Highlights
-
•
Pediococcus pentosaceus and Enterococcus faecium blocked foodbone pathogenic biofilm formations.
-
•
Nisin Production against planktonic and biofilm forms of tested foodborne pathogens.
-
•
Pediococcus pentosaceus and Enterococcus faecium could form biofilms.
Keywords: Lactic Acid Bacteria, biofilm, foodborne pathogen, isolation, antimicrobial compound
Abstract
Pediococcus pentosaceus and Enterococcus faecium isolated from fermented fish and chicken represented the potential probiotic properties against Bacillus cereus ATCC 11778, Escherichia coli ATCC 8739, and Salmonella enterica subsp. enterica serovar Typhimurium ATCC 13311. Isolated Lactic Acid Bacteria were tested for physiological characteristics, antimicrobial activity of crude supernatant containing 0.5- 1.3% w/ v nisin against planktonic and biofilm of foodborne pathogens, biofilm forming ability, auto-aggregation, co-aggregation with all tested pathogens, bacterial survival in acid and bile salt conditions, hemolytic activity, and minimal inhibitory concentration of antibiotics. Isolates were also identified using 16S rRNA sequencing. LAB showed antimicrobial activities against planktonic and biofilm forms of all tested foodborne pathogens. All LAB could develop biofilms to prevent biofilm formations of all tested pathogens through the co-aggregation process. They showed 6-8% tolerance to bile salt, were partially resistant to low pH, hemolysis negative, and antibiotic susceptibility to the level allowed by European Food Safety Authority.
1. Introduction
Lactic Acid Bacteria (LAB) are used for the production of many fermented foods. They have been characterised as non-spore forming, gram positive rods or cocci, which are catalase-negative that share many biochemical and physiological properties [1]. The natural reservoir of LAB is green plant materials. LAB have been isolated from various fermented food products and from human and animal guts [2]. They are a group of bacteria that are of specific interest to the food industry based on their ability to undertake anaerobic fermentation and their probiotic characteristics. They are part of the healthy microbiota of the human gut, as they prevent food spoilage and growth of pathogenic microorganisms. Some are considered probiotics, which are beneficial microbes that colonise the gut of humans and animals. This group of microorganisms have been reported to tolerate acid and bile, and are able to adhere to epithelial surfaces. They also show antagonistic activity towards intestinal pathogens, and other antimicrobial properties [3]. LAB are varied based on morphology (rods, cocci, tetrads), mode of glucose fermentation, substrate spectrum, growth at different temperatures (15 and 45°C), ability to grow at high salt concentrations, and acid, alkaline or ethanol tolerance. They are widely used for this purpose and are generally regarded as safe organisms. Their ability to produce organic acids, including lactic and acetic acid, as well as the bacteriocins produced by some strains, is mainly responsible for their preservation effect [4].
Biofilms are a natural form of cell immobilisation that results from microbial attachment to solid supports either biotics or abiotic surfaces in submerged environments. The presence of the high cell density in the biofilm enables the bacterial in the biofilm to withstand stresses, such as pH change or starvation. The presence of pathogenic and spoilage biofilms are relevant risk factor in the food industry by resisting against cleaning and disinfection processes. Biofilms can develop on any type of surface, including plastic, metal, glass, soil particles, wood, biotic surfaces and stainless steel [5,6]. Salmonella are a group of important pathogenic bacteria that are transmitted by food. They are capable of adhering and forming biofilm on metal, glass, or rubber surfaces [7]. S. Typhimurium causes gastroenteritis in humans and other mammals, which can lead to bloody diarrhea. E. coli has been characterised as causing diarrhea. Outbreaks of E. coli infections have been primarily associated with eating undercooked meat. Cells of E. coli embedded under the extracellular polymeric substance (EPS) of a biofilm structure have been found to persist after treatment with chlorine [8]. B. cereus is a foodborne pathogen that frequently contaminate food production plants or grains. This pathogenic infection can cause the foodborne diseases including endophthalmitis, meningitis, or periodontitis [9]. B. cereus is resistant to various environments through the formation of spores and of biofilms. It is capable of forming biofilms on industrial equipment, especially on the stainless steel components used in the food industry [10]. Biofilms of these foodborne pathogens have been found to be resistant to various levels of biocides, particularly due to the formation of the EPS matrix. This explains why available disinfectants that are mostly effective against planktonic cells are not active against biofilm cells [11].
LAB have been found to form biofilms on biotic and abiotic material to serve industrial purposes or function as antagonistic effectors against various foodborne pathogens in either planktonic or biofilm mode of growth [12]. Isolated LAB from Brazilian foods, Lactococcus lactis, Lactobacillus sakei, and Lactobacillus curvatis have been found to reduce the biofilm formation of Listeria monocytogenes, Escherichia coli O157:H7, and Salmonella enterica subsp. enterica serovar Typhimurium through co- aggregation, not attributed to bacteriocin production [13]. Therefore, the use of probiotic bacteria or their active compounds is a promising method to control pathogenic biofilm formation on food industrial surfaces in the future.
This project aims to isolate LAB from fermented chicken and fish to prevent the biofilm formation of pathogenic microbes. We also tested their ability to create antagonistic conditions against common foodborne pathogens (S. Typhimurium, E. coli, and B. cereus). The ability of the isolated LAB to block foodborne pathogenic biofilm formation through the co-aggregation was also determined, and their microbial characteristics, including bile salt tolerance, acidic pH tolerance, haemolysis activity, and antibiotic susceptibilities were quantified. The isolated LABs from fermented meats would be generally recognized as safe to be applied in the food industry against the foodborne pathogens. Different regions represent different indigenous microbes therefore, this would be considered as novelty of this work to lead the future perspective of using isolated indigenous LABs as probiotics or utilizing their antagonistic activities against foodborne pathogenic biofilms.
2. Material and Methods
2.1. Bacterial strains and culture conditions
The isolated LAB were grown on deMan Rogosa and Sharpe (MRS) agar at 37°C. B. cereus ATCC 11778 was grown in nutrient agar or nutrient broth at 30°C. E. coli ATCC 8739 and S. enterica subsp. enterica serovar Typhimurium ATCC 1331 (American Type Culture Collection, ATCC) were cultured in nutrient agar or nutrient broth at 37°C. All cultures have been grown overnight with absorbance (A600 nm) approximately 1.0 prior all tests. All strains were maintained at -80°C in the appropriate cultivation broth containing 20% (v/v) glycerol.
