Abstract
The hypothalamus is a crucial organ for the maintenance of appropriate body fat storage. Neurons in the hypothalamic arcuate nucleus (ARH) detect energy shortage or surplus via the circulating concentrations of metabolic hormones and nutrients, and then coordinate energy intake and expenditure to maintain energy homeostasis. Malfunction or loss of hypothalamic ARH neurons results in obesity. Accumulated evidence suggests that hypothalamic inflammation is a key pathological mechanism that links chronic overconsumption of a high-fat diet (HFD) with the development of obesity and related metabolic complications. Interestingly, overnutrition-induced hypothalamic inflammation occurs specifically in the ARH, where microglia initiate an inflammatory response by releasing proinflammatory cytokines and chemokines in response to excessive fatty acid flux. Upon more prolonged HFD consumption, astrocytes and perivascular macrophages become involved and sustain hypothalamic inflammation. ARH neurons are victims of hypothalamic inflammation, but they may actively participate in hypothalamic inflammation by sending quiescence or stress signals to surrounding glia. In this mini-review, we describe the current state of knowledge regarding the contributions of neurons and glia, and their interactions, to HFD-induced hypothalamic inflammation.
Keywords: glia, hypothalamus, inflammation, neurons, obesity
INTRODUCTION
The hypothalamus is known to be a key center for the control of body weight (Roh et al., 2016; Schwartz, 2006). Of the various regions of the hypothalamus, the arcuate nucleus (ARH) is of paramount importance because neurons in this location are specialized to sense metabolic signals from the periphery, such as leptin, insulin, ghrelin, and glucose, and to regulate energy intake and expenditure (Schwartz, 2006). Two groups of ARH neurons are considered to be critical regulators of energy balance. One is proopiomelanocortin (POMC)-producing neurons, which are activated by the anorexigenic hormone leptin or under the conditions of energy surplus. POMC neuronal activation causes a negative energy balance by suppressing food intake or by stimulating energy expenditure. The other is the group of neurons producing both agouti-related protein (AGRP) and neuropeptide Y (NPY). In contrast to POMC neurons, these neurons are activated by the hunger hormone ghrelin but inhibited by leptin. Activation of AGRP/NPY neurons leads to a positive energy balance by promoting food-seeking behavior and by suppressing energy consumption (Schwartz, 2006).
Obesity is defined as excessive fat storage as a result of a chronic surplus of energy intake relative to energy expenditure. Given the critical role of the hypothalamus in energy homeostasis, obesity can be regarded as a metabolic disorder associated with hypothalamic dysfunction. Obese humans and animals display low-grade inflammation in multiple organs, including in the hypothalamus (Lumeng and Saltiel, 2011). Increased expression of proinflammatory cytokines, such as interleukin-1β (IL-1β), IL-6, and tumor necrosis factor α (TNF-α), and activation of inflammatory signaling pathways have been observed in the hypothalamus of obese animals compared with lean animals (Cai and Liu, 2011; De Souza et al., 2005; Jais and Bruning, 2017). Moreover, artificial activation of inflammatory signaling pathways in the hypothalamus of lean animals, especially in NPY/AGRP neurons, leads to obesity and the dysregulation of glucose metabolism (Jais and Bruning, 2017; Jung and Kim, 2013). This experimental evidence indicates that inflammation is not the pathological endpoint of obesity, but rather it is an active player that drives the progression of diet-induced obesity (DIO) and the development of metabolic complications. In ARH POMC and NPY/AGRP neurons, activation of inflammatory signaling pathways (the toll-like receptor 4 [TLR4], myeloid differentiation factor 88 [Myd-88], c-Jun N-terminal kinase [JNK], and inhibitor of κB kinase-β [IKKβ]-nuclear factor-κB [NF-κB] signaling) disrupts the leptin and insulin signaling pathways (janus kinase 2 [JAK2]-signal transducer and activator of transcription-3 [STAT3] signaling and phosphatidylinositol 3-kinase [PI3K]-Akt signaling), thereby hampering the sensing of metabolic signals and the normal regulation of energy homeostasis by these neurons (Cai and Liu, 2011; Park et al., 2019). Therefore, hypothalamic inflammation is thought to be a key mechanism of overnutrition-induced ARH neuronal dysfunction and the resultant obesity (Cai and Liu, 2011). The biological processes and mediators that are involved in or orchestrate hypothalamic inflammation are still being identified. In this mini-review, we discuss the molecular mechanisms of hypothalamic inflammation, with a focus on the major cellular contributors and their interactions.
