Abstract
In January 2020, Novel Coronavirus Disease 2019 (COVID-19) resulted in a global pandemic, creating uncertainty toward the management of liver transplantation (LT) programs. Lombardy has been the most affected region in Italy: the current mortality rate of COVID-19 patients is 18.3% (10 022 deaths; April 10th) with hospitals in Lombardy having to expand the total number of ICU beds from 724 to 1381 to accommodate infected patients. There has been a drastic decrease in liver donors. From February 23rd until April 10th, 17 LTs were performed in Lombardy. Mean donor age was 49 years (range 18-74) whereas mean recipient age was 55 (13-69); mean MELD score was 12 (6-24). All donors underwent screening for SARS-CoV-2 prior to LT. Two patients tested positive after LT, and one patient died for COVID on POD 30. Sixteen patients are alive after an average of 30 days post-LT (range 3-46). 10 patients have been discharged. This study has found no specific reason concerning the safety of recipients, to stop LT programs. Several key lessons from our experience are reported. However, due to the complex circumstances which surround the viral outbreak, the cessation or a reduction in LT activity is a pragmatic requirement.
KEYWORDS: bronchoalveolar lavage (BAL), clinical research/practice, critical care/intensive care management, epidemiology, infection and infectious agents – viral, liver transplantation/hepatology, organ allocation
Abbreviations: BAL, brochoalveolar lavage; CNT, Centro Nazionale Trapianti; CoV, coronavirus; COVID, coronavirus disease; CPAP, continuous positive airway pressure; ICU, intensive care unit; INMI, Istituto Nazionale Malattie Infettive; LT, liver transplantation; MERS, Middle East respiratory syndrome; NITp, North Italy Transplant program; POD, postoperative day; SARS, severe acute respiratory syndrome; WHO, World Health Organization
1. INTRODUCTION
In December 2019, the world saw the initial reports of a new respiratory illness in patients from Wuhan, China. The disease rapidly spread throughout China and was later identified as the Novel Coronavirus Disease 2019 (COVID-19), resulting in a global pandemic.
The first cases of COVID 19 in Italy were confirmed in 2 Chinese tourists on January 30, 2020. On February 18, the first documented secondary transmission was identified in an Italian citizen and shortly afterwards, Northern Italy experienced an alarming acceleration in the number of confirmed cases.
Liver transplantation (LT) is a well-established procedure in Italy for end-stage liver diseases, with 1031 patients on the waiting list in December 2019.1
Lombardy, the most highly affected region in Italy, is a region where there are currently four active liver transplant programs (Ospedale Niguarda in Milan, Fondazione IRCCS Policlinico in Milan, Istituto dei Tumori in Milan, and Ospedali Riuniti in Bergamo).
There is currently a large degree of uncertainty toward the management of LT programs during the coronavirus outbreak. Our aim is therefore to share our observations on the coronavirus outbreak in order to demonstrate the impact that such a disease may have on liver transplant programs.
Specifically, we aim to identify:
-
1
If the outbreak of coronavirus has led to a decrease in the number of LTs, and the reasons for this.
-
2
The effect of the coronavirus outbreak, whether direct or indirect on the survival of liver transplant patients, including their risk of infection with resulting morbidity and measures to be kept to prevent SARS-CoV-2 infections in LT patients.
2. PATIENTS AND METHODS
Regional (Lombardy) data on patients with confirmed COVID-19 were gathered prospectively from January 30, 2020 onward.
We compared data regarding liver donations in NITp area (Lombardy, Veneto, Liguria, Friuli Venezia Giulia, Trento, and Marche), to the number of COVID positive patients in Lombardy, through the North Italy Transplant program (NITp) Database.
We analyzed the number of liver donations performed in Lombardy from January to March 2020. We calculated percent reduction and 95% confidence intervals (95% CI) of mean weekly counts of donors (referred and recovered) in the 4 weeks period from February 23 (period 1) with those in the previous 8 weeks (period 0), using univariate Poisson regression. Statistical analysis was performed with Stata 16 (StataCorp. 2019).
