Abstract
The ongoing outbreak of the novel coronavirus (SARS‐CoV‐2) infection is creating serious challenges for health laboratories that seek to identify viral infections as early as possible, optimally at the earliest appearance of symptom. Indeed, there is urgent need to develop and deploy robust diagnostic methodologies not only to use in health laboratory environments but also directly in places where humans circulate and spread the virus such as airports, trains, boats, and any public aggregation places. The success of a reliable and sensitive asymptomatic diagnosis relies on the identification and measurement of informative biomarkers from human host and virus in a rapid, sensitive, and inexpensive manner. The objective of this article is to describe an innovative multidisciplinary approach to develop an efficient, inexpensive, and easy‐to‐use portable instrument (bCUBE® by Hyris Ltd) that can be employed as a surveillance system for the emergency caused by SARS‐CoV‐2. A solution for Coronavirus testing, compliant with CDC guidelines, is scheduled to be released in the next weeks. In addition, we will describe a workflow and path of an integrated multi‐omic approach that will lead to host and pathogen biomarker discovery in order to train the instrument to provide reliable results based on a specific biomarker's fingerprint of SARS‐CoV‐2 infection.
Keywords: coronavirus, device, instrument, portable, SARS‐CoV‐2 detection
1. INTRODUCTION
The January 30, 2020, the World Health Organization (WHO) declared a global health emergency for an outbreak of a novel Coronavirus (SARS‐CoV‐2) that occurred in the city of Wuhan (Hubei province, China). 1 The rapid spread to more than 25 other countries across the world (Centers of Disease Control and Prevention, 2020) and the high number of contagions (over 70 000) in only 1 month after the first case report on December 31, 2019, is alarming for all the world's human populations (https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Although the fatality rate of SARS‐CoV‐2 is around 4%, lower than previous SARS‐CoV (severe acute respiratory syndrome coronavirus and MERS‐CoV [Middle East respiratory syndrome coronavirus], WHO has highlighted the importance of strengthening collaborative efforts among scientists to develop effective interventions for controlling and preventing the epidemic). 2 , 3 , 4 Coronaviruses belong to the family Coronaviridae and are characterized by a positive‐sense single‐stranded RNA genome that was released via the community online resource virological.org on 10 January (Wuhan‐Hu‐1, GenBank accession number MN908947). After that, four other genomes were deposited on 12 January in the viral sequence database of the Global Initiative on Sharing All Influenza Data (GISAID). The genome sequence showed that the virus is closely related to the coronavirus causing severe acute respiratory syndrome (SARS), agent of the 2002/03 outbreak of SARS in humans. This virus family includes a large number of viruses present in rhinolophid bats in both Europe and Asia. These viruses are pathogen of animals such as birds and mammals. In humans, they typically induce mild respiratory infections, generally observed in the common cold.
A workflow of the coronavirus primary screening has been shown in Figure 1. A reliable diagnosis of the viral infection is one the most urgent priorities for public health management of the disease spread. Usually in acute respiratory infection, RT‐PCR is traditionally employed to identify viruses in respiratory secretions. A robust detection technology is usually based on real‐time RT‐PCR because it is highly sensitive and specific, although it requires infrastructure, skilled personnel and at least 4‐6 hours for analysis. Currently available techniques do not provide results before several hours or even days, and the tests require a well‐equipped laboratory and trained personnel. Indeed, these methods are not efficient to rapidly screen a high number of individuals in places where thousands of people transit per hours. These methods are effective in confirming the infections when symptoms are already present but they are completely inefficient to identify infections at asymptomatic stage. The development of a means for a quick and simple detection of this virus in humans is a worldwide priority considering that infections have been observed in world areas such as Africa where the scarce health assistance will not be able to control the spread. Indeed, it is important to generate new ideas that will allow the development of new detection prototypes able to quickly identify infected humans and prevent the virus from spreading.
FIGURE 1.