MICROGLIA
Microglia are the principal type of immune cell residing in the brain parenchyma. They are key players in brain homeostasis because they prune unnecessary synapses and remove invading microorganisms, dead cell debris, and toxic metabolites through phagocytosis (Kierdorf and Prinz, 2017). Notably, hypothalamic microglia are readily activated in response to the consumption of a high-fat diet (HFD) (Gao et al., 2014; Thaler et al., 2012). Once activated, they accumulate and undergo the following morphological changes: their cell bodies enlarge, and their ramifying processes thicken and shorten. In activated microglia, ionized calcium-binding adapter molecule 1 (Iba1) expression increases, but the expression of other microglial markers, such as transmembrane protein 119 (TMEM119) and P2Y purinoreceptor 12 (P2Y12), significantly decrease (Valdearcos et al., 2017).
Notably, the consumption of an HFD or saturated fatty acids (SFA) for only 3 days is sufficient to activate microglia in mice (Thaler et al., 2012; Valdearcos et al., 2014). Moreover, a single oral administration of milk fat, which contains the SFA palmitate, activates hypothalamic microglia (Valdearcos et al., 2014). By contrast, the administration of olive oil, which contains the monounsaturated fatty acid oleic acid, does not activate hypothalamic microglia (Valdearcos et al., 2014). These results indicate that 1) ARH microglia respond very rapidly to HFD-feeding and 2) the type of fatty acids they encounter determine their activation. Interestingly, HFD-induced microglial activation is restricted to the ARH (Lee et al., 2018; Thaler et al., 2012; Valdearcos et al., 2014). This region is close to the median eminence, a circumventricular organ that is not encompassed by the blood–brain barrier (BBB). Furthermore, blood vessels in the ARH become permeable during long-term HFD-feeding (Lee et al., 2019), which means that circulating SFAs can more easily access the ARH (Lee et al., 2018; Valdearcos et al., 2017).
During the course of HFD-feeding, microglial expansion precedes weight gain (Jais and Bruning, 2017; Thaler et al., 2012). Furthermore, microglial depletion using the colony-stimulating factor 1 receptor (CSF1R) inhibitor PLX5622 or clodronate-containing liposomes prevent HFD-induced obesity (Valdearcos et al., 2017). Consistent with this, the inhibition of IKKβ/NF-κB signaling in CX3C chemokine receptor 1 (CX3CR1)-expressing microglia ameliorates DIO and hypothalamic inflammation (Valdearcos et al., 2017). These experimental data suggest that the activation of inflammatory signaling in microglia may be essential for weight gain and the development of obesity during HFD-feeding (Valdearcos et al., 2017). Activated microglia may induce obesity by causing hypothalamic neuronal dysfunction or injury. They secrete proinflammatory cytokines, which activate inflammatory signaling in adjacent neurons, which in turn induce neuronal insulin and leptin resistance (Cai and Liu, 2011; Jais and Bruning, 2017). Prolonged microglial activation may also cause hypothalamic neuronal apoptosis, especially of anorexigenic/catabolic POMC neurons (Moraes et al., 2009).
ASTROCYTES
Astrocytes are another glial cell type that is abundant in the central nervous system (CNS) and supports neuronal functions in many ways (Benarroch, 2005). They store glycogen, provide lactate to neurons under glucose-deprived conditions, and also control neuronal activity by regulating synaptic function and plasticity (Abbott et al., 2006; Benarroch, 2005; Verkhratsky and Nedergaard, 2018). Furthermore, astrocytic foot processes cover blood vessels and help to maintain the integrity of the BBB.
Prolonged consumption of an HFD increased the numbers of glial fibrillary acidic protein (GFAP)+ astrocytes, a process that is referred to as reactive astrogliosis (Horvath et al., 2010; Thaler et al., 2012). HFD consumption, as well as the activation of NF-κB signaling, induces morphological changes in hypothalamic astrocytes, principally, a shortening of high-order branching of their processes (Zhang et al., 2017). Moreover, activation of astrocytic NF-κB signaling induces glucose intolerance and hypertension, even in chow diet-fed rodents, and these mice consume more food and gain more weight (Zhang et al., 2017). By contrast, the inhibition of astrocytic NF-κB signaling reduces food intake and weight gain during HFD-feeding. Consistent with this, the inhibition of astrocytic NF-κB signaling by tamoxifen-inducible IKKβ deletion in GFAP-Cre/ERT2 mice reduces HFD-induced hypothalamic inflammation and reactive astrogliosis, and attenuates DIO and glucose intolerance (Douglass et al., 2017). Interestingly, to obtain these effects, mice must consume an HFD for 6 weeks before the induction of Cre-loxP recombination. These data suggest that the activation of inflammatory signaling in astrocytes plays a role in the later phase of hypothalamic inflammation during the development of DIO.