We prospectively collected data on LTs performed in Lombardy before and after the onset of the outbreak.
Data regarding donors (age, laboratory data, ICU days, BMI, city of residence, graft), recipients (age, gender, basic laboratory data, MELD score, BMI, disease, city of residence, immunosuppression, hospitalization days, outcome, surgical and medical complications, tests for coronavirus), LT (date, ischemia time), and retransplantation procedures performed in the Lombardy Region were collected prospectively from February 23, 2020 onward.
The survival of liver-transplanted patients from February 23, 2020 to April 10, 2020 was documented.
The study was approved by the Ethical Committee of the promoting center.
2.1. Timeline of events
The first two confirmed cases of COVID-19 in Italy were identified on January 30, 2020 at the Istituto Nazionale Malattie Infettive Lazzaro Spallanzani (INMI) in Rome, one of the leading tertiary centers for infectious diseases in Italy. The patients were reported to be 2 Chinese tourists who were isolated on January 29 and discharged on February 26. In response, Italy declared a state of emergency, suspending all incoming and outgoing flights to China.
The first documented case of secondary transmission was identified on February 18 in Codogno, a town of 15 000 inhabitants southeast of Milan within the Lombardy region of Northern Italy.
Afterward, the number of confirmed cases of COVID-19 slowly grew, until February 23, when suddenly 121 new cases were confirmed.
On February 24 the National Department of Transplantation (Centro Nazionale Trapianti: CNT) issued a directive2 stating that all deceased donors from 5 Northern Regions of Italy (Lombardy, Veneto, Piemonte, Emilia Romagna, and Trento) were to be tested for SARS-CoV-2 prior to transplantation. Broncho Alveolar Lavage (BAL), if positive, would result in the transplantation process to be aborted. On March 6, the recommendation was extended to donors in all Italian regions. On March 16, the CNT released a new directive, stating that additionally, all recipients were to be screened prior to a transplantation via nasal pharyngeal swabbing.
From February 24, infections and deaths from SARS-CoV-2 in Lombardy rose sharply (see Figure 1).
FIGURE 1.
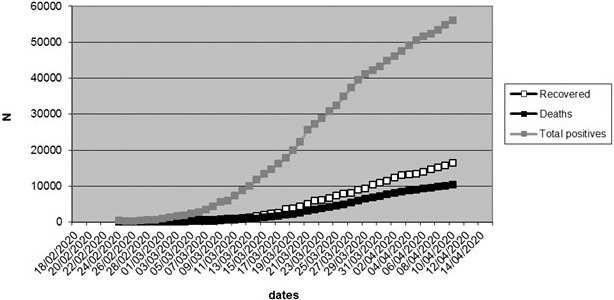
The number of confirmed SARS-CoV-2 cases in Lombardy
The Councils of Ministers enacted new 9 decree-law3 (from February 23 to March 22) in order to contain the outbreak. It was initially decided that more than 50 000 people from 11 different municipalities in Northern Italy were to be quarantined; however, shortly afterward, this was expanded, leading to the cessation of all commercial activity across Italy (March 21), with the exception of supermarkets and pharmacies.
3. RESULTS
From February 23, 2020 until March 22, liver donation in the NITp area decreased. The comparison of data from the January to end of March indicates a drastic reduction in the donor rate. Donors referred and recovered before and after the onset of the outbreak in Lombardy are reported in Figure 2. Referred donors dropped from 9.6 (period 0) to 4.0 (period 1) per week (−58%, 95% CI: −44% to −73%). Recovered donors dropped from 5.4 (period 0) to 3.0 (period 1) per week (−46%, 95% CI: −71% to +6%).
FIGURE 2.