2.2. Isolation of Lactic Acid Bacteria
Lactic Acid Bacteria were isolated by the direct plating method from homemade fermented chicken and fish. The samples were ground in 0.1% (w/v) peptone and incubated at 37 °C overnight to enrich the number of microbes. Homogenized samples were serially diluted 10-fold and spread on deMan Rogosa and Sharpe (MRS) agar supplemented with 0.5% (w/v) CaCO3, which was added as an indicator for acid production. The isolates were characterised based on gram staining, morphology observation, spore staining, motility, and the catalase test according to Bergey’s manual of determinative bacteriology. Gram positive rods or cocci that were non-spore forming, non-motile, and catalase negative were isolated and identified as LAB [14].
2.3. HPLC analysis on nisin production
The isolated LAB was grown in MRS for overnight at 37 °C. Cells were removed by centrifugation at 1,200 rpm for 5 minutes. Culture supernatant was filtered through a 0.22 μm membrane filter (Millipore Corp., Bedford, MA). The concentration of nisin in the culture supernatant was analysed by HPLC (Shimadzu) using Inertsil C18 column (5 μm, 250 mm × 4.6 mm) maintained at 40 °C. The mobile phase was 1% trichloroacetic acid at the flow rate 2 ml/ min with a UV detector at a wavelength of 280 nm. The standard solution used were 0.5%, 1.5% and 2% w/ v standard nisin (Sigma Chemical Co., USA).
2.4. Bacterial strain analysis
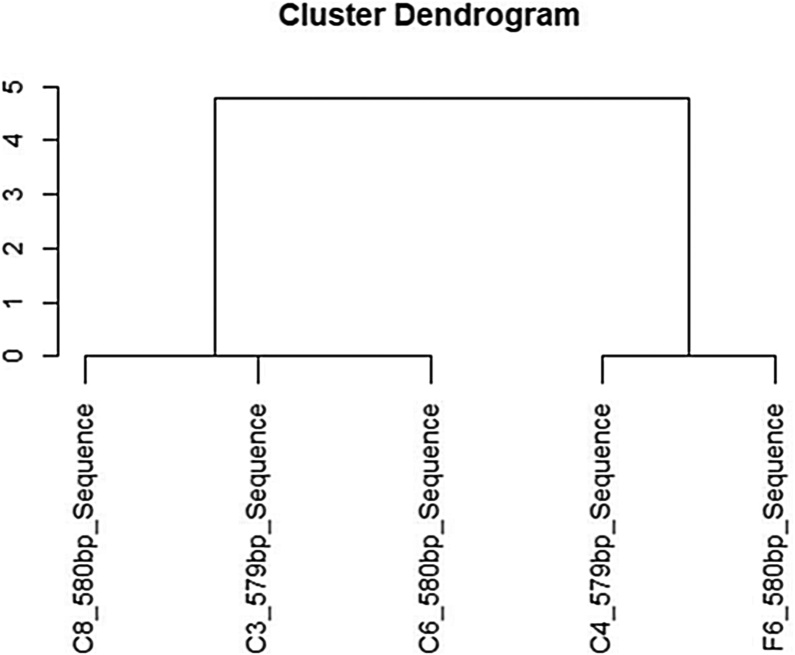
Isolated LAB were identified by 16S rRNA gene sequencing. The universal primers 1492R (5' TACGGYTACCTTGTTACGACTT 3') and 27 F (5' AGAGTTTGATCMTGGCTCAG 3') were used for the sequencing analysis [15]. The sequencing and analysis was done by Genscript, Co, Ltd. The resulting sequence was analysed through the National Center for Biotechnology Information (NCBI) GenBank database. The dendrogram was prepared using the R programming Language.
2.5. Antimicrobial activity of the isolated LABs
The isolates were tested on their ability to inhibit the growth of indicator strains S. Typhimurium, E. coli, and B. cereus. The isolates were cultured in MRS broth and incubated at 37 °C for another 24 hours. Then each culture was centrifuged at 1,200 rpm for 5 minutes to remove cell pellets. The supernatant from each culture was collected and filtered with a 0.45 microfilter. The antimicrobial activities of all LAB isolates supernatants were tested against the three pathogens under neutralized and non-neutralized conditions using disc diffusion method.
2.6. Biofilm forming ability of LAB isolates
The biofilm forming ability of selected isolates was demonstrated using qualitative and quantitative analysis. The quantitative analysis was performed using crystal violet staining (0.1% crystal violet dye) based on bacterial attachment to a polystyrene surface normalised to the (absorbance (A600 nm). To initiate biofilm formation in each isolate, an overnight culture with an OD600 measurement of approximately 1.0 was inoculated into 2 ml of 1:5 diluted MRS medium. The biofilms were allowed to develop under static conditions at 30 °C for 3 consecutive days when the medium was replaced each day. The bacterial attachment was measured each day using 1% w/v crystal violet staining for 20 minutes and then destained with 95% ethanol. The optical density of the destained material was measured at absorbance (A600 nm) using a spectrophotometer. The biofilm forming abilities of all LAB strains were compared based on the intensity of the destained crystal violet [16]. The experiment was performed in triplicate, and the results were statistically analyzed by ANOVA with Duncan’s multiple range test. The null hypothesis was accepted or rejected with 95% confidence interval (p < 0.05).
The qualitative analysis was performed using real time visualisation under a flow cell system [16]. Biofilms of all stains were grown on polystyrene surfaces in flow cells at room temperature. The flow cell channels were filled with 1:5 diluted MRS medium and then inoculated with overnight cultures (OD600 about 1.0). After the inoculation, cultures were incubated statically for 4 hours to facilitate the initial attachment on the flow cell surface. After the initial attachment, the flow was restored at a flow rate of 0.2 ml/ min. Biofilm development of LAB was visualised and captured using bright-field microscopy attached with a digital camera (dino-eye model AM423x) after 24 hours of cultivation at 300x magnification.