When cultured in palmitate‐containing medium, astrocytes accumulate lipid droplets (Kwon et al., 2017), and these lipid‐laden cells display higher expression of the reactive astrocyte marker GFAP, and inflammatory cytokines/chemokines, such as TNFα, IL‐1β, IL‐6, and chemokine C-C motif ligand-2 (CCL2) (also called monocyte chemoattractant protein-1 [MCP-1]). Moreover, astrocytes have been shown to release proinflammatory biomolecules (Allan et al., 2001; Kwon et al., 2017), although the level of secretion of proinflammatory cytokines by astrocytes may be less than that by activated microglia (Valdearcos et al., 2014). As an alternative mechanism, reactive astrocytes may promote the development of obesity by modifying neurotransmitter release or uptake. For example, astrocytic NF-κB activation increases hypothalamic extracellular GABA levels, which significantly contributes to higher food intake and weight gain (Zhang et al., 2017).
On the other hand, astrocytes secrete vascular endothelial growth factor (VEGF)-A under neuroinflammatory conditions (Argaw et al., 2012). VEGF-A binds to VEGF receptor 2 (VEGFR2) on endothelial cells and increases BBB permeability (Argaw et al., 2012). Therefore, it is possible that astrocyte-derived VEGF-A induces the BBB hyperpermeability observed in the ARH of DIO mice (Langlet et al., 2013; Lee et al., 2019) and contributes to hypothalamic inflammation by allowing the free entry of circulating SFAs and immune cells. This possibility needs to be tested in the future.
PERIVASCULAR MACROPHAGES
Perivascular macrophages are brain-resident myeloid cells that are found at the interface of brain microvessels and the parenchyma (Serrats et al., 2010). These cells express receptors that are required for pathogen recognition, phagocytosis, cytokine signaling, and antigen presentation (Serrats et al., 2010; Williams et al., 2001) and coordinate innate and adaptive immune responses to pathogens and other immune stimulants that enter the CNS via blood vessels (Williams et al., 2001).
Similarly to microgliosis and reactive astrogliosis, lysozyme-M (LysM)+ or CD169+ macrophages accumulate and are activated in the ARH of HFD-fed obese mice (Lee et al., 2018). The HFD-induced activation and accumulation of perivascular macrophages are evident after 2 weeks of HFD-feeding; therefore, these cells are activated later than microglia during the course of HFD feeing (Lee et al., 2018). Once activated, perivascular macrophages migrate from the perivascular space to the ARH parenchyma (Lee et al., 2018) and simultaneously they undergo a morphological change from linear to reactive microglia-like cells. Moreover, upon exposure to an HFD, perivascular macrophages in the ARH express high levels of inducible nitric oxide synthase (iNOS) (Lee et al., 2018) and may release a large amount of NO. Inhibition of hypothalamic iNOS in ARH macrophages significantly abrogates various aspects of HFD-induced hypothalamic inflammation, including proinflammatory cytokine overproduction, microgliosis, astrogliosis, macrophage activation/accumulation, and vascular hyperpermeability (Lee et al., 2018). Moreover, it improves hypothalamic leptin and systemic insulin resistance and glucose intolerance, despite having no effect on obesity (Lee et al., 2018). These results indicate that perivascular macrophage-derived NO mediates HFD-induced hypothalamic inflammation and glucose dysregulation.
MICROGLIA–ASTROCYTE INTERACTIONS
Interactions between glial cells, and especially between microglia and astrocytes, have been implicated in brain health and diseases (Jha et al., 2019), and their cross-talk may play an important role in HFD-induced hypothalamic inflammation. As discussed above, microglia are the versatile cell type to be activated in response to an SFA influx into the ARH (Thaler et al., 2012; Valdearcos et al., 2014). Activated microglia may in turn urge astrocytes to change their phenotype from the resting (non-reactive) to the reactive type by secreting proinflammatory molecules, such as IL-1α, TNFα, and complement (C)1q, as demonstrated in lipopolysaccharide-induced neuroinflammation (Liddelow et al., 2017).
Conversely, activated astrocytes may regulate microglial activity and contribute to sustained microglial activation. In support of this proposition, the treatment of microglia with conditioned medium of palmitate-treated reactive astrocytes increases the expression of the microglial chemokine CCL2, which promotes microglial migration (Kwon et al., 2017). The recruited microglia may then interact with neighboring astrocytes and further exacerbate the hypothalamic inflammation and neuronal injury (Kwon et al., 2017). Another chemotactic factor, CXCL12/stromal cell-derived factor (SDF)-1, may also be involved in the astrocytic–microglial interaction during HFD-induced hypothalamic inflammation and weight gain. The expression of CXCL12 and its receptors CXCR4 and CXCR7 is elevated in hypothalamus of DIO mice. Moreover, central administration of CXCL12 increases the expression of hypothalamic orexigenic neuropeptides and caloric intake (Poon et al., 2016).