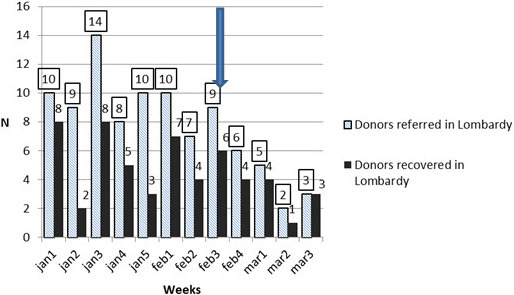
Donors referred and recovered in Lombardy before and after the onset of outbreak (the arrow shows the beginning of the outbreak) [Color figure can be viewed at wileyonlinelibrary.com]
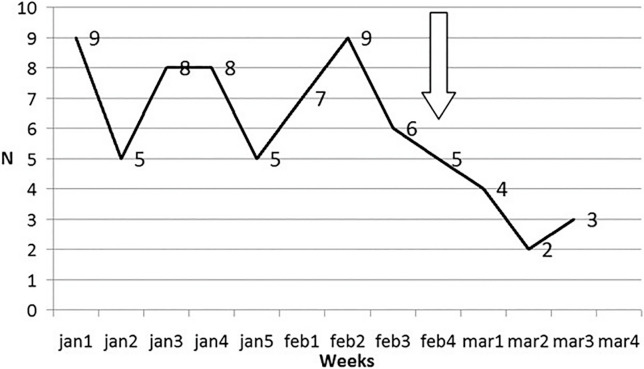
LTs in Lombardy also decreased ( Figure 3) from the 4th week of February onward.
FIGURE 3.
Liver transplantations in Lombardy before and after the onset of outbreak (the arrow shows the beginning of the outbreak)
In Italy there are approximately 5090 ICU beds.4 The number of curative care beds5 in Italy in 2017 were estimated at 2.62 per 100 000 inhabitants, and 3.72 in the European Union. Existing data on curative care beds for comparison have reported that Germany has 6.01/100 000 inhabitants. Current European data on ICU beds do not allow for the direct comparison of different countries.
In mid-February there were 724 ICU beds in the Lombardy region. In response to the outbreak, by March 19 this was increased to 1250. Then, a reported 1381 patients were being treated in ICU by April 4 indicative of an acute influx of COVID-19 patients requiring treatment in secondary care ( Figures 4 and 5).
FIGURE 4.
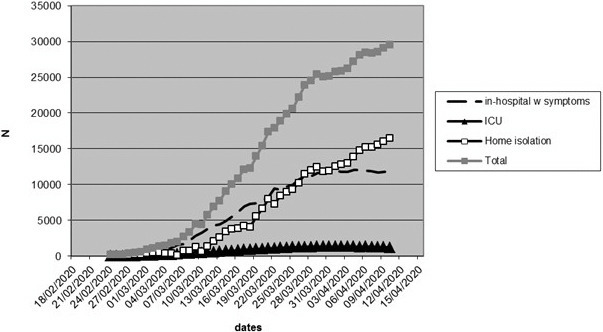
The treatment location of SARS-CoV-2-infected patients in Lombardy
FIGURE 5.
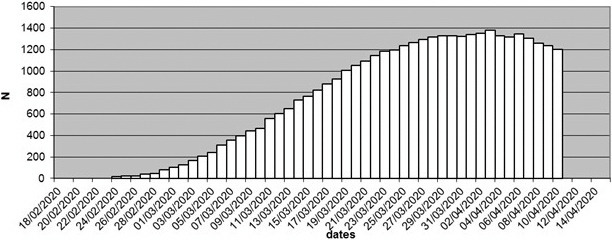
Intensive care beds with COVID patients in Lombardy during SARS-CoV-2 outbreak
The current mortality rate in COVID patients in Italy is 12.7% (18 279 deaths; April 10) and 18.3% (10 022 deaths) in Lombardy.
