Abstract
Background
Antiviral drugs are administered in patients with severe COVID‐19 respiratory syndrome, including those treated with direct oral anticoagulants (DOACs). Concomitant administration of antiviral agents has the potential to increase their plasma concentration. A series of patients managed in the Cremona Thrombosis Center were admitted at Cremona Hospital for SARS‐CoV‐2 and started antiviral drugs without stopping DOAC therapy. DOAC plasma levels were measured in hospital and results compared with those recorded before hospitalization.
Methods
All consecutive patients on DOACs were candidates for administration of antiviral agents (lopinavir, ritonavir, or darunavir). Plasma samples for DOAC measurement were collected 2to 4 days after starting antiviral treatment, at 12 hours from the last dose intake in patients on dabigatran and apixaban, and at 24 hours in those on rivaroxaban and edoxaban. For each patient, C‐trough DOAC level, expressed as ng/mL, was compared with the one measured before hospitalization.
Results
Of the 1039 patients hospitalized between February 22 and March 15, 2020 with COVID‐19 pneumonia and candidates for antiviral therapy, 32 were on treatment with a DOAC. DOAC was stopped in 20 and continued in the remaining 12. On average, C‐trough levels were 6.14 times higher during hospitalization than in the pre‐hospitalization period.
Conclusion
DOAC patients treated with antiviral drugs show an alarming increase in DOAC plasma levels. In order to prevent bleeding complications, we believe that physicians should consider withholding DOACs from patients with SARS‐CoV‐2 and replacing them with alternative parenteral antithrombotic strategies for as long as antiviral agents are deemed necessary and until discharge.
Keywords: anticoagulant, antiviral agents, COVID‐19, DOAC, plasma level
Essential
-
•
Antiviral agents potentially increases DOAC (direct oral anticoagulant) plasma levels.
-
•
Patients on DOAC with severe COVID‐19 respiratory syndrome started antiviral drugs in hospital.
-
•
DOAC plasma levels were measured and compared with those recorded before hospitalization.
-
•
DOAC patients treated with antiviral drugs show an alarming increase in DOAC plasma levels.
-
•
Physicians should consider withholding DOACs, replacing with parenteral anticoagulant drugs.
Alt-text: Unlabelled Box
1. INTRODUCTION
The World Health Organization on March 11, 2020 declared the novel coronavirus infection COVID‐19 a global pandemic.1., 2. Italy, particularly the area of Cremona located in the northern region of the country, was notified as the first European country in which severe acute respiratory syndrome due to SARS‐CoV‐2 was spreading.2 Currently, we are observing an increasing number of patients treated with direct oral anticoagulants (DOACs)—dabigatran, apixaban, rivaroxaban, and edoxaban—hospitalized with severe COVID‐19 infection. DOACs are indicated for the prevention of stroke and systemic embolism in patients with non‐valvular atrial fibrillation (NVAF) and for the prevention and treatment of venous thromboembolism.3 At present DOACs are administered at fixed dose without indications for dose adjustment based on laboratory testing,3., 4. even if a high inter‐individual variability in drug blood levels was shown and an association between DOAC plasma levels and thrombotic and bleeding complications was observed.5., 6., 7., 8., 9., 10., 11., 12.
Patients treated with DOACs should receive multiple drug treatment during hospitalization for severe COVID‐19 respiratory syndrome that may include antiviral therapies (lopinavir/ritonavir, darunavir), tocilizumab (humanized monoclonal antibody against the interleukin‐6 receptor), chloroquine or hydroxychloroquine, antibiotics, steroids, nonsteroidal anti‐inflammatory drugs, bronchodilators, and immunosuppressive drugs.13., 14., 15., 16. As previously reported, antiviral therapies strongly interact with DOACs, because both are substrates of the P‐glycoprotein and/or cytochrome P450‐based metabolic pathways.17., 18. Therefore the concomitant administration of DOACs and antiviral drugs has the potential to sharply increase DOAC anticoagulant plasma level, thus increasing hemorrhagic risk.
In addition to multiple drug‐drug interactions, also metabolic alterations induced by the acute disease can cause unpredictable and unstable DOAC anticoagulant effects, exposing patients to the risk of uncontrolled bleeding or thrombotic complications.17., 18., 19., 20.
A series of patients, chronically managed in the Cremona Thrombosis Center for anticoagulant treatment with DOAC, were hospitalized at Cremona Hospital for severe SARS‐CoV‐2 respiratory syndrome. They started antiviral drugs without stopping DOAC therapy.
During hospitalization DOAC plasma levels were measured and the results were compared with those recorded in the same patients at the Thrombosis Center before hospitalization.
2. METHODS
All consecutive patients admitted to Cremona Hospital (Northern Italy) with COVID‐19 pneumonia were eligible for this investigation, provided they were on anticoagulant treatment with a DOAC (apixaban, rivaroxaban, edoxaban, or dabigatran) for prevention or treatment of cardiovascular disorders and were candidates for administration of antiviral agents (lopinavir, ritonavir, or darunavir).
Plasma samples were collected within 2 to 4 days after starting antiviral treatment, at 12 hours from the last dose intake in patients on dabigatran and apixaban, and at 24 hours in those on rivaroxaban and edoxaban. DOAC levels, expressed as drug concentration‐equivalent (ng/mL), were measured using echarin chromogenic assay calibrated for dabigatran, and specific anti‐factor Xa (FXa) assays calibrated for apixaban and rivaroxaban (Stago).21
For each patient, C‐trough DOAC level was compared with the one measured at our Thrombosis Center before hospitalization where a structured follow‐up is applied, including periodical clinical evaluation, laboratory tests for renal function, blood cell count, and DOAC plasma measurement at steady state.11., 12.
The study has been approved by the local Ethics Committee. All patients gave their written informed consent before enrolment and the research was conducted according to the World Medical Association Declaration of Helsinki.
3. RESULTS
Of the 1039 patients hospitalized between February 22 and March 15, 2020 with COVID‐19 pneumonia and candidates for antiviral therapy, 32 were on treatment with a DOAC. Based on the decision of attending physicians, the drug was discontinued in 20, and continued in the remaining 12 (5 patients on apixaban, 3 patients on rivaroxaban, 3 patients on edoxaban, and 1 patient on dabigatran). Eight out of twelve were males, mean age was 80 years (69‐89 years); half of them were on low DOAC doses. They all had concomitant administration of hydroxychloroquine and azithromycin or levofloxacin.
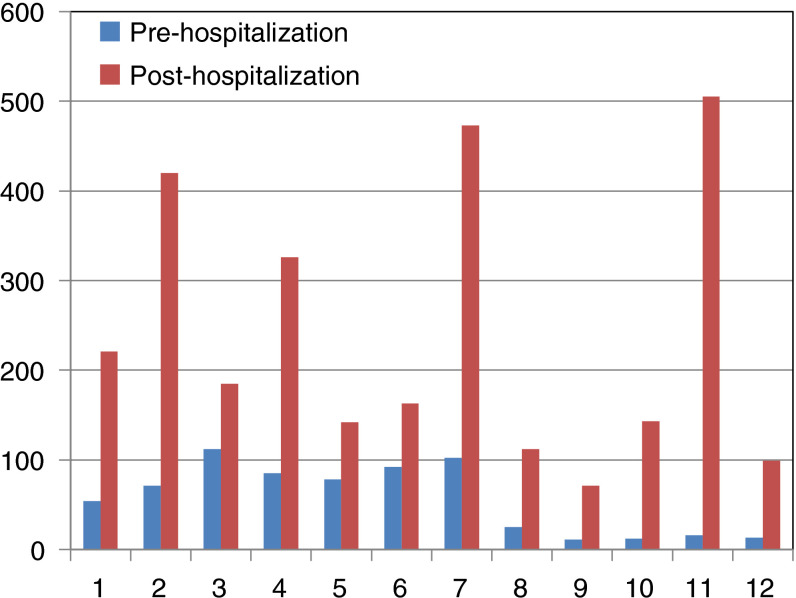
Table 1 details for each patient the main characteristics as well as C‐trough DOAC plasma levels before and during hospitalization, also shown in Figure 1 . On average, C‐trough levels were 6.14 times higher during hospitalization than in pre‐hospitalization period.
Table 1.
Main clinical characteristics and C‐trough DOAC levels in the cohort of patients
| Number | Age | Sex | Clinical indication | DOAC | Dose mg/d | Antiviral drugs | C‐trough (ng/mL) Pre‐hospitalization | C‐trough (ng/mL) In‐hospital | Δ % |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 86 | M | NVAF | Dabigatran | 110 × 2 | Lopinavir/Ritonavir | 54 | 221 | +309.3 |
| 2 | 89 | F | NVAF | Apixaban | 2.5 × 2 | Lopinavir/Ritonavir | 71 | 420 | +491.5 |
| 3 | 74 | M | NVAF | Apixaban | 5 × 2 | Darunavir/Ritonavir | 112 | 185 | +65.2 |
| 4 | 69 | F | NVAF | Apixaban | 5 × 2 | Lopinavir/Ritonavir | 85 | 326 | +283.5 |
| 5 | 77 | M | VTE | Apixaban | 5 × 2 | Darunavir/Ritonavir | 78 | 142 | +82 |
| 6 | 73 | M | NVAF | Apixaban | 5 × 2 | Lopinavir/Ritonavir | 92 | 163 | +77.2 |
| 7 | 80 | F | NVAF | Edoxaban | 60 | Lopinavir/Ritonavir | 102 | 473 | +363.7 |
| 8 | 89 | M | NVAF | Edoxaban | 30 | Darunavir/Ritonavir | 25 | 112 | +348 |
| 9 | 85 | M | NVAF | Edoxaban | 30 | Darunavir/Ritonavir | 11 | 71 | +545.5 |
| 10 | 82 | M | NVAF | Rivaroxaban | 15 | Lopinavir/Ritonavir | 12 | 143 | +1091.7 |
| 11 | 77 | M | NVAF | Rivaroxaban | 20 | Lopinavir/Ritonavir | 16 | 505 | +3056.2 |
| 12 | 79 | F | NVAF | Rivaroxaban | 15 | Lopinavir/Ritonavir | 13 | 99 | +661.5 |
Figure 1.
Changes in C‐trough plasma levels, expressed as ng/mL, between pre‐ and in‐hospital, in the 12 patients observed
4. DISCUSSION
In the emergency critical situation, such as we experienced in the first weeks of the outbreak of COVID‐19 pandemic in our hospital, we had the opportunity to observe and learn from direct experience on DOAC patients. The pandemic SARS‐COV‐2 is causing hospitalization of thousands of persons, especially the elderly, many of whom are treated with oral anticoagulants for cardiovascular diseases. Treatment of COVID‐19 infection is currently based on antiviral and immunosuppressive drugs.13., 14., 15., 16., 17., 18. Several drugs, particularly those that strongly interact with P‐glycoprotein and/or cytochrome P450‐based metabolic pathways, such as antiviral agents, can modify DOAC pharmacokinetic and pharmacodynamic profiles, consequently changing their plasma anticoagulant activity.7., 8., 9., 10., 17., 18., 19.
In all 12 examined patients, an alarming increase in DOAC plasma levels compared to pre‐hospitalization was observed after hospital admission. Although we cannot exclude a possible role of concomitant drugs or disease‐related organ dysfunctions, our results are consistent with those coming from several studies addressing interferences between DOAC and antiviral agents.17., 18., 19., 20. Although the potential of antiviral agents to increase the plasmatic concentration of DOACs, as well as their bleeding risk, is well known there is no agreement on the most appropriate clinical management in these circumstances. Accordingly, some physicians continue DOAC treatment while others do not, as was the case in our hospital.
An alternative option could be adjusting dosage (where available) according to plasma values, as is often done in situations like bleeding and thromboembolic complications, need of surgery, or invasive procedure.22 However, we think that in critical conditions, such as severe SARS‐CoV‐2 respiratory syndrome, DOAC level adjustment is both impractical and unlikely to offer the desirable protection against thromboembolic and hemorrhagic complications.
In order to prevent bleeding complications, we believe that physicians should consider withholding DOACs from patients with severe SARS‐CoV‐2 infection and replacing them with alternative parenteral antithrombotic strategies for as long as antiviral agents are deemed necessary and until discharge.
CONFLICTS OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
S. Testa: study design, first draft manuscript preparation; O. Paoletti: patient identification and manuscript approval; C. Dellanoce: patient identification and data analysis; M. Betti, G. Danzi, R. Morandini, M. Tala: acquisition of data; M. Giorgi Pierfranceschi, A. Pan: acquisition of data and manuscript approval; G. Palareti, P. Prandoni: final manuscript revision and approval.
Footnotes
Manuscript handled by: David Lillicrap
Final decision: David Lillicrap, 17 April 2020
REFERENCES
- 1.Phelan A.L., Katz R., Gostin L.O. The novel coronavirus originating in wuhan, china: challenges for global health governance. JAMA. 2020;323(8):709. doi: 10.1001/jama.2020.1097. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO). Coronavirus disease 2019 (COVID‐19) Situation Report – 51. 2020.
- 3.Ageno W., Gallus A.S., Wittkowsky A., Crowther M., Hylek E.M., Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence‐based clinical practice guidelines. Chest. 2012;141:e44S–88S. doi: 10.1378/chest.11-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holbrook A., Schulman S., Witt D.M., et al. Evidence‐based management of anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 Suppl):e152S–e184S. doi: 10.1378/chest.11-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Testa S., Tripodi A., Legnani C., et al. Plasma levels of direct oral anticoagulants in real life patients with atrial fibrillation: Results observed in four anticoagulation clinics. Thromb Res. 2016;137:178–183. doi: 10.1016/j.thromres.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Reilly P.A., Lehr T., Haertter S., et al. The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE‐LY Trial (Randomized Evaluation of Long‐Term Anticoagulation Therapy) J Am Coll Cardiol. 2014;63(4):321–328. doi: 10.1016/j.jacc.2013.07.104. [DOI] [PubMed] [Google Scholar]
- 7.(EMA) EMA. Pradaxa ‐ summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐Product_Information/human/000829/WC500041059.pdf. Accessed 26 September 2017.
- 8.(EMA) EMA. Xarelto ‐ Summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/000944/WC500057108.pdf. Accessed 26 September 2017.
- 9.(EMA) EMA. Eliquis ‐ Summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/002148/WC500107728.pdf. Accessed 26 September 2017.
- 10.(EMA) EMA. Lixiana ‐ summary of product characteristics. European Medicines Agency. Lixiana ‐ summary of product characteristics. 2017. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/002629/WC500189045.pdf. Accessed 26 September 2017.
- 11.Testa S., Legnani C., Antonucci E., et al. Palareti G; Coordinator of START2‐Register. Drug levels and bleeding complications in atrial fibrillation patients treated with direct oral anticoagulants. J Thromb Haemost. 2019;17(7):1064–1072. doi: 10.1111/jth.14457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Testa S., Paoletti O., Legnani C., et al. Low drug levels and thrombotic complications in high‐risk atrial fibrillation patients treated with direct oral anticoagulants. J Thromb Haemost. 2018;16(5):842–848. doi: 10.1111/jth.14001. [DOI] [PubMed] [Google Scholar]
- 13.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019‐nCoV) Nat Rev Drug Discov. 2020;19(3):149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 14.Cao B., Wang Y., Wen D., et al. A trial of lopinavir‐ritonavir in adults hospitalized with severe Covid‐19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devaux C.A., Rolain J.M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID‐19? Int J Antimicrob Agents. 2020;12 doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and coronavirus disease‐2019 (COVID‐19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3) doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foerster K.I., Hermann S., Mikus G., et al. Drug‐drug interactions with direct oral anticoagulants. Clin Pharmacokinet. 2020 doi: 10.1007/s40262-020-00879-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steffel J., Verhamme P., Potpara T.S., et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non‐vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39(16):1330–1393. doi: 10.1093/eurheartj/ehy136. [DOI] [PubMed] [Google Scholar]
- 19.Mueck W., Kubitza D., Becka M. Co‐administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol. 2013;76(3):455–466. doi: 10.1111/bcp.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong I.Y., Kim R.B. Importance of pharmacokinetic profile and variability as determinants of dose and response to dabigatran, rivaroxaban, and apixaban. Can J Cardiol. 2013;29(7 Suppl):S24–S33. doi: 10.1016/j.cjca.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Gosselin R.C., Adcock D.M., Bates S.M., et al. International Council forStandardization in Haematology (ICSH) recommendations for laboratory measurement of direct oral anticoagulants. Thromb Haemost. 2018;118(3):437–450. doi: 10.1055/s-0038-1627480. [DOI] [PubMed] [Google Scholar]
- 22.Tripodi A., Ageno W., Ciaccio M., et al. Position paper on laboratory testing for patients on direct oral anticoagulants. A consensus document from the SISET, FCSA, SIBioC and SIPMeL. Blood Transfus. 2018;16(5):462–470. doi: 10.2450/2017.0124-17. [DOI] [PMC free article] [PubMed] [Google Scholar]