We recently reported the involvement of CD137 (also called tumor necrosis factor receptor superfamily member 9 [TNFRSF9] or 4-1BB) and/or its ligand CD137L (also called TNFSF9 or 4-1BBL) in hypothalamic astrocytic–microglial cross-talk under DIO conditions (Kim et al., 2018). Binding of CD137 to CD137L transmits the bidirectional signals in astrocytes and microglia that are in contact (Shao and Schwarz, 2011). Activation of CD137 signaling in astrocytes increases astrocyte reactivity, and the stimulation of microglial CD137L signaling leads to microglial activation (Kim et al., 2018). Co-culture of astrocytes and microglia results in an increase in inflammatory cytokine production, whereas inhibition of the interaction between CD137 and CD137L using a CD137-neutralizing antibody inhibits this effect. Consistent with this, in an in vivo study, CD137 depletion reduces hypothalamic astrocyte/microglia activation during HFD consumption. These findings suggest that CD137/CD137L may serve as an important mediator of overnutrition-induced hypothalamic inflammation.
MICROGLIA–NEURON INTERACTIONS
Neurons and microglia communicate with one another through the secretion of bioactive molecules or direct contact (Szepesi et al., 2018). The secretory repertoire of each is altered during inflammation, such that there is greater secretion of proinflammatory cytokines, chemokines, reactive oxygen species, and ATP, but lower secretion of neurotropic factors and anti-inflammatory cytokines (Mizuno, 2015; Szepesi et al., 2018).
Under normal conditions, microglia remain in a quiescent state, which limits unproductive inflammatory responses, and neurons play an active role in this process. One well-known microglial–neuronal interaction is mediated by chemokine C-X3-C motif ligand-1 (CX3CL1)/fractalkine and its receptor CX3CR1, which are members of the chemokine–chemokine receptor family (Paolicelli et al., 2014). Microglia express CX3CR1, which binds neuronal CX3CL1 (Paolicelli et al., 2014). This CX3CR1–CX3CL1 interaction relays neuronal ‘off’ signals to microglia to maintain them in their resting state (Biber et al., 2007). Consistent with this, CX3CR1 deficiency worsens neurodegeneration because of greater microglial-mediated neurotoxicity (Cardona et al., 2006). Likewise, CX3CL1–CX3CR1 signaling protects against obesity-induced hypothalamic inflammation (Dorfman et al., 2017).
The expression of both CX3CL1 and CX3CR1 was found to be lower in the hypothalamus of HFD-fed male mice, and overexpression of CX3CL1 or CX3CL1 in the hypothalamus prevented DIO in these mice. Interestingly, female mice showed no reduction in hypothalamic CX3CL1–CX3CR1 expression during HFD-feeding (Dorfman et al., 2017). The ability of female mice to maintain CX3CR1–CX3CL1 signaling under HFD-fed conditions may account for their resistance to HFD-induced hypothalamic inflammation and obesity.
ASTROCYTE–NEURON INTERACTIONS
Bidirectional interactions between astrocytes and neurons are important for the homeostatic control of neuronal activity, metabolism, synaptic transmission, and neurotransmitter synthesis, as well as for the defense against oxidative stress and neuroinflammation (Kirchhoff et al., 2001; Ricci et al., 2009). Astrocytic–neuronal interactions have been shown to be protective against glutamate-induced neurotoxicity, and these effects are mediated by CCL6 and its receptor CCR1 (Nakagawa et al., 2019). Moreover, a recent study revealed a novel mechanism of astrocyte-mediated neuroprotection (Ioannou et al., 2019). Hyperactivated neurons generate peroxidated fatty acids but expel them in the form of apolipoprotein E (ApoE)-containing small lipid particles because neurons have a limited capacity for mitochondrial lipid oxidation. The neighboring astrocytes take up these lipid particles and then either store them as lipid droplets or oxidize them in their mitochondria. This neuronal–astrocytic cooperation in lipid metabolism appear to be critical for neuronal health, and the disruption of this may predispose neurons to lipotoxicity.
By contrast, under pathological conditions, reactive astrocytes release proinflammatory mediators that induce neuronal injury (Allan et al., 2001; Ricci et al., 2009). For instance, reactive astrocytes secrete CCL2, which triggers hypothalamic inflammation by binding to CCR2 on neurons and glia (Kwon et al., 2017). The deletion of CCL2 and CCR2 retards neuronal loss under neuroinflammatory conditions (Allen et al., 2013; Tian et al., 2017), and thus CCL2-CCR2 signaling may contribute to hypothalamic neuronal dysfunction in obesity.