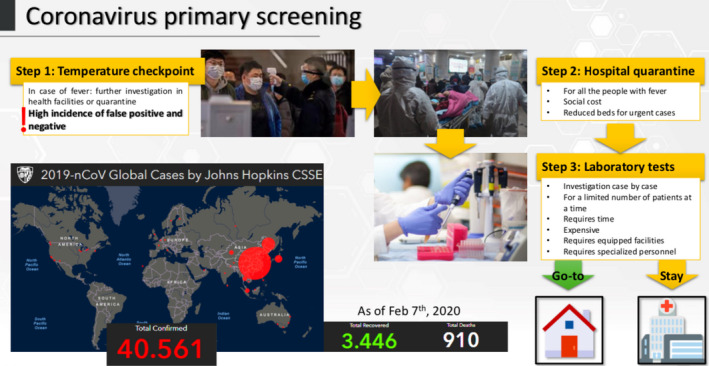
Steps of current coronavirus primary screening
2. BIOMARKER IDENTIFICATION
A large number of possible host‐pathogen biomarkers (transcripts, miRNAs, proteins, metabolites) may be analyzed through different omic tools. Here, we propose the application of a portable instrument based on the analysis of molecular responses at transcript level. The proposed approach consists in the adaptation of an existing infield portable technology currently used for real‐time detection of viral agents in plants, for detection of SARS‐CoV‐2 in confined and contaminated environments (for example, airports, buildings, and boats). It links translational genomics to technologies, potentially enhancing the discovery of SARS‐CoV‐2‐specific biomarkers while bridging gaps in conventional human virus detection and molecular diagnostics methods. An analysis at two complementary levels (host transcriptional responses and viral nucleic acid presence) may allow clarification of the pathogenetic mechanisms of the viral agent identifying strict and complex cause‐effect relationships occurring in the host‐pathogen interactions. Traditional approaches are based on pathogen DNA/RNA and protein detection using, respectively, PCR‐based or protein‐based methods in traditional laboratory instruments. However, this kind of method is not useful in the quest to reduce the spread of SARS‐CoV‐2 infections because: (a) The virus is not uniformly distributed within human tissues and is therefore often missed when at low titers undetectable with current real‐time PCR technologies; (b) the virus has high incubation times (14‐30 days) at asymptomatic conditions having the capability to be highly contagious at this stage; (c) the analysis with traditional methods takes time for sample transport, extraction and analysis (16‐24 hours at least) reducing the speed of viral spread counteracting actions; and (d) the need of skilled personnel to perform analysis. The proposed strategy is focused on the analysis of human responses that are triggered during viral infection with specific emphasis on the immune responses that are upregulated rapidly after infection and are locally at the site of infection (nose nostrils and also at a distance (lungs and other organs). Biomarkers that are associated with such responses can be found both locally and at certain distance from nostrils. At the early‐stage event of the host‐pathogen interaction, a “stress condition” typically occurs such as a “inflammatory response” due to the activation of recognition mechanisms of pathogen‐associated and pathogen‐induced virulence factors. These early asymptomatic responses are typically linked with changes in both host and the viral agent at transcriptional and the post‐transcriptional levels with specific, mRNA, miRNA, non‐coding RNA, proteins, and metabolites activated in response to the pathogen presence.