2.7. Auto aggregation and co-aggregation study of LAB
Aggregation abilities of isolated LAB were studied as described by Collado et al. [17]. Bacterial cells from an overnight culture were harvested by centrifugation for 2 minutes [13] and washed twice with phosphate buffer pH 7.0 and pellets were re-suspended in the same buffer. The number of bacteria were approximately at 107- 108 CFU/ml. The optical density (OD600) of the homogenized bacterial suspension was recorded and then monitored again at 24 hours after incubation at 37°C under the static condition. The percent of aggregation was evaluated as (1- (Atime/A0) ×100) where Atime represents the absorbance of the mixture at 24 hours and A0 absorbance at time 0.
Co-aggregation of pathogenic microbes with LAB strains was analysed based on the analysis of absorbance value (OD) of suspended cultures after the overnight co-aggregation with E. coli, S. Typhimurium, or B. cereus as previously described [13,17]. LAB bacterial suspensions were prepared as described above and mixed with equal volumes (500 μl) of the cultures of the pathogens. Mixtures were then incubated at 37°C for 24 hours. The percentage of co-aggregation was evaluated as (Apathogen + ALAB)/2 – (Amix)/(Apathogen + ALAB)/s) × 100. Where Apathogen and ALAB represent the absorbance in the tubes containing only the pathogen or the LAB strain respectively and Amix represented the absorbance of the mixture after 24 hours incubations [18]. The experiment was performed in triplicate, and the results were statistically analyzed by ANOVA with Duncan’s multiple range test as above.
2.8. Analysis on the tolerance to bile salts and acidic pH
LAB strains were evaluated on bile salt tolerance (0-10%) and survival rate at low pH (1.5-3) using MRS based medium at various bile concentrations and acidity levels to observe the bacterial survival at each specific condition [19]. The bile salt containing MRS agars (2- 20% bile salt) were inoculated with 100 μl of LABs cultures at 106- 107CFU/ ml and incubated at 37°C for 2 days. The plates were determined for the growth of bacterial lawns to indicate the resistance to the specific concentration of bile salt concentration.
For acid tolerance, MRS broth was prepared at different pH conditions (1.5-6.5) with 5 M HCl. Then 1 ml of overnight LAB culture (OD600 of approximately 0.2-0.3 containing 106- 107 CFU/ml) was added to 19 ml acidic MRS broth. The samples were then incubated at 37℃ for 24 hours. The growths were measured by spectrophotometry at 600 nm. A t-test was performed at a 95% confidence interval in order to determine the statistical significance (p < 0.05).
2.9. Hemolytic Activity
Hemolytic activity test was carried out on blood agar plates containing 5% (v/v) horse blood (purchased from Thermo scientific, Oxoid microbiology products) and incubated at 30°C for 24- 48 hours. A clear zone appeared around the colony was indicated as hemolytic activity [20].
2.10. Antibiotic susceptibility analysis
Antibiotic susceptibility was analysed by a broth microdilution protocol [21]. Antibiotics employed in this study were ampicillin, ciprofloxacin, clindamycin, gentamycin, kanamycin, streptomycin, erythromycin, vanomycin, tetracycline, and chloramphenicol. All antibiotics were purchased from Sigma-Aldrich, USA. Antibiotic stocks were prepared at 40 and 4 and two-fold microdilutions were performed in 96 wells. One hundred microliters of sterile MRS were loaded in each well for dilution with the antibiotic stocks. The starting cell concentration of LAB cultures was CFU/ml as recommended by the European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and infectious diseases (ESCMID). The plates were incubated at for 24 hours and absorbance (A595 nm) [22].
2.11. Antimicrobial activity test of crude supernatant from LABs against biofilm of pathogenic strains
The overnight LAB cultures were centrifuged to collect cell pellets and supernatants. The pH of the supernatants were adjusted to neutral and filtered with a 0.45 microfilter. The supernatant (1 ml) was mixed with 1 ml of an overnight culture of B. cereus, E. coli, or S. Typhimurium (OD containing CFU/ml). The culture with the crude LAB supernatant was incubated at for 24 hours. Any resulting biofilm was then stained with crystal violet. The optical density at 600 nm was measured using a spectrophotometer, as described above. The biofilm forming ability under each specific condition was compared based on the intensity of the crystal violet staining. A t-test was performed at a 95% confidence interval in order to determine the statistical significance (p < 0.05).
3. Results and discussion
Five Lactic Acid Bacteria (LAB) were isolated from fermented chicken and fish. The isolates were coded as follow; C3, C4, C6, C8 and F6. From 16S rRNA gene sequencing analysis of all isolated LAB, C4 and F6 were found to be Enterococcus faecium strain FM11-2 with 99% similarity, while C3, C6, and C8 were found to be Pediococcus pentosaceus strain PP 04 with 99% similarity (Fig. 1). The crude supernatant from isolated LAB cultures were evaluated on their inhibitory effects on the three foodborne pathogens including B. cereus ATCC 11778, E. coli ATCC 8739, and S. Typhimurium ATCC 13311 by determining the inhibitory zone using the paper disc method. The neutralized and non- neutralized crude supernatants were tested for their antimicrobial activity against all tested foodborne pathogens (Table1). LAB crude extracts had a relatively broad spectrum of activity against all foodborne pathogens with different inhibitory levels. The neutralized crude supernatants from all isolates had a similar level of antimicrobial activity as the non-neutralized crude supernatant, which implies that the antimicrobial activities of all isolated LAB strains were not altered by excluding the effect of organic acids. Lactic Acid Bacteria could produce antimicrobial compounds including organic acids, diacetyl, hydrogen peroxide, ethanol, reuterin, bacteriocins, and bactericidal proteins. The neutralized crude supernatants of C3 and C8, in turn, resulted in increase the antimicrobial activities against B. cereus and E. coil than non- neutralized crude supernatant while the active compounds would probably be dominated under the neutralized condition. LAB have been found to inhibit spoilage and pathogenic microorganisms by producing anti-microbial metabolites [23]. Based on the above work, it appears that the antimicrobial activities of all isolated LAB resulted from their produced active compounds rather than the acidity of the crude extracts. In our work, nisin productions were produced from all 5 isolated in the level of 0.5- 1.3% w/v.
Fig. 1.
Dendrogram analysis of sequence of all isolated Lactic Acid Bacteria (LAB). The dendrogram was prepared using the R programming Language.
Table 1.