CONCLUSIONS AND FUTURE PERSPECTIVES
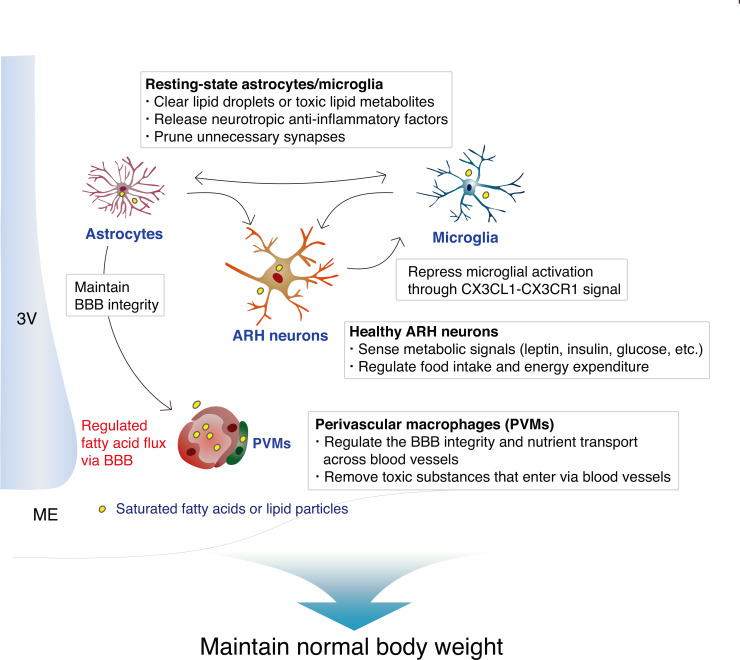
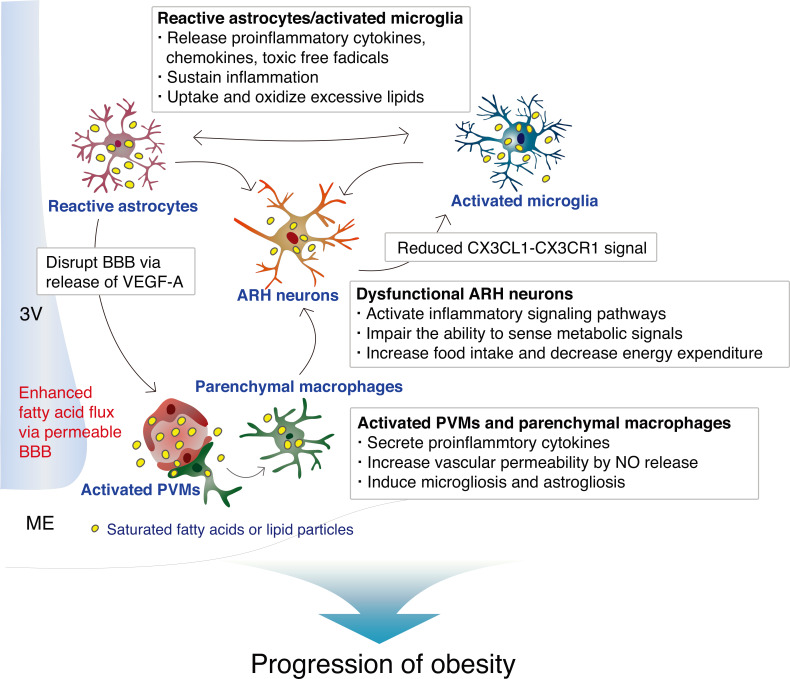
We have summarized the proven and possible interactions between hypothalamic ARH neurons and glia in normal homeostatic condition in Fig. 1, and during obesity-associated inflammation in Fig. 2. It is noteworthy that the hypothalamus is one of the most sensitive organs to SFA-induced inflammation/immune activation, and that hypothalamic inflammation is not a consequence of established obesity, but rather a significant contributor to the development of obesity.
Fig. 1. Homeostatic interactions between neurons and glia (microglia, astrocytes, and perivascular macrophages) in the hypothalamic ARH.
3V, third ventricle; ME, median eminence.
Fig. 2. Hypothalamic neuronal– glial and glial–glial interactions during obesity-associated inflammation induced by long-term consumption of a high-fat diet.
3V, third ventricle; ME, median eminence; NO, nitric oxide.
As shown in cortical neurons, hypothalamic neurons might have a limited capacity to oxidize fatty acids in their mitochondria or to store them in lipid droplets (Ioannou et al., 2019). They can expel surplus fatty acids in the form of small lipid particles, which are then taken up and oxidized by surrounding astrocytes and microglia (Ioannou et al., 2019). In this situation, microglia and astrocytes may be transformed from their quiescent state to a reactive state to handle lipid overload. Hence, the early stages of HFD-induced hypothalamic inflammation may operate to protect neurons from lipotoxicity. This hypothesis also provides a good explanation for the restriction of HFD-induced hypothalamic inflammation to the mediobasal part of the ARH because blood vessels in this area become permeable during fasting and HFD-feeding (Langlet et al., 2013; Lee et al., 2019), which allows the free entry of circulating fatty acids. ARH neurons, which primarily detect changes in the systemic metabolic state (Schwartz, 2006), are readily exposed to lipotoxicity, and thus hypothalamic inflammation may be part of a neuroprotective mechanism for these critically important neurons. If this fatty acid influx subsides, hypothalamic inflammation resolves and activated microglia and astrocytes return to their quiescent states.