Through the comparison of transcriptomic signatures under asymptomatic and symptomatic conditions, genes associated with the beginning of disease symptoms can be identified. The molecular processes precede symptom's appearance, when human host activate more physiological responses, leading to disease‐associated phenotypic modifications and metabolic problems linked to pathogen virulence factors. The human response itself may lead to detrimental effects, exaggerating the direct pathogen action or inducing mutually negative actions. Prevention of SARS‐CoV‐2 disease spread may require: (a) development of portable instruments for rapid, easy and reliable detection of viral infections at a presymptomatic stage; (b) development therapeutic methods for destruction of SARS‐CoV‐2 before irreversible negative effects on human health has occurred (these approaches may focus on targeting of human immune system); and (c) identification of pathways and bioactive compounds that can stimulate the innate immune responses. Indeed, the proposed approach is to identify and analyze biomarkers modulated in either host or pathogen at early stages of infection. When any eukaryotic host is subjected to stress, an “induced stress response” occurs similarly to the host “inflammatory response” to pathogen‐associated and pathogen‐induced virulence factors. 5 If the induced stress response is enhanced, tissue injury can happen, causing to irreversible effects at asymptomatic stages, because it is often too late to avoid tissue and organ damages once symptoms appear. During the early stages of this condition, the induction of immune stress‐related genes and pathways commonly associated with pathogen infection and physiological disorders occur and these genes are typically associated with general early state of stress response. Other differentially regulated genes at the presymptomatic stage are stress specific and may work as useful biomarkers for the early diagnosis of the host health conditions. These responses include changes in the key metabolic pathways associated with key indicators of early stages of infection of known pathogens. Usually, this stress condition usually anticipates the manifestation of symptoms associated with diseases. For this reason, a focus on the analysis of key genes, transcripts, proteins, metabolites, or pathways could be used to monitor the human health status. These induced biological molecules may be used as biomarkers for both stress identification and recovery. The identification of molecules that are early host responses associated in a reversible stress status using portable instruments is the first stage for asymptomatic diagnosis. The biomarker's identification needs the analysis of large data sets through web‐based applications that queries large public databases of information on transcripts, proteins, or metabolites modulated in specific biological conditions (ie, Genevestigator).
Here, we propose the employment of an instrument that is already widely used for plant pathogens 6 , 7 and could be easily adapted also for the detection of SARS‐CoV‐2. The idea is to use a portable DNA‐based detection devices not only targeting viral RNA but also analyze a pool of early responsive host transcripts. This transcriptional fingerprint would greatly enhance early asymptomatic detection of a viral such as SARS‐CoV‐2 having more than 14 days of asymptomatic stage. This complementary approach on host and pathogen will overcome the limits of an approach aiming at only target the pathogen DNA that is unreliable at asymptomatic stage, especially in case of pathogen with systemic diffusion such as viruses. A molecular analysis of early human responses may complement traditional methods based on the identification of the pathogen presence, enhancing the detection of first infections at asymptomatic stage. Surely, the expression analysis of a single gene of any host does not allow the specific diagnose of infections because there are no host genes strictly specific for a specific pathogen. However, a pool of responsive host genes might be employed as host biomarkers to quickly identify early alarm stress state, better than aspecific methods currently used such the measurement of body temperature for fever detection. A biomarker can be defined as a biological characteristic that can be analyzed and considered as an indicator of a particular physiological such as pathogenic processes, or responses to a therapeutic intervention. In omic sciences, a “biomarker profile” includes a series of transcriptomic, proteomic, and/or metabolomic features linked with a particular stress, development of physiological condition. Combined, these characteristics could become a biomarker of pathogenic condition assisting in diagnosis and therapy. A systems‐based approach could gain insight into underlying biological regulatory network (BRN) governing interactions between hosts and pathogens. Heterogeneous omic data sets could be integrated to define a biological regulatory network underlying early host and pathogen biomarkers for asymptomatic detection of the viral infections. To assist the biomarkers discovery, the vast amount of transcriptomic data available in public databases in response to human coronaviruses will allow a deep meta‐analysis using previously customized bioinformatics pipelines Ref. [8, 9, 10, 11]. This work consists of re‐analyzing raw data obtained by independent experiments using a normalization and standardization procedure included in a bioinformatics pipeline. This work will provide differentially regulated genes from each study finding commonalities and differences in genes, genet sets, pathways, and gene/protein networks to clarify molecular mechanisms in host‐pathogen interactions. 12
The genome sequence of SARS‐CoV‐2 is closely related to that of SARS‐CoV. Like SARS‐CoV and MERS‐CoV, SARS‐CoV‐2 belongs to the Betacoronavirus genus), 4 , 13 having a genome size of ~30 kilobases, similarly with the other coronaviruses, encoding for multiple structural and non‐structural proteins. 14 , 15 In addition, SARS‐CoV‐2 seems to have similar cell entry mechanism and type of human cell receptor. 4 , 16 , 17 T cells respond well against the structural proteins that are most immunogenic compared to the non‐structural proteins. 18 Among them, the S and N proteins have showed to be the most dominant and long‐lasting by T‐cell responses. 19 Indeed, as possible targets for pathogen nucleic acid detection for bCUBE® we may suggest the following: proteins including spike (S) proteins, envelope (E) proteins, membrane (M) proteins, and nucleocapsid (N) proteins. No mutation in SARS‐CoV‐2 sequences (as of February 6, 2020) has been observed in comparison with the experimentally determined SARS‐CoV‐derived B‐cell epitopes (both linear and discontinuous) implying that these epitopes have the potential to elicit a cross‐reactive/effective responses against SARS‐CoV‐2). 20 Indeed as host response targets, we may suggest to test IFNγ TNFα, IL‐2, as suggested by previous data. 18 , 21 Indeed, S peptides showed the highest percentages of IFNγ producing cells.