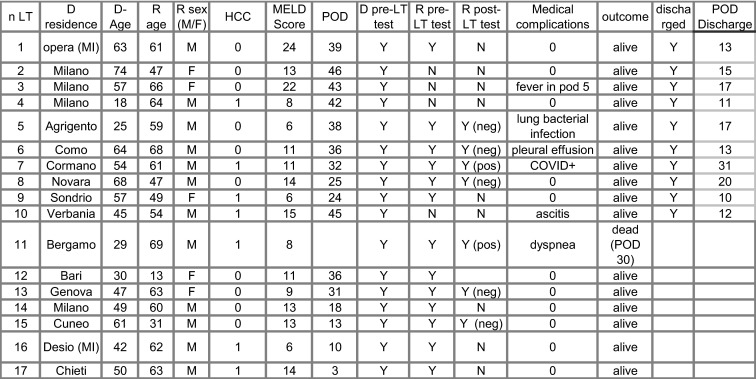
From February 23 to April 10, 16 LTs and 1 retransplantation were performed in Lombardy in 16 adults and 1 pediatric patient. All donors except 1 and all recipients except 3 were from Northern Italy. Mean donor age was 49 years (range 18-74) whereas recipient mean age was 55 (13-69); 6 patients were affected by hepatocellular carcinoma. One was HIV pos; the mean MELD score was 12 (6-24). All donors underwent screening for SARS-CoV-2 prior to LT with BAL and 13 recipients were tested with nasal pharyngeal swabbing prior to LT. All patients except 4 were treated with Basiliximab, Prednisolone, and Tacrolimus after LT. Medical complications occurred in 6 patients and in 5 patients repeated screening for coronavirus was performed. Of these 5 patients, 2 patients, males, aged 61 and 69, resulted positive. The first patient manifested with fever on postoperative day (POD) 9 but had a normal chest x-ray findings. The second patient, HIV pos, had positivity for SARS-CoV-2 on POD 22 and died with COVID-19 on POD 30; 16 patients are currently alive after a mean time of 30 days post-LT (range 3-46). A total of 10 patients have been discharged after a mean time of 16 days (range 11-31) and are being follow-up as outpatients.
Data regarding donors, recipients, and transplantation procedures are reported in Figure 6.
FIGURE 6.
Main donor and recipient data of LTs performed during SARS-CoV-2 outbreak in Lombardy. All “D residences” are towns from Northern Italy except for Agrigento. D, donor; R, recipient; Y, yes; N, no; F, female; M, male; POD, postoperative day
4. DISCUSSION
The World Health Organization (WHO) declared COVID-19 a Public Health Emergency of International Concern as of February 1, 2020 and declared as a pandemic on March 11, 2020.
Over recent decades6 coronavirus has been responsible for several outbreaks, including the 2002 SARS (Severe acute respiratory syndrome) outbreak in Guandong Province, China, and the 2012 MERS (Middle East respiratory syndrome) outbreak in Saudi Arabia.
The current SARS-CoV-2 is highly contagious and as to date (April 10, 2020) 1 687 857 cases have been identified worldwide with 102 198 deaths. The mortality rate in closed cases (recovered and deceased) is 21%.7
A total of 81 907 cases have been reported in China since the outbreak (April 10, 2020)7; however, the rapid doubling time of cases and spread in other countries has been alarming.
For example in Italy, only a handful of cases were reported on January 30, but by February 23, Italy had reported 121 cases8 and by April 10, 147 577 cases.