However, upon persistent exposure to an HFD, higher levels of inflammation may be induced to dispose of large amounts of fatty acids, fat metabolites, or cellular debris. At this stage, the microglia may be more highly activated and accumulate as a result of interactions with astrocytes and neurons. By releasing proinflammatory cytokines and chemokines, they may attract other types of immune cell, such as perivascular macrophages, into the fat-overloaded ARH parenchyma (Lee et al., 2018; Valdearcos et al., 2017). Circulating immune cells may also be recruited through the permeable ARH vessels, although the precise profiles of the infiltrating immune cells require further characterization. The released cytokines and dietary SFAs activate inflammatory signaling pathways in the hypothalamic neurons that impede the ability of neurons to sense peripheral metabolic signals (Cai and Liu, 2011; Jais and Bruning, 2017). In the later stages of persistent or repeated hypothalamic inflammation, some of these neurons degenerate and die (Moraes et al., 2009). Together, these changes may lead to an impairment in the hypothalamic control of energy balance and the development of morbid obesity.
The exact mechanisms of the neuronal–glial interactions during the different stages of overnutrition-induced hypothalamic inflammation have yet to be determined. Moreover, further studies are needed to better understand the influence of hormones (leptin, insulin, and gut and sex hormones) and genetic factors on neuronal–glial interactions during the course of hypothalamic inflammation. Hypothalamic inflammation may share common features with other neuroinflammatory disorders and also have characteristics similar to those of obesity-associated inflammation in peripheral metabolic organs. A better understanding of the specific roles of the cellular contributors to hypothalamic inflammation and the mode of their interactions should help us to treat or prevent obesity more effectively.
ACKNOWLEDGMENTS
This study was supported by grants from the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT of Korea (2018R1D1A1B07041643, 2018R1C1B6005102, 2020R1A2C3004843), and the Asan Institute for Life Sciences (2018-326, 2019-IP0855). We thank the Scientific Publications Team at Asan Medical Center and Mark Cleasby, Ph.D., of Bioedit Inc., for their editorial assistance in preparing this manuscript.
Footnotes
AUTHOR CONTRIBUTIONS
C.H.L., R.Y., and M.S.K. wrote the manuscript. K.S. discussed the content of the manuscript.
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to disclose.
REFERENCES
- Abbott N.J., Rönnbäck L., Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Allan S.M., Harrison D.C., Read S., Collins B., Parsons A.A., Philpott K., Rothwell N.J. Selective increases in cytokine expression in the rat brain in response to striatal injection of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate and interleukin-1. Brain Res. Mol. Brain Res. 2001;93:180–189. doi: 10.1016/S0169-328X(01)00211-X. [DOI] [PubMed] [Google Scholar]
- Allen A.R., Eilertson K., Sharma S., Schneider D., Baure J., Allen B., Rosi S., Raber J., Fike J.R. Effects of radiation combined injury on hippocampal function are modulated in mice deficient in chemokine receptor 2 (CCR2) Radiat. Res. 2013;180:78–88. doi: 10.1667/RR3344.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argaw A.T., Asp L., Zhang J., Navrazhina K., Pham T., Mariani J.N., Mahase S., Dutta D.J., Seto J., Kramer E.G., et al. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J. Clin. Invest. 2012;122:2454–2468. doi: 10.1172/JCI60842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch E.E. Neuron-astrocyte interactions: partnership for normal function and disease in the central nervous system. Mayo Clin Proc. 2005;80:1326–1338. doi: 10.4065/80.10.1326. [DOI] [PubMed] [Google Scholar]