Proteomics may be also used to validate the discovery of transcriptional markers analyzing the same infection stages considered at transcript level. A possible technique could be the isobaric Tags for Relative and Absolute Quantitation (iTRAQ), typically employed in quantitative proteomics using tandem mass spectrometry to analyze quantitative measurements of protein amount from different sources in a single experiment. Briefly, once proteins are extracted from the same collected tissues (nostril tissues), they may be precipitated using ProteoExtractTM Protein Precipitation kit (Calbiochem), dehydrated, resuspended, and digested with tripsin and generated peptides analyzed using a system composed by QExactive mass spectrometer, an Easy‐LC and a nanospray ionization source. Data could be acquired using a data‐dependent ms/ms method and raw data should be analyzed using X!Tandem, visualized using Scaffold Proteome software and proteins identified using Uniprot databases and cRAP database. The use of proteomics should enable identification not only viral proteins but also host proteins modulated by early infection (at the same way of transcriptomics, a complement approach host‐pathogen is required). Some of the identified pathogen virulence proteins may be selected for further validation through expression in model animals to validate their virulence phenotype. Synthetic virulence genes should be designed to insure higher in vivo expression through codon's optimization (ie, using DNAworks software, http://mcl1.ncifcrf.gov/dnaworks). Signal peptides could be analyzed by SignalP 4.0 server, and N‐Glycosylation sites could be predicted using NetNglyc1.0 (http://www.cbs.dtu.dk/services/NetNglyc/). This approach has been successful to determine more than 4500 proteins in plants including both host and pathogen. 7 Considering the systemic presence of SARS‐CoV‐2, the identification of specific genes from peripheral blood and different tissues would be very helpful to discover tissue‐specific host biomarkers. The viral infection is typically perceived by the host before that the viral nucleic acid can be detected by traditional methods such as quantitative real‐time PCR.
3. PORTABLE INSTRUMENT FOR CORONAVIRUS INFIELD DETECTION
Here, we propose the use of the portable device such as bCUBE®2.0 (developed by Hyris Ltd).
The device is a miniaturized device that is able to perform both temperature cycles and isothermal analysis, enabling a wide array of nucleic acid detection methods such as qPCR and isothermal amplifications (Figure 2), with a power consumption of maximum 60 W. The instrument runs custom thermal cycling protocols and performs real‐time PCR analyses. The instrument, sized 100 × 100 × 120 mm with a weight of 1.15 kg, is certified to both European and North American standards. Thanks to a live sync to a central database, it can be monitored and controlled remotely from any device or in mobile with a Smartphone. The analysis can be performed using customized cartridges to analyze in multiplex way up to 16 or 36 samples of 10‐25 μL in one run, on two detection channels (FAM/HEX).
FIGURE 2.
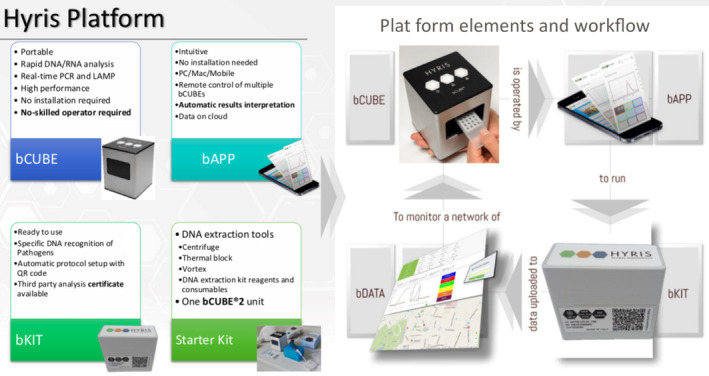
Components of Hyris platform: bCUBE®, bAPP, bKit, and starter kit
The key features of this system are the following:
viral detection in 1.5 hours using RT‐PCR
automatic result interpretation
high resolution melting and isothermal amplification options
wireless or wired connectivity.