Antimicrobial activity of isolated Lactic Acid Bacteria from fermented chicken (code as C) and fish (Code as F). The culture supernatants (non-neutralized and neutralized) were tested against S. Typhimurium, E. coli and B. cereus using the paper disc method (+ diameter of clear zone 0.7- 0.9 cm, ++ diameter of clear zone 1.0- 1.2 cm, +++ diameter of clear zone 1.3- 1.5 cm).
| Lactic acid | Supernatant |
Neutralized Supernatant |
||||
|---|---|---|---|---|---|---|
| B. cereus | S. Typhimurium | E. coli | B. cereus | S. Typhimurium | E. coli | |
| C3 | ++ | + | ++ | +++ | + | ++ |
| C4 | +++ | + | ++ | ++ | + | ++ |
| C6 | + | ++ | ++ | + | ++ | ++ |
| C8 | ++ | ++ | + | ++ | + | ++ |
| F6 | + | ++ | ++ | + | ++ | ++ |
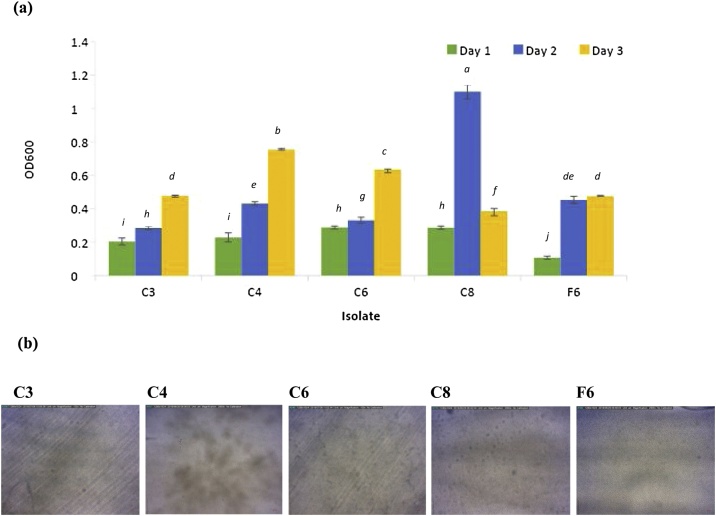
LAB biofilms have been reported as one of the barrier effect against the development of spoilage and pathogenic microorganisms [24]. Crystal violet staining of attached cells can be used as an indirect method to determine the amount of bacterial attachment. The amount of bacterial attachment can be further determined by spectrophotometer [25]. The intensity of the crystal dye was correlated to the level of the bacterial attachment or biofilm formation on polystyrene (PS) tubes from day 1 toward day 3. The amount of bacterial attachment on PS surface for LAB strains C3, C4, C6, and F6 increased steadily from day 1 to day 3 except for strain C8 (Fig. 2-a). There was a large reduction in bacterial attachment for strain C8 on day 3; however, attachment was much higher in this strain in comparison to others on day 2. Previously, Lactobacillus plantarum subsp. plantarum JCM1149 and Lactobacillus brevis JCM1059 have been reported to adhere to a glass surface [12]. From our study, this is the first time to reveal LAB biofilm morphology under the flow cell system. Our result illustrated the differences in the biofilm forming phenomena among all LAB isolates when observed under the bright- field microscopy (Fig. 2-b). Microscopy techniques have been used as a way to directly visualise biofilm development and real time observation was performed using a flow cell system. After one day of inoculation, LAB strain C4 produced a highly developed heterogeneous biofilm on surfaces, while the others only formed a thin layer. The biofilm formed by the C4 strain may be more beneficial in industrial applications, and, due to its high cell density, may be further used for bacteriocin production. From our result, LAB strain C4 also developed a significant amount of exopolysaccharide (EPS) where the previous study reported that EPS could enhance the colonisation of probiotic bacteria in the gastrointestinal tract, therefore benefitting consumer health [26].
Fig. 2.
(a) Quantification of crystal violet staining to indirectly measure the amount of surface attached biomass (OD600) of LAB isolates C3, C4, C6, C8, and F6. Error bar indicates the standard deviation (n = 3). Bars marked with a, b, and c had the three highest activity levels. Based on ANOVA, the different letters represent a significant difference of p < 0.05.(b) Bright-field microscopy of flow cell inoculated with different LAB strains and the subsequent biofilm attachment and morphology after one day of growth at ambient temperature with a constant flow rate of 0.2 ml/ minute. The scale bar represents 0.1 mm while the pictures were captured at 300 × .