- Biber K., Neumann H., Inoue K., Boddeke H.W. Neuronal 'On' and 'Off' signals control microglia. Trends Neurosci. 2007;30:596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Cai D., Liu T. Hypothalamic inflammation: a double-edged sword to nutritional diseases. Ann. N. Y. Acad. Sci. 2011;1243:E1–E39. doi: 10.1111/j.1749-6632.2011.06388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona A.E., Pioro E.P., Sasse M.E., Kostenko V., Cardona S.M., Dijkstra I.M., Huang D., Kidd G., Dombrowski S., Dutta R., et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- De Souza C.T., Araujo E.P., Bordin S., Ashimine R., Zollner R.L., Boschero A.C., Saad M.J., Velloso L.A. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- Dorfman M.D., Krull J.E., Douglass J.D., Fasnacht R., Lara-Lince F., Meek T.H., Shi X., Damian V., Nguyen H.T., Matsen M.E., et al. Sex differences in microglial CX3CR1 signalling determine obesity susceptibility in mice. Nat. Commun. 2017;8:14556. doi: 10.1038/ncomms14556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass J.D., Dorfman M.D., Fasnacht R., Shaffer L.D., Thaler J.P. Astrocyte IKKbeta/NF-kappaB signaling is required for diet-induced obesity and hypothalamic inflammation. Mol. Metab. 2017;6:366–373. doi: 10.1016/j.molmet.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Ottaway N., Schriever S.C., Legutko B., Garcia-Caceres C., de la Fuente E., Mergen C., Bour S., Thaler J.P., Seeley R.J., et al. Hormones and diet, but not body weight, control hypothalamic microglial activity. Glia. 2014;62:17–25. doi: 10.1002/glia.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath T.L., Sarman B., García-Cáceres C., Enriori P.J., Sotonyi P., Shanabrough M., Borok E., Argente J., Chowen J.A., Perez-Tilve D., et al. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc. Natl. Acad. Sci. U. S. A. 2010;107:14875–14880. doi: 10.1073/pnas.1004282107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou M.S., Jackson J., Sheu S.H., Chang C.L., Weigel A.V., Liu H., Pasolli H.A., Xu C.S., Pang S., Matthies D., et al. Neuron-astrocyte metabolic coupling protects against activity-induced fatty acid toxicity. Cell. 2019;177:1522–1535.:e14. doi: 10.1016/j.cell.2019.04.001. [DOI] [PubMed] [Google Scholar]
- Jais A., Brüning J.C. Hypothalamic inflammation in obesity and metabolic disease. J. Clin. Invest. 2017;127:24–32. doi: 10.1172/JCI88878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha M.K., Jo M., Kim J.H., Suk K. Microglia-astrocyte crosstalk: an intimate molecular conversation. Neuroscientist. 2019;25:227–240. doi: 10.1177/1073858418783959. [DOI] [PubMed] [Google Scholar]
- Jung C.H., Kim M.S. Molecular mechanisms of central leptin resistance in obesity. Arch. Pharm. Res. 2013;36:201–207. doi: 10.1007/s12272-013-0020-y. [DOI] [PubMed] [Google Scholar]
- Kierdorf K., Prinz M. Microglia in steady state. J. Clin. Invest. 2017;127:3201–3209. doi: 10.1172/JCI90602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Kwon Y.H., Kim C.S., Tu T.H., Kim B.S., Joe Y., Chung H.T., Goto T., Kawada T., Park T., et al. The involvement of 4-1BB/4-1BBL signaling in glial cell-mediated hypothalamic inflammation in obesity. FEBS Open Bio. 2018;8:843–853. doi: 10.1002/2211-5463.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff F., Dringen R., Giaume C. Pathways of neuron-astrocyte interactions and their possible role in neuroprotection. Eur. Arch. Psychiatry Clin. Neurosci. 2001;251:159–169. doi: 10.1007/s004060170036. [DOI] [PubMed] [Google Scholar]
- Kwon Y.H., Kim J., Kim C.S., Tu T.H., Kim M.S., Suk K., Kim D.H., Lee B.J., Choi H.S., Park T., et al. Hypothalamic lipid-laden astrocytes induce microglia migration and activation. FEBS Lett. 2017;591:1742–1751. doi: 10.1002/1873-3468.12691. [DOI] [PubMed] [Google Scholar]
- Langlet F., Levin B.E., Luquet S., Mazzone M., Messina A., Dunn-Meynell A.A., Balland E., Lacombe A., Mazur D., Carmeliet P., et al. Tanycytic VEGF-A boosts blood-hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting. Cell Metab. 2013;17:607–617. doi: 10.1016/j.cmet.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.H., Kim H.J., Lee Y.S., Kang G.M., Lim H.S., Lee S.H., Song D.K., Kwon O., Hwang I., Son M., et al. Hypothalamic macrophage inducible nitric oxide synthase mediates obesity-associated hypothalamic inflammation. Cell Rep. 2018;25:934–946.:e5. doi: 10.1016/j.celrep.2018.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.H., Shin S.H., Kang G.M., Kim S., Kim J., Yu R., Kim M.S. Cellular source of hypothalamic macrophage accumulation in diet-induced obesity. J. Neuroinflammation. 2019;16:221. doi: 10.1186/s12974-019-1607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow S.A., Guttenplan K.A., Clarke L.E., Bennett F.C., Bohlen C.J., Schirmer L., Bennett M.L., Munch A.E., Chung W.S., Peterson T.C., et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng C.N., Saltiel A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T. Neuron-microglia interactions in neuroinflammation. Clin. Exp. Neuroimmunol. 