The bCUBE® 2.0 can be controlled by the Hyris bAPP, a multiplatform GUI that works on smartphones, tablets, laptops, and PCs with typical operating systems (Figure 3). Key features are the following:
manage one or multiple bCUBEs on the field or remotely
manage and configure access privileges in your network
run custom protocols for RD or use public “Global Recipes” for validated applications
design and perform analysis and tests using either real‐time PCR or isothermal protocols
use the data for internal QA, or to generate and receive in real time third party Certificates
aggregate and perform in depth statistical, process and quality controls leveraging also on correlated data like timestamps and geolocalization of samples.
FIGURE 3.
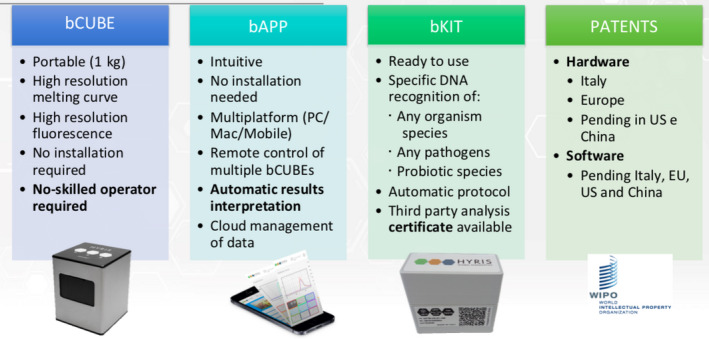
Additional details of the components of the portable system
This miniaturized instrument has been recently proposed for highly threatening pathogen outbreak in agriculture such as Xylella fastidiosa in olive. 6 The instrument has typically been used for the detection of plant viral infections directly in field. The novelty is the possibility to use the detection principles and methodologies from the infield detection of plant virus in crops to quick and portable detection of a dangerous viral agent in humans such as SARS‐CoV‐2. The instrument analyzes mRNA in <2 hours directly in the field, without the need of laboratory infrastructures, through the performance of a qRT‐PCR analysis. Gene expression could be conducted collecting samples from human nostrils with tools such as cotton swabs. Confirmation may be performed blood samples. RNA may be extracted from these collection instruments with a quick procedure using simplified extraction procedures.
The typical workflow for the use of the instrument for pathogen detection is shown in Figure 4. Results may be provided in approximately 2 hours. At the moment, the developed software and hardware are used to target the pathogen DNA, but a next step could be the analysis of host Xf‐regulated genes discovered by meta‐analysis works. Implementation and validation tests of the results obtained directly by the portable device could be conducted analyzing the same tissues with traditional laboratory equipment. This work would verify the consistency of the field results delivered by the instrument.
FIGURE 4.