4.1. Liver transplantation and the COVID-19 outbreak
The first LT in Italy dates back to 1983, and to date (December 2019) 24 518 LT have been performed.1
There are currently 21 active liver transplant units, and according to European Liver Transplant Registry9 these units are operating at the rate of more than 1000 liver transplants per year. In 2019, 1302 liver transplants were performed, including 1278 liver transplants from brain-dead donors.10
As the impact of COVID-19 during the start of the outbreak was not well established, the National Department of Transplantation (Centro Nazionale Trapianti: CNT) opted to continue with LTs, as they are regarded as a life-saving procedure. These procedures were to continue, despite concerns regarding the safety from these donors. Initially the national guidelines stated that only donors had to be screened with BAL for SARS-CoV-2. Donor screening was performed in all cases and negative in all donors. Based on data from Lombardy, the most at risk age group for mortality are those over the age of 5011 with worse prognosis over the age 70. CT chest scans of donors are not always readily available during the current pandemic. However, when available, may represent a further diagnostic advantage. Screening for COVID-19 in recipients has only recently been (March 16) suggested in Italian national protocols; however, prior to these regulations most transplant units were already performing recipient testing for SARS-CoV-2. We believe that additionally testing the recipient is the best practice as the immunosuppression posttransplant may allow for uncontrolled viral proliferation in previously undiagnosed patients.
Testing performed through BAL samples in donors is likely more sensitive,12 and allows the exclusion of disease in at risk donors. The same cannot be said for recipients who undergo testing via nasal pharyngeal swabbing. A single negative test is likely to be unreliable and the results from BAL cannot be logistically obtained prior to LT due to such strict time constraints. It is therefore a possibility that unrecognized positive patients may undergo LT. If this occurs, the patient may represent an unrecognized super-spreader13 after LT.
We believe that BAL in recipients, even if nasal pharyngeal swabbing is negative, may still represent an important diagnostic test, when the recipient is intubated, either performed at the end of LT, or immediately before. In our first patient who tested positive 9 days after LT, only nasal pharyngeal swabbing was performed; therefore, it is impossible to know if infection was present prior LT or if it was contracted afterwards during recovery in hospital. We therefore recommend BAL in all LT recipients, even if the result may only be available during or soon after LT. This allows for reliable information to be retrieved, aiding both in the prevention of disease in health-care workers and in the management of the patient. In case of BAL positivity during LT, the patient should be transferred in a COVID ICU after LT. The second patient was found to be positive on postoperative 22, therefore it is likely that this represents infection posttransplant.
In recipients a further diagnostic tool for COVID-19 should be a chest CT scan at arrival in the hospital for LT. In case of suspect COVID-19 at pre-LT CT scan, LT should not be performed.
The debate regarding the impact of immunosuppression is currently wide open14 as some authors believe that immunosuppression may be beneficial in COVID-19.
The full natural history of COVID-19 is currently unknown; however, the use biologics such as Basiliximab, a monoclonal antibody to the α chain (CD25) of the IL-2 receptor of T cells, may act toward the attenuation of cytokine storm syndrome.14 The impact of biologics and their associated benefits effects in COVID-19 has yet to be reported. The patient who died among our cases was not treated with basiliximab.
4.2. Current literature on organ transplant and coronavirus
In the literature there have been few papers published regarding LT during outbreaks of coronavirus disease. In the first publication15 during the recent outbreak of SARS-CoV-2, several measures including screening of medical staff, the creation of a donation protocol, and the documentation of pre/postoperative management measures were reported by the Organ Transplant Center, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, University of Electronic Science and Technology in Western China. However, to date no results regarding LTs have been reported.
Michaels,13 on the basis of two cases of kidney transplanted people during the MERS outbreak in 2014 reported by AlGhamdi16 suggests that “due to the need for immunosuppression in solid organ transplant recipients, they may be anticipated to have more intense and prolonged shedding of virus thus potentially increasing the risk of transmission to contacts including health-care workers.”
The two cases of MERS CoV infections in 2014 reported by AlGhamdi were two renal transplant recipients with variable clinical presentations and outcomes. The first patient presented with progressive respiratory symptoms, acute renal failure and died. The second survived. The authors state that although a few changes to the transplant program were made in response to the MERS-CoV endemic, transplant procedures were ultimately stopped during the epidemic.16
In Canada 2003, Kumar17 reported the case of a 74-year-old male who died of SARS 18 days after the onset of symptoms. The patient had undergone LT 9 years prior and as such, due to an outbreak of SARS in the greater Toronto area, all programs in the city were temporarily closed. Prior to restarting the transplant program, a donor SARS screening tool was performed due to risk of coronavirus transmission from undiagnosed organ donors.