2015;6:225–231. doi: 10.1111/cen3.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes J.C., Coope A., Morari J., Cintra D.E., Roman E.A., Pauli J.R., Romanatto T., Carvalheira J.B., Oliveira A.L., Saad M.J., et al. High-fat diet induces apoptosis of hypothalamic neurons. PLoS One. 2009;4:e5045. doi: 10.1371/journal.pone.0005045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S., Izumi Y., Takada-Takatori Y., Akaike A., Kume T. Increased CCL6 expression in astrocytes and neuronal protection from neuron-astrocyte interactions. Biochem. Biophys. Res. Commun. 2019;519:777–782. doi: 10.1016/j.bbrc.2019.09.030. [DOI] [PubMed] [Google Scholar]
- Paolicelli R.C., Bisht K., Tremblay M.È. Fractalkine regulation of microglial physiology and consequences on the brain and behavior. Front. Cell. Neurosci. 2014;8:129. doi: 10.3389/fncel.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Joe Y., Ryter S.W., Surh Y.J., Chung H.T. Similarities and distinctions in the effects of metformin and carbon monoxide in immunometabolism. Mol. Cells. 2019;42:292–300. doi: 10.14348/molcells.2019.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon K., Barson J.R., Ho H.T., Leibowitz S.F. Relationship of the chemokine, CXCL12, to effects of dietary fat on feeding-related behaviors and hypothalamic neuropeptide systems. Front. Behav. Neurosci. 2016;10:51. doi: 10.3389/fnbeh.2016.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci G., Volpi L., Pasquali L., Petrozzi L., Siciliano G. Astrocyte-neuron interactions in neurological disorders. J. Biol. Phys. 2009;35:317–336. doi: 10.1007/s10867-009-9157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh E., Song D.K., Kim M.S. Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp. Mol. Med. 2016;48:e216. doi: 10.1038/emm.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M.W. Central nervous system regulation of food intake. Obesity (Silver Spring) 2006;14(Suppl 1):1s–8s. doi: 10.1038/oby.2006.275. [DOI] [PubMed] [Google Scholar]
- Serrats J., Schiltz J.C., Garcia-Bueno B., van Rooijen N., Reyes T.M., Sawchenko P.E. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron. 2010;65:94–106. doi: 10.1016/j.neuron.2009.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z., Schwarz H. CD137 ligand, a member of the tumor necrosis factor family, regulates immune responses via reverse signal transduction. J. Leukoc. Biol. 2011;89:21–29. doi: 10.1189/jlb.0510315. [DOI] [PubMed] [Google Scholar]
- Szepesi Z., Manouchehrian O., Bachiller S., Deierborg T. Bidirectional microglia-neuron communication in health and disease. Front. Cell. Neurosci. 2018;12:323. doi: 10.3389/fncel.2018.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler J.P., Yi C.X., Schur E.A., Guyenet S.J., Hwang B.H., Dietrich M.O., Zhao X., Sarruf D.A., Izgur V., Maravilla K.R., et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian D.S., Peng J., Murugan M., Feng L.J., Liu J.L., Eyo U.B., Zhou L.J., Mogilevsky R., Wang W., Wu L.J. Chemokine CCL2-CCR2 signaling induces neuronal cell death via STAT3 activation and IL-1beta production after status epilepticus. J. Neurosci. 2017;37:7878–7892. doi: 10.1523/JNEUROSCI.0315-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdearcos M., Douglass J.D., Robblee M.M., Dorfman M.D., Stifler D.R., Bennett M.L., Gerritse I., Fasnacht R., Barres B.A., Thaler J.P., et al. Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab. 2017;26:185–197.:e3. doi: 10.1016/j.cmet.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdearcos M., Robblee M.M., Benjamin D.I., Nomura D.K., Xu A.W., Koliwad S.K. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 2014;9:2124–2138. doi: 10.1016/j.celrep.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A., Nedergaard M. Physiology of astroglia. Physiol. Rev. 2018;98:239–389. doi: 10.1152/physrev.00042.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K., Alvarez X., Lackner A.A. Central nervous system perivascular cells are immunoregulatory cells that connect the CNS with the peripheral immune system. Glia. 2001;36:156–164. doi: 10.1002/glia.1105. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Reichel J.M., Han C., Zuniga-Hertz J.P., Cai D. Astrocytic process plasticity and IKKbeta/NF-kappaB in central control of blood glucose, blood pressure, and body weight. Cell Metab. 2017;25:1091–1102.:e4. doi: 10.1016/j.cmet.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]