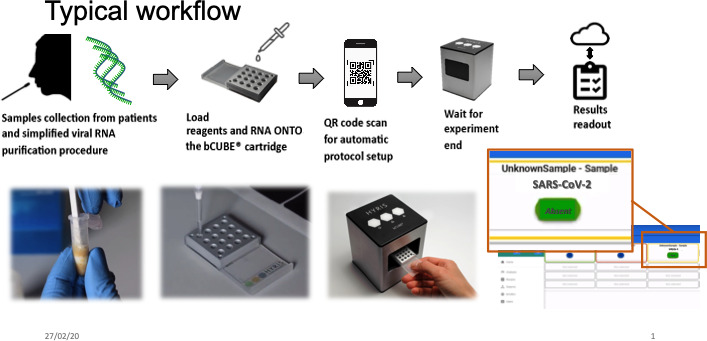
Typical workflow for the use of bCUBE® for detection of pathogens from DANN extraction to results readout
4. CONCLUSIONS
The proposed instrument fits well when a quick, easy, reliable and in‐house early detection (and confirmation) is required to rapidly decontaminate environments and initiate quarantine of infected individuals. The workflow for a secondary coronavirus screening using bCUBE® is shown in Figure 5 . The system is able to detect not only viruses, but also specific bacteria, proteins, and DNA molecules, an increased or reduced concentration of which in a person's saliva, blood and other tissues (ie, nostrils) provide the diagnosis of the SARS‐CoV‐2 infections. The most important aspect of this tool is the extreme ease of use and the automatic interpretation of the results. The app has an intuitive interface and can guide the operator through every step of the setup and analysis. Initiation in the analysis is simple and the automatic result interpretation engine can assist the operator in determining the outcome. The supplied power source works at both 110 V and 220 V, and the power cord can be easily interchanged with the one compatible with the standard of your destination country. Real‐time and remote control of the bCUBEs is allowed thanks to the connection to Hyris bDATA® service. The instrument is well suitable to work on boats and other quarantine environments for the possibility to have results in short time. It also fits well in the airports after a quick scan of other symptoms (ie, fever) in order to confirm the presence of the viral infections.
FIGURE 5.
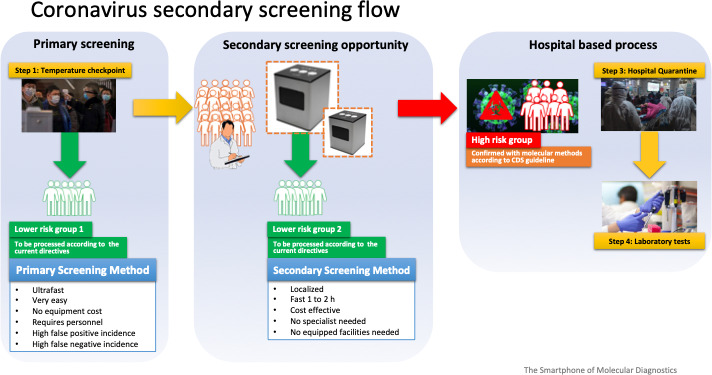
Possible workflow of Coronavirus secondary screening using bCUBE® portable instrument
CONFLICT OF INTEREST
We certify that Lorenzo Colombo, Stefano Lo Priore, Isabella Della Noce and Simone Romano are employees of Hyris Ltd. that produces the bCUBE® instrument described in this article.
Martinelli F, Perrone A, Della Noce I, Colombo L, Lo Priore S, Romano S. Application of a portable instrument for rapid and reliable detection of SARS‐CoV‐2 infection in any environment. Immunol Rev. 2020;295(Suppl. 1):4–10. 10.1111/imr.12857
REFERENCES
- 1. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu X, Wang X‐J. Potential inhibitors for SARS‐CoV‐2 coronavirus M protease from clinically approved medicines. BioRxiv. 2020;01(29):924100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou P, Yang X‐L, Wang X‐G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dandekar AM, Martinelli F, Davis C, et al. Analysis of early host responses for asymptomatic disease detection and management of specialty crops. Crit Rev in Immunol. 2010;30:277‐289. [DOI] [PubMed] [Google Scholar]
- 6. Martinelli F, Marchese A, Giovino A, et al. Infield and early detection of Xylella fastidiosa infections in olive using a portable instrument. Front Plant Sci. 2019;9:2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martinelli F, Reagan RL, Dolan D, et al. Proteomic analysis highlights the role of detoxification pathways in increased tolerance to Huanglongbing disease. BMC Plant Biol. 2016;16:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Balan B, Caruso T, Martinelli F. Transcriptomic responses to biotic stresses in Malus x domestica: a meta‐analysis study. Sci Rep. 2018;8(1): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Balan B, Ibanez AM, Dandekar AM, et al. Identifying host molecular features strongly linked with responses to Huanglongbing disease in citrus leaves. Front Plant Sci. 2018;9:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Balan B, Caruso T, Martinelli F. