cell. Biol. 1998;18:5457–5464. doi: 10.1128/MCB.18.9.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagami S., Inokuchi Ji J., Kabayama K., Yoshimura H., Kitamura F., Uemura S., Ogawa C., Ishii A., Saito M., Ohtsuka Y., et al. Ganglioside GM3 participates in the pathological conditions of insulin resistance. J. Biol. Chem. 2002;277:3085–3092. doi: 10.1074/jbc.M103705200. [DOI] [PubMed] [Google Scholar]
- Takabe K., Spiegel S. Export of sphingosine-1-phosphate and cancer progression. J. Lipid Res. 2014;55:1839–1846. doi: 10.1194/jlr.R046656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi C.M., Kondo T., Sajan M., Luo J., Bronson R., Asano T., Farese R., Cantley L.C., Kahn C.R. Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via Akt and PKClambda/zeta. Cell Metab. 2006;3:343–353. doi: 10.1016/j.cmet.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Turner N., Kowalski G.M., Leslie S.J., Risis S., Yang C., Lee-Young R.S., Babb J.R., Meikle P.J., Lancaster G.I., Henstridge D.C., et al. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia. 2013;56:1638–1648. doi: 10.1007/s00125-013-2913-1. [DOI] [PubMed] [Google Scholar]
- Turpin S.M., Nicholls H.T., Willmes D.M., Mourier A., Brodesser S., Wunderlich C.M., Mauer J., Xu E., Hammerschmidt P., Brönneke H.S., et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014;20:678–686. doi: 10.1016/j.cmet.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Unger R.H. Lipid overload and overflow: metabolic trauma and the metabolic syndrome. Trends Endocrinol. Metab. 2003;14:398–403. doi: 10.1016/j.tem.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Ussher J.R., Koves T.R., Cadete V.J.J., Zhang L., Jaswal J.S., Swyrd S.J., Lopaschuk D.G., Proctor S.D., Keung W., Muoio D.M., et al. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes. 2010;59:2453–2464. doi: 10.2337/db09-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma M.K., Yateesh A.N., Neelima K., Pawar N., Sandhya K., Poornima J., Lakshmi M.N., Yogeshwari S., Pallavi P.M., Oommen A.M., et al. Inhibition of neutral sphingomyelinases in skeletal muscle attenuates fatty-acid induced defects in metabolism and stress. SpringerPlus. 2014;3:255–212. doi: 10.1186/2193-1801-3-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.N., O'Brien L., Brindley D.N. Effects of cell-permeable ceramides and tumor necrosis factor-alpha on insulin signaling and glucose uptake in 3T3-L1 adipocytes. Diabetes. 1998;47:24–31. doi: 10.2337/diab.47.1.24. [DOI] [PubMed] [Google Scholar]
- Wang R., Ding Q., De Assuncao T.M., Mounajjed T., Maiers J.L., Dou C., Cao S., Yaqoob U., Huebert R.C., Shah V.H. Hepatic stellate cell selective disruption of dynamin-2 GTPase increases murine fibrogenesis through up-regulation of sphingosine-1 phosphate-induced cell migration. Am. J. Pathol. 2017a;187:134–145. doi: 10.1016/j.ajpath.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Harashima S.I., Liu Y., Usui R., Inagaki N. Sphingosine kinase 1-interacting protein is a novel regulator of glucose-stimulated insulin secretion. Sci. Rep. 2017b;7:779. doi: 10.1038/s41598-017-00900-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt M.J., Barnett A.C., Bruce C.R., Schenk S., Horowitz J.F., Hoy A.J. Regulation of plasma ceramide levels with fatty acid oversupply: evidence that the liver detects and secretes de novo synthesised ceramide. Diabetologia. 2012;55:2741–2746. doi: 10.1007/s00125-012-2649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J.Y., Holland W.L., Kusminski C.M., Sun K., Sharma A.X., Pearson M.J., Sifuentes A.J., McDonald J.G., Gordillo R., Scherer P.E. Targeted induction of ceramide degradation leads to improved systemic metabolism and reduced hepatic steatosis. Cell Metab. 2015;22:266–278. doi: 10.1016/j.cmet.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiu L., Chang N., Yang L., Liu X., Yang L., Ge J., Li L. Intracellular sphingosine 1-phosphate contributes to collagen expression of hepatic myofibroblasts in human liver fibrosis independent of its receptors. Am. J. Pathol. 2015;185:387–398. doi: 10.1016/j.ajpath.2014.09.023. [DOI] [PubMed] [Google Scholar]
- Xu W., Lu C., Zhang F., Shao J., Zheng S. Dihydroartemisinin restricts hepatic stellate cell contraction via an FXR-S1PR2-dependent mechanism. IUBMB Life. 2016;68:376–387. doi: 10.1002/iub.1492. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Hashiramoto A., Haluzik M., Mizukami H., Beck S., Norton A., Kono M., Tsuji S., Daniotti J.L., Werth N., et al. Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc. Natl. Acad. Sci. U. S. A. 2003;100:3445–3449. doi: 10.1073/pnas.0635898100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Badeanlou L., Bielawski J., Roberts A.J., Hannun Y.A., Samad F. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 2009;297:E211–E224. doi: 10.1152/ajpendo.91014.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Chang N., Liu X., Han Z., Zhu T., Li C., Yang L., Li L. Bone marrow-derived mesenchymal stem cells differentiate to hepatic myofibroblasts by transforming growth factor-beta1 via sphingosine kinase/sphingosine 1-phosphate (S1P)/S1P receptor axis. Am. J. Pathol. 2012;181:85–97. doi: 10.1016/j.ajpath.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Yang L., Han Z., Tian L., Mai P., Zhang Y., Wang L., Li L. Sphingosine 1-phosphate receptor 2 and 3 mediate bone marrow-derived monocyte/macrophage motility in cholestatic liver injury in mice. Sci. Rep. 2015;5:13423. doi: 10.1038/srep13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Yue S., Yang L., Liu X., Han Z., Zhang Y., Li L. Sphingosine kinase/sphingosine 1-phosphate (S1P)/S1P receptor axis is involved in liver fibrosis-associated angiogenesis. J. Hepatol. 2013;59:114–123. doi: 10.1016/j.jhep.2013.02.021. [DOI] [PubMed] [Google Scholar]
- Yano M., Watanabe K., Yamamoto T., Ikeda K., Senokuchi T., Lu M., Kadomatsu T., Tsukano H., Ikawa M., Okabe M., et al. Mitochondrial dysfunction and increased reactive oxygen species impair insulin secretion in sphingomyelin synthase 1-null mice. J. Biol. Chem. 2011;286:3992–4002. doi: 10.1074/jbc.M110.179176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying W., Riopel M., Bandyopadhyay G., Dong Y., Birmingham A., Seo J.B., Ofrecio J.M., Wollam J., Hernandez-Carretero A., Fu W., et al. Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell. 2017;171:372–384.:e12. doi: 10.1016/j.cell.2017.08.035. [DOI] [PubMed] [Google Scholar]
- Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- Zhao H., Przybylska M., Wu I.H., Zhang J., Maniatis P., Pacheco J., Piepenhagen P., Copeland D., Arbeeny C., Shayman J.A., et al. Inhibiting glycosphingolipid synthesis ameliorates hepatic steatosis in obese mice. Hepatology. 2009;50:85–93. doi: 10.1002/hep.22970. [DOI] [PubMed] [Google Scholar]
- Zhao H., Przybylska M., Wu I.H., Zhang J., Siegel C., Komarnitsky S., Yew N.S., Cheng S.H. Inhibiting glycosphingolipid synthesis improves glycemic control and insulin sensitivity in animal models of type 2 diabetes. Diabetes. 2007;56:1210–1218. doi: 10.2337/db06-0719. [DOI] [PubMed] [Google Scholar]
- Zigdon H., Kogot-Levin A., Park J.W., Goldschmidt R., Kelly S., Merrill A.H., Scherz A., Pewzner-Jung Y., Saada A., Futerman A.H. Ablation of ceramide synthase 2 causes chronic oxidative stress due to disruption of the mitochondrial respiratory chain. J. Biol. Chem. 2013;288:4947–4956. doi: 10.1074/jbc.M112.402719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinda M.J., Vlahos C.J., Lai M.T. Ceramide induces the dephosphorylation and inhibition of constitutively activated Akt in PTEN negative U87mg cells. Biochem. Biophys. Res. Commun. 2001;280:1107–1115. doi: 10.1006/bbrc.2000.4248. [DOI] [PubMed] [Google Scholar]