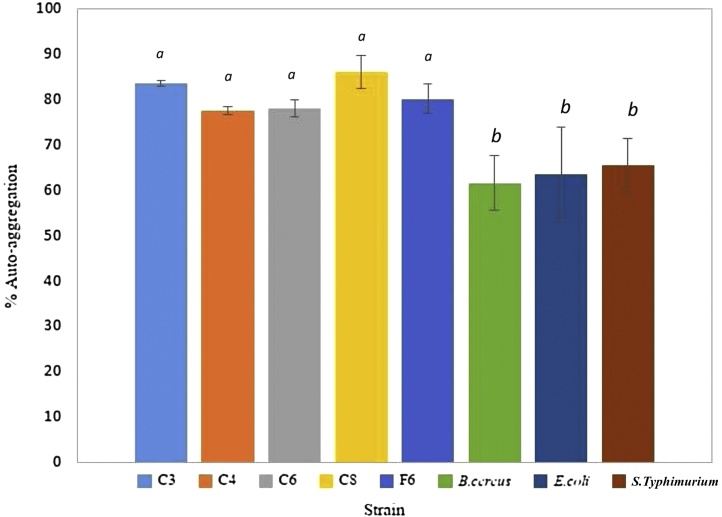
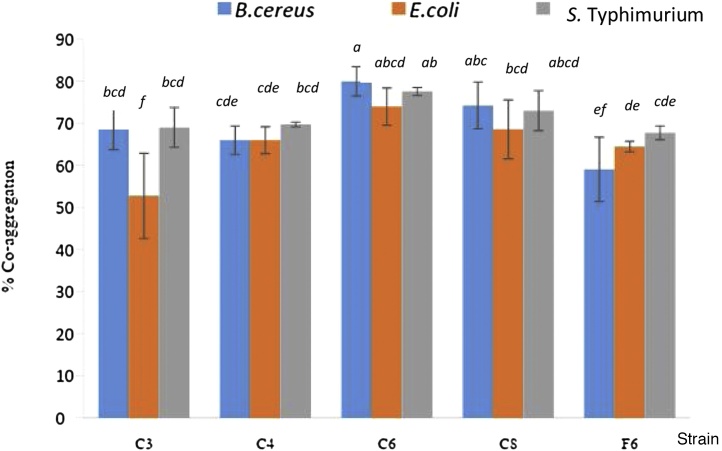
LAB strain C8 showed the highest level of auto-aggregation (86%) (Fig. 3). However, there was no significantly different amount of auto-aggregation when all 5 strains of LAB isolates were compared. Each LAB isolate tested had auto-aggregation levels in the range of 78-86%. Percent levels of auto-aggregation in B. cereus, E.coli, and S. Typhimurium were only in the range of 61-65%. From this study, isolated LABs showed a higher ability to auto-aggregate than the tested foodborne pathogens. Percent auto- aggregation levels in all isolated were found to be higher than the bacteriocin producing strains Lactococcus lactis VB69 and VB94 that were found to have 30- 50% auto-aggregation [13]. Aggregation rate plays a significant role in the prevention of colonisation on industrial surfaces or intestinal tracts by pathogenic bacteria [18]. The amount of inhibition was also found to correlate to the percentage of auto-aggregation [27]. Polystyrene tubes have a hydrophobic surface that has been reported to provide the strongest level of bacterial attachment or biofilm formation [27]. Therefore, we firstly report on the correlation between LAB biofilm formations or auto-aggregations that could enhance the production of inhibitory substances. All LAB strains tested co-aggregated with B. cereus, E. coli, and S. Typhimurium (Fig. 4). LAB strain C6 showed the highest co-aggregation with all foodborne pathogens (74- 80% co-aggregation). Co-aggregation plays a significant role in preventing pathogenic colonisation of industrial surfaces [18], as well as interfering with the ability of the pathogen to infect and colonise host cells, especially in the gastrointestinal tract..
Fig. 3.
Auto-aggregation of Lactic Acid Bacteria strains cells re-suspended in phosphate buffer, pH 7.0 evaluated after 24 hours incubation at 37°C. Data represents the mean of three replicates. Error bars represented standard deviation. Bars marked with “a” had highest activity levels. Based on ANOVA, the different letters represent a significant difference of p < 0.05.
Fig. 4.
Co-aggregation of Lactic Acid Bacteria strains with B. cereus, E. coli, and S.Typhimurium after 24 hours incubation at 37°C in phosphate buffer, pH 7.0. Data represents the mean of three replicates. Error bars represented standard deviation. Bars marked with a, b, and c had the three highest activity levels. Based on ANOVA, the different letters represent a significant difference of p < 0.05.
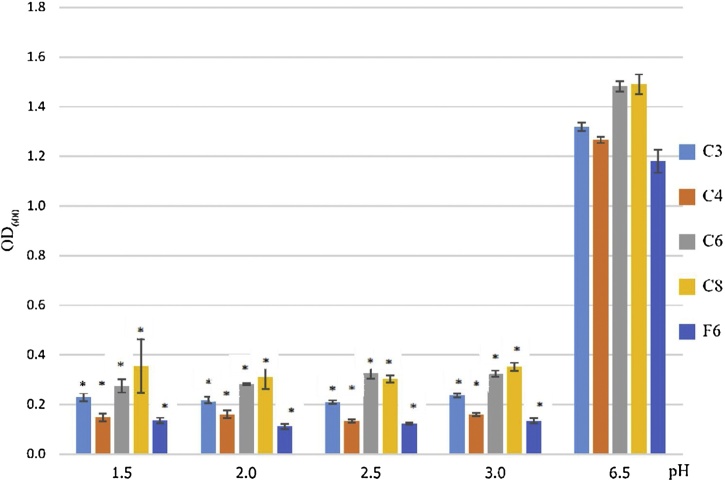
All isolated LAB were able to tolerate bile in the concentration range of between 6- 8% and did not show a positive result for hemolytic activity. All tested LAB isolates could survive well at pH 6.5 but at lower pH levels of 1.5- 3.0, comparable to the stomach’s pH range, LAB strain C6 and C8 showed higher survival rates than other LAB strains. All LAB strains tolerated bile salts and acidic pH, which shows that they may be able to tolerate conditions in the human digestive system (Fig. 5). Other probiotic strains illustrated the toleration to the bile salts of 4% [13]. The above factors show that the isolated LAB strains have the potential to be used as probiotics [28]. The antibiotic susceptibility analysis gave the MIC level for ciprofloxacin (CIP), chloramphenicol (CMP), vancomycin (VAN), erythromycin (ERY), streptomycin (STR), clindamycin (CLI), gentamicin (GEN), ampicillin (AMP), tetracycline (TET), and kanamycin (KAN) for all isolated strains (Table 2). The MIC of chloramphenicol, vancomycin, streptomycin, gentamicin, ampicillin, and kanamycin did not higher than that suggested by the European Food Safety Authority (EFSA) for all LAB isolates [29]. Some LAB isolated strains were resistant to several antibiotics; C3 was resistant to erythromycin, clindamycin, and tetracycline and F6 was resistant to erythromycin and tetracycline. C4, C6 and C8 were susceptible to all tested antibiotics except tetracycline, they would meet this standard EFSA requirement. However, The EFSA requires that bacteria must lack antimicrobial resistance when used in food products. A previous study has indicated that naturally occurring Lactic Acid Bacteria could be resistant to various antimicrobial agents [22]. The natural resistance to antibiotic is probably due to the cell wall structure, membrane permeability or efflux mechanism [30]. However, further genetic studies are needed to confirm whether this resistance is due to the acquiring of antimicrobial determinant prior the use as probiotics [31].
Fig. 5.
Growth of isolated Lactic Acid Bacteria at the different pH condition range pH 1.5-6.5 when incubated at 37℃ for 24 hours. The two tailed t-test was used to compare the significant difference in the growth based on OD600 and marked with asterisks when p < 0.05 (standard deviation n = 3).
Table 2.