Chui18 reported that during the 2003 SARS outbreak in Hong Kong, no LTs were performed, as the intensive care unit was dedicated to the care of SARS patients. The author reminds that “the SARS outbreak demonstrated the vulnerability of an organ transplantation service. The human and financial costs are significant.”
While closely related to SARS-CoV and MERS-CoV, the mortality associated with COVID-19 may be lower than what is reported. However, caution is still required as we do not yet know the full impact of the infection as it spreads to more diverse populations.
4.3. Limiting factors for liver transplantation
Although authorities in Italy have not formally halted the transplant programs across Lombardy, several factors have contributed to a temporary decrease in LTs:
-
1
There has been an overwhelming influx of COVID-19 patients to ICU beds. This has affected both the identification process for potential donors, as well as the availability of beds for recipients.
-
2
ICU doctors have been redeployed in the care of COVID-19 patients, and thus there has been a paucity of ICU specialists for the liver transplant programs.
-
3
There are concerns toward the risk of nosocomial COVID-19 infection in recipients, especially considering the potential contact with asymptomatic carriers within the hospital. Separation of Units into COVID-19-positive and COVID-19-negative patients is mandatory, so that in case of difficulty to obtain that, a LT should not be performed as the risk of infection is too high. For the same reason, from the beginning of the outbreak our hospital has prohibited family/visitors at the bedside. Patients in medical units should wear face coverings such as doctors, nurses, and health-care workers. Moreover, the transfer of patients to radiology units for a chest X-ray represents an additional infection risk. Thus a bedside chest ultrasound may be a useful alternative in these situations. All at risk staff including doctors, nurses, health-care workers, ambulances, endoscopy units, and radiology units should be routinely screened for coronavirus.
-
4
Close clinical monitoring is mandatory if an LT program is intended to continue; no serial testing for SARS-CoV-2 in asymptomatic recipients should be performed in the postoperative period unless there are clinical manifestations such as fever or elevated CRP of undetermined origin. In that case a nasal pharyngeal swabbing test should be performed. If clinical doubts persist it is better to perform a chest CT scan and if positive LT patients should be transferred in a COVID Unit.
-
5
As already reported by others there are concerns regarding the safety of the procurement teams,13 who may be exposed to potentially infected patients during the procurement process. Ideally the procurement team should not be sent to the donor hospital advising the local team at the donor hospital for organ retrieval and sending to the recipient hospital.
If organization and the situation at a local level permits, there must be a careful risk/benefit analysis for performing transplantation, taking into consideration the recipient’s risk of dying of end-stage liver disease or cancer vs the risk of COVID-19. Therefore, during an outbreak, we advise the avoidance of performing LTs in nonurgent cases, with LT reserved to those patients with true end-stage liver disease and extremely poor prognosis.
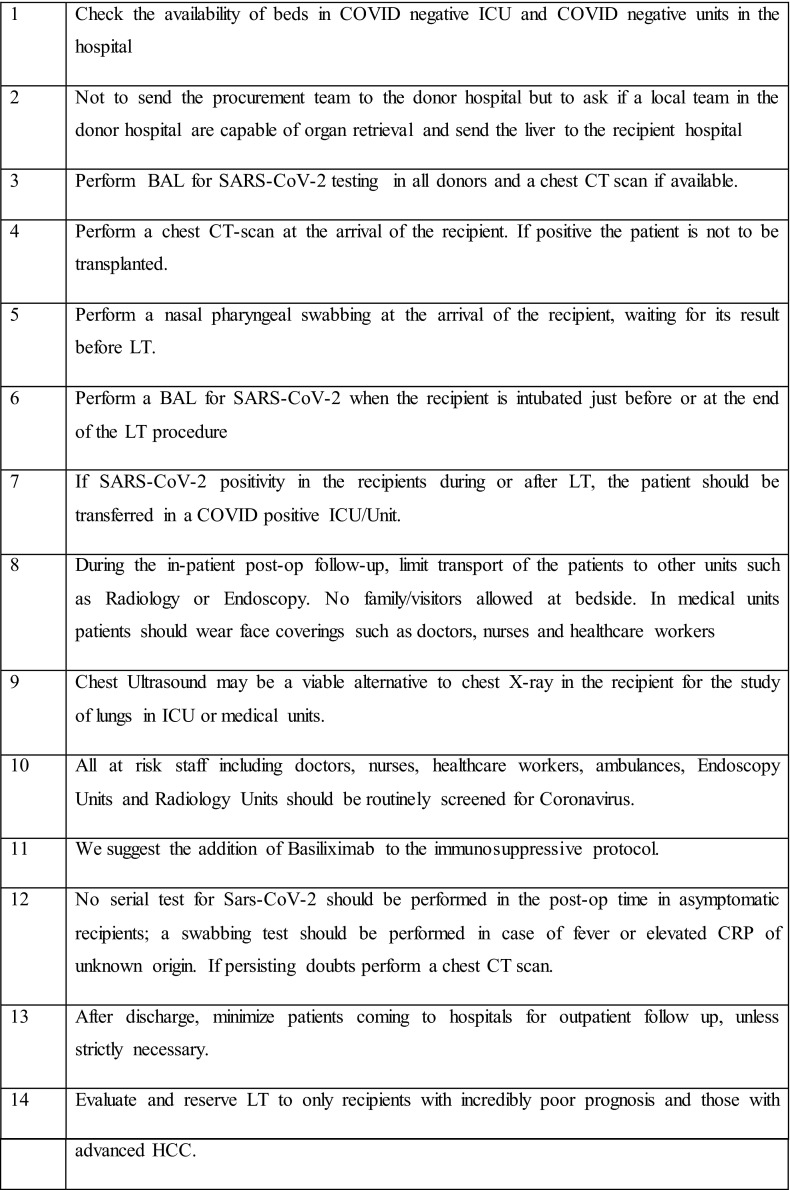
A table summarizing key lessons that we learnt from our experience is reported in Figure 7.
FIGURE 7.
Suggested measures for liver transplantation during SARS-CoV-2 outbreak
4.4. Limits of this study
The primary limit of our study is the short follow-up time for our patients. If serious complications are reported in the future, though the adoption of all measures to obviate them, then the evidence surrounding the management of liver transplant programs during outbreaks should be reconsidered.
Furthermore, it should be noted that data concerning the number of infected patients in Italy from March 1 onward is not likely to be representative of the true number of cases, as testing was limited to only patients who were exhibiting symptoms.
5. CONCLUSION
During the current ongoing outbreak of SARS-CoV-2 in Lombardy, Italy, there is a drastic decrease in the number of LTs performed. The main reasons behind this are the lack of ICU beds, organizational difficulties for ICU workers and concerns for infection in both patients and staff.
On the basis of our results, this study has found no specific reason concerning the safety of liver recipients to stop LT programs. However, due to the complex circumstances which surround the viral outbreak, a rapid cessation or limitation of activity is often required pragmatically.
Over the short follow-up period, the current survival of liver-transplanted patients during the coronavirus outbreak is quite good. However, the positivity of two patients after LT and the death of one of them, raises important questions regarding the facilitation of liver transplants during an outbreak. We suggest that only patients with true end-stage liver disease and extremely poor prognosis should undergo LT. We believe that COVID-19 protocols should include screening for both donors and recipients prior to LT. Testing both patient populations advocates not only for the safety of the recipients but also of the health workers too. BAL is the preferred method for recipient screening, either performed prior or immediately after the transplant procedure as this allows for rapid availability of reliable results. Different measures and precautions are to be taken in order to promote the safety of LT during the coronavirus outbreak.
Acknowledgments
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
Data are available from the authors on request.
REFERENCES
- 1.CNT. Attività di donazione e trapianto di organi. http://www.trapianti.salute.gov.it/imgs/C_17_cntPubblicazioni_351_allegato.pdf. Published 2020. Accessed April 10, 2020.
- 2.CNT. Aggiornamento delle misure di prevenzione. http://www.trapianti.salute.gov.it/imgs/C_17_cntAvvisi_225_0_file.pdf. Published 2020. Accessed April 10, 2020.
- 3.Il_Sole_24_ore. Coronavirus, tutti i provvedimenti economici e sanitari varati dal Governo. https://www.ilsole24ore.com/art/elenco-provvedimenti-ADyvx9C. Published 2020. Accessed April 10, 2020.
- 4.AGI. Annuario Statistico del Servizio SanitarioNazionale. https://www.agi.it/fact-checking/news/2020-03-06/coronavirus-posti-letto-ospedali-7343251/. Published 2020. Accessed April 10, 2020.
- 5.Eurostat. Hospital beds by type of care. https://appsso.Eurostat.ec.europa.eu/nui/submitViewTableAction.do. Published 2017. Accessed April 10, 2020.
- 6.Peeri NC, Shrestha N, Rahman MS, et al. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? [published online ahead of print 2020]. Int J Epidemiol. 10.1093/ije/dyaa033 [DOI] [PMC free article] [PubMed]
- 7.Worldometers. Covid-19 Coronavirus outbreak. https://www.worldometers.info/. Published 2020. Accessed April 10, 2020.
- 8.Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395(10231):1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ELTR. European Liver Transplant Registry. http://www.eltr.eu/. Accessed April 10, 2020.
- 10.Min.Salute. http://www.trapianti.salute.gov.it/trapianti/archivioDatiCnt.jsp. Published 2020. Accessed April 10, 2020.
- 11.GEDIVISUAL. Decessi complessivi per classi d’età. https://lab.gedidigital.it/gedi-visual/2020/coronavirus-i-contagi-in-italia/?ref=RHPPTP-BH-I249591240-C12-P2-S1.8-L. Published 2020. Accessed April 10, 2020.
- 12.Loeffelholz MJ, Tang YW. Laboratory diagnosis of emerging human coronavirus infections - the state of the art. Emerg. Microb Infect. 2020;9(1):747–756. doi: 10.1080/22221751.2020.1745095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michaels MG, La Hoz RM, Danziger-Isakov L, et al. Coronavirus disease 2019: implications of emerging infections for transplantations [published online ahead of print February 24, 2020]. Am J Transplant. 10.1111/ajt.15832 [DOI] [PMC free article] [PubMed]
- 14.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan L, Zeng J, Yang H. Challenges and countermeasures for organ donation during the SARS-CoV-2 epidemic: the experience of Sichuan Provincial People’s Hospital. Intensive Care Med. 2020. 10.1007/s00134-020-05978-8 [DOI] [PMC free article] [PubMed]
- 16.AlGhamdi M, Mushtaq F, Awn N, Shalhoub S. MERS CoV infection in two renal transplant recipients: case report. Am J Transplant. 2015;15(4):1101–1104. doi: 10.1111/ajt.13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar D, Tellier R, Draker R, Levy G, Humar A. Severe Acute Respiratory Syndrome (SARS) in a liver transplant recipient and guidelines for donor SARS screening. Am J Transplant. 2003;3(8):977–981. doi: 10.1034/j.1600-6143.2003.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chui AK, Rao AR, Chan HL, Hui AY. Impact of severe acute respiratory syndrome on liver transplantation service. Transpl Proc. 2004;36(8):2302–2303. doi: 10.1016/j.transproceed.2004.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the authors on request.