Determination of minimal inhibitory concentration (MIC) of antibiotics against Lactic Acid Bacterial strains where the experiment was performed triplicate. CIP, ciprofloxacin; CMP, chloramphenicol; VAN, vancomycin; ERY, erythromycin, STR, streptomycin; CLI, clindamycin; GEN, gentamicin; AMP, ampicillin; TET, tetracycline; and KAN, kanamycin.
| LAB strains | MICs ( |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CIP | CMP | VAN | ERY | STR | CLI | GEN | AMP | TET | KAN | |
| C3 | 20 | 2 | 20 | 2.5 | 10 | 10 | 2.5 | 2 | 10 | 20 |
| C4 | 20 | 0.5 | 20 | 0.5 | 20 | 0.5 | 2 | 2.5 | 10 | 20 |
| C6 | 20 | 0.5 | 20 | 0.5 | 10 | 0.5 | 5 | 0.5 | 10 | 10 |
| C8 | 20 | 0.5 | 20 | 0.5 | 10 | 0.5 | 2 | 0.5 | 20 | 10 |
| F6 | 1 | 2.5 | 20 | 5 | 10 | 0.5 | 5 | 0.5 | 20 | 20 |
CIP, ciprofloxacin; CMP, chloramphenicol; VAN, vancomycin; ERY, erythromycin, STR, streptomycin; CLI, clindamycin; GEN, gentamicin; AMP, ampicillin; TET, tetracycline; and KAN, kanamycin.
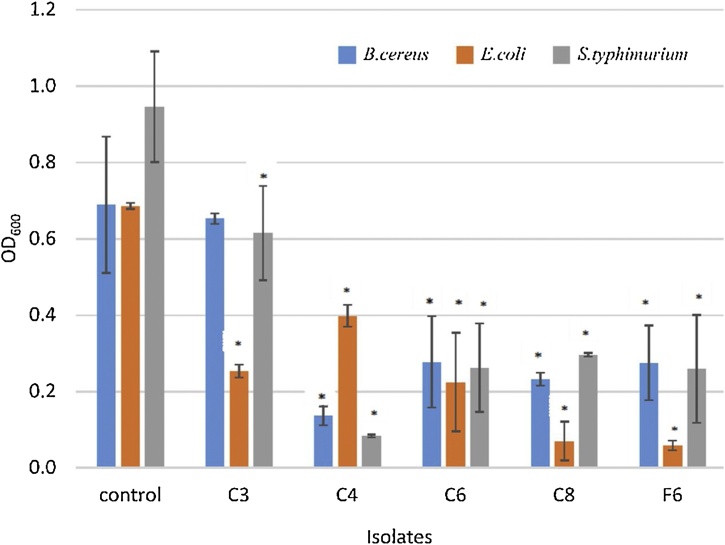
Crude supernatants from all LAB strains were isolated and analysed to determine their effect on biofilm formations of B. cereus, E. coli, and S. typhimurium. The assay was quantitatively analysed using crystal violet staining. Crude supernatants from LAB isolates C4, C6, C8, and F6 showed significant effects on the biofilm formation of B. cereus (Fig. 6). Biofilm development in E. coli and S. typhimurium was significantly affected by the crude supernatants of isolates C3, C4, C6, C8, and F6 to different levels. The C3 crude supernatant produced a significant effect only on gram negative bacterial biofilm formation, while the other supernatants provided broad spectrum effects over biofilms of gram positive and gram negative foodborne pathogens. Isolates C6, C8, and F6 produced a similar level of inhibition against biofilm formation for all pathogens. Inhibition occurred despite any organic acid present in the supernatant being neutralised. The ability of LAB to reduce B. cereus, E. coli, and S. typhimurium biofilms could be mediated by blocking pathogenic biofilm formation and the presence of antimicrobial metabolites. The active compounds produced by the LAB strains could inhibit biofilm formation in B. cereus, E. coli, and S. typhimurium within 24 hours. L. lactis has been reported to produce bacteriocins against biofilm formations of Listeria monocytogenes, S. typhimurium, and E. coli [13].
Fig. 6.
Antimicrobial activities of the crude supernatant from Lactic Acid Bacterial isolates (C3, C4, C6, C8, and F6) against biofilm formations of B. cereus, E. coli, and S. Typhimurium. The crystal violet staining the attached cells after 24 hours exposure to the crude supernatant from the isolates. The two tailed t-test was used to compare the significant difference in the growth based on OD600 and marked with asterisks when p < 0.05 (standard deviation n = 3).
Enterococcus strains have been used in human systemic health as probiotic supplements and functional food to prevent gastrointestinal diseases. Enterococci are facultative anaerobic, gram-positive cocci that form a part of animal and human gastrointestinal tracts. They are also frequently found in fermented food such as cheese and meat, vegetables and olives [32,[33], [34]]. E. faecium has been found in the faeces of healthy humans, a factor that led to its use as a probiotic. It has been found to inhibit the biofilm formation of streptococci, which normally creates oral biofilms [35]. There are various strains of P. pentosaceus that exhibit broad spectrum antimicrobial activities against foodborne pathogens, and also prevent adhesion of pathogenic microbes to surfaces [36]. According to the previous study, P. pentosaceus have been foundto produce antimicrobial compounds against planktonic cultures and biofilm formations of B. cereus, E. coli, and S. typhimurium. Isolated LAB strains could form biofilms and some strains developed a thick EPS. Biofilms of LAB strains could also prevent the adhesion of foodborne pathogens on the tested PS surface. This was mediated through co-aggregation during biofilm formation, in which the LAB isolates were at an advantage due to their higher levels of auto-aggregation. These LAB strains showed partial probiotic characteristics and would not show a risk to the consumer based on their negative hemolytic tests and antibiotic sensitivities. Based on the above work, these LAB strains could inhibit the growth of foodborne pathogens and minimise the rate of pathogenic bacterial adhesion. However, the isolated LABs from this work still need other assessments of probiotic properties including the amplification of known Enterocin genes, adhesion ability to human intestinal cells, cholesterol assimilation, bsh acivity, lipolytic activity, toleration under stimulated gastrointestinal tract, potentiality of yogurt culture, homo/ heterofermentative characterization and antioxidative activity [[37], [38], [39]].
4. Conclusions
LAB isolated from fermented food were analysed for their potential to develop protective biofilms and possible application as probiotics. They were able to produce active compounds that inhibit biofilm development and cell proliferation of various foodborne pathogens. Further work needs to be carried out to determine the mechanisms by which LAB biofilms and their active compounds inhibit foodborne pathogens. In future, we hope to use these LAB in vivo as probiotics for human and animal feed supplements, however, other assessments of probiotic properties must be performed prior to confer the usage. The antimicrobial activity of the isolated LAB could also be considered for use in the food processing industry for sanitizing purposes.
Declaration of Competing Interest
The authors have no conflict of interests.
CRediT authorship contribution statement
Tatsaporn Todhanakasem: Conceptualization, Methodology, Funding acquisition, Writing - review & editing, Formal analysis, Validation. Kornkanok Ketbumrung: Investigation, Data curation.
Acknowledgements
This work was financially supported by Assumption University Research Grant (RP 60010).
The authors are grateful to Dr. Pattanop Kanokratana, Enzyme laboratory, National Center for Genetic Engineering and Biotechnology (BIOTEC), Pathumthani, Thailand for his technical assistance.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2020.e00477.
Contributor Information
Todhanakasem Tatsaporn, Email: tatsaporntdh@au.edu.
Ketbumrung Kornkanok, Email: kornkanok-m@outlook.com.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Abriouel H., Benomar N., Cobo A., Caballero N., Fuentes M.Á.F., Pérez-Pulido R., Gálvez A. Characterization of Lactic Acid Bacteria from naturally-fermented Manzanilla Aloreña green table olives. Food Microbiol. 2012;32:308–316. doi: 10.1016/j.fm.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Sornplang P., Leelavatch V., Sukon P., Yowarach S. Antibiotic resistance of Lactic Acid Bacteria isolated from a fermented fish product, pla-chom. Res J Microbiol. 2011;6:898–903. [Google Scholar]
- 3.Wan L.Y.M., Chen Z.J., Shah N.P., El-Nezami H. Modulation of intestinal epithelial defense responses by probiotic bacteria. Crit. Rev. Food Sci. Nutr. 2016;56:2628–2641. doi: 10.1080/10408398.2014.905450. [DOI] [PubMed] [Google Scholar]
- 4.Lindgren S.E., Dobrogosz W.J. Antagonistic activities of Lactic Acid Bacteria in food and feed fermentations. FEMS Microbiol. Rev. 1990;7:149–163. doi: 10.1111/j.1574-6968.1990.tb04885.x. [DOI] [PubMed] [Google Scholar]
- 5.Gandhi M., Chikindas M.L. Listeria: a foodborne pathogen that knows how to survive. Int. J. Food Microbiol. 2007;113:1–15. doi: 10.1016/j.ijfoodmicro.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Todhanakasem T. Microbial biofilm in the industry. Afr J Microbiol Res. 2013;7:1625–1634. [Google Scholar]
- 7.Hood S.K., Zottola E.A. Adherence to stainless steel by foodborne microorganisms during growth in model food systems. Int. J. Food Microbiol. 1997;37:145–153. doi: 10.1016/s0168-1605(97)00071-8. [DOI] [PubMed] [Google Scholar]
- 8.Ryu J.H., Beuchat L.R. L.R., Biofilm formation and sporulation by Bacillus cereus on a stainless steel surface and subsequent resistance of vegetative cells and spores to chlorine, chlorine dioxide, and a peroxyacetic acid–based sanitizer. J. Food Prot. 2005;68:2614–2622. doi: 10.4315/0362-028x-68.12.2614. [DOI] [PubMed] [Google Scholar]
- 9.Tourasse N.J., Helgason E., Økstad O.A., Hegna I.K., KolstØ A. The Bacillus cereus group: novel aspects of population structure and genome dynamics. J. Appl. Microbiol. 2006;101:579–593. doi: 10.1111/j.1365-2672.2006.03087.x. [DOI] [PubMed] [Google Scholar]
- 10.Kearns D.B., Chu F., Branda S.S., Kolter R., Losick R. A master regulator for biofilm formation by Bacillus subtilis. Mol. Microbiol. 2005;55:739–749. doi: 10.1111/j.1365-2958.2004.04440.x. [DOI] [PubMed] [Google Scholar]
- 11.Van Houdt R., Michiels C.W. Biofilm formation and the food industry, a focus on the bacterial outer surface. J. Appl. Microbiol. 2010;109:1117–1131. doi: 10.1111/j.1365-2672.2010.04756.x. [DOI] [PubMed] [Google Scholar]
- 12.Kubota H., Senda S., Nomura N., Tokuda H., Uchiyama H. Biofilm formation by Lactic Acid Bacteria and resistance to environmental stress. J. Biosci. Bioeng. 2008;106:381–386. doi: 10.1263/jbb.106.381. [DOI] [PubMed] [Google Scholar]
- 13.Gómez N.C., Ramiro J.M.P., Quecan B.X.V., de Melo Franco B.D.G. Use of potential probiotic Lactic Acid Bacteria (LAB) biofilms for the control of Listeria monocytogenes, Salmonella typhimurium, and Escherivachia coli O157: H7 biofilms formation. Front. Microbiol. 2016;7:863. doi: 10.3389/fmicb.2016.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garriga M., Pascual M., Monfort J.M., Hugas M. Selection of lactobacilli for chicken probiotic adjuncts. J. Appl. Microbiol. 1988;84:125–132. doi: 10.1046/j.1365-2672.1997.00329.x. [DOI] [PubMed] [Google Scholar]
- 15.Maya K., Singh R.S., Upadhyay S.N., Dubey S.K. Kinetic analysis reveals bacterial efficacy for biodegradation of chlorpyrifos and its hydrolyzing metabolite TCP. Process Biochem. 2011;46:2130–2136. [Google Scholar]
- 16.Todhanakasem T., Young G.M. Loss of flagellum-based motility by Listeria monocytogenes results in formation of hyperbiofilms. J. Bacteriol. 2008;190:6030–6034. doi: 10.1128/JB.00155-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collado M.C., Meriluoto J., Salminen S. Adhesion and aggregation properties of probiotic and pathogen strains. Eur. food Res. Technol. 2008;226:1065–1073. [Google Scholar]
- 18.García-Cayuela T., Korany A.M., Bustos I., de Cadiñanos L.P.G., Requena T., Peláez C., Martínez-Cuesta M.C. Adhesion abilities of dairy Lactobacillus plantarum strains showing an aggregation phenotype. Food Res. Int. 2014;57:44–50. [Google Scholar]
- 19.Millette M., Cornut G., Dupont C., Shareck F., Archambault D., Lacroix M. Capacity of human nisin-and pediocin-producing Lactic Acid Bacteria to reduce intestinal colonization by vancomycin-resistant enterococci. Appl. Environ. Microbiol. 2008;74:1997–2003. doi: 10.1128/AEM.02150-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carrillo P.G., Mardaraz C., Pitta-Alvarez S.I., Giulietti A.M. Isolation and selection of biosurfactant-producing bacteria. World J. Microbiol. Biotechnol. 1996;12:82–84. doi: 10.1007/BF00327807. [DOI] [PubMed] [Google Scholar]
- 21.Muñoz M.D.C.C., Benomar N., Lerma L.L., Gálvez A., Abriouel H. Antibiotic resistance of Lactobacillus pentosus and Leuconostoc pseudomesenteroides isolated from naturally-fermented Aloreña table olives throughout fermentation process. Inter. J. Food Microbiol. 2014;172:110–118. doi: 10.1016/j.ijfoodmicro.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 22.Danielsen M., Wind A. Susceptibility of Lactobacillus spp. to antimicrobial agents. Int. J. Food Microbiol. 2003;82:1–11. doi: 10.1016/s0168-1605(02)00254-4. [DOI] [PubMed] [Google Scholar]
- 23.Savadogo A., Ouattara A.T.C., Bassole H.N.I., Traore S.A. Bacteriocins and Lactic Acid Bacteria-a minireview. African J. Biotechnol. 2006;5 [Google Scholar]
- 24.König H., Unden G., Fröhlich J. Must and in Wine. Springer; 2009. Biology of Microorganisms on Grapes. [Google Scholar]
- 25.Djordjevic D., Wiedmann M., McLandsborough L.A. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl. Environ. Microbiol. 2002;68:2950–2958. doi: 10.1128/AEM.68.6.2950-2958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.German B., Schiffrin E.J., Reniero R., Mollet B., Pfeifer A., Neeser J.R. The development of functional foods: lessons from the gut. Trends Biotechnol. 1999;17:492–499. doi: 10.1016/s0167-7799(99)01380-3. [DOI] [PubMed] [Google Scholar]
- 27.Di Bonaventura G., Piccolomini R., Paludi D., D’orio V., Vergara A., Conter M., Ianieri A. Influence of temperature on biofilm formation by Listeria monocytogenes on various food‐contact surfaces: relationship with motility and cell surface hydrophobicity. J. Appl. Microbiol. 2008;104:1552–1561. doi: 10.1111/j.1365-2672.2007.03688.x. [DOI] [PubMed] [Google Scholar]
- 28.Corzo G., Gilliland S.E. Bile salt hydrolase activity of three strains of Lactobacillus acidophilus. J. Dairy Sci. 1999;82:472–480. doi: 10.3168/jds.S0022-0302(99)75256-2. [DOI] [PubMed] [Google Scholar]
- 29.(FEEDAP) E. on A. and P. or S. used in A.F. Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 2012;10:2740. [Google Scholar]
- 30.Ammor M.S., Flórez A.B., Mayo B. Antibiotic resistance in non-enterococcal Lactic Acid Bacteria and bifidobacteria. Food Microbiol. 2007;24:559–570. doi: 10.1016/j.fm.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 31.van Reenen C.A., Dicks L.M.T. Horizontal gene transfer amongst probiotic Lactic Acid Bacteria and other intestinal microbiota: what are the possibilities?, A review. Arch. Microbiol. 2011;193:157–168. doi: 10.1007/s00203-010-0668-3. [DOI] [PubMed] [Google Scholar]
- 32.De Vrese M., Offick B. Bioactive Foods in Promoting Health. Elsevier; 2011. Probiotics and prebiotics: effects on diarrhea; pp. 205–227. [Google Scholar]
- 33.Marteau P., Messing B., Arrigoni E., Briet F., Flourié B., Morin M.C., Rambaud J.C. Do patients with short-bowel syndrome need a lactose-free diet? Nutrition. 1997;13:13–16. doi: 10.1016/s0899-9007(97)90872-8. [DOI] [PubMed] [Google Scholar]
- 34.Marteau P.R., Vrese M., de Cellier C.J., Schrezenmeir J. Protection from gastrointestinal diseases with the use of probiotics Am. J. Clin. Nutr. 2011;73:430s–436s. doi: 10.1093/ajcn/73.2.430s. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki N., Yoneda M., Hatano Y., Iwamoto T., Masuo Y., Hirofuji T. Enterococcus faecium WB2000 inhibits biofilm formation by oral cariogenic streptococci. Int. J. Dent. 2011 doi: 10.1155/2011/834151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bajpai V.K., Han J.H., Rather I.A., Park C., Lim J., Paek W.K., Lee J.S., Yoon J.I., Park Y.H. Characterization and antibacterial potential of Lactic Acid Bacterium Pediococcus pentosaceus 4I1 isolated from freshwater fish Zacco koreanus. Front Microbiol. 2016;7:2037. doi: 10.3389/fmicb.2016.02037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adnan M., Patel M., Hadi S. Functional and health promoting inherent attributes of Enterococcus hirae F2 as a novel probiotic isolated from the digestive tract of the freshwater fish. Catla catla. PeerJ. 2017;5:e3085. doi: 10.7717/peerj.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alshammari E., Patel M., Sachidanandan M., Kumar P., Adnan M. Potential Evaluation and Health Fostering Intrinsic Traits of Novel Probiotic Strain Enterococcus durans F3 Isolated from the Gut of Fresh Water Fish Catla catla. Food Sci. Anim. Resour. 2019;39:844. doi: 10.5851/kosfa.2019.e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hegarty J.W., Guinane C.M., Ross R.P., Hill C., Cotter P.D. Bacteriocin production: a relatively unharnessed probiotic trait? F1000Research. 2016;5 doi: 10.12688/f1000research.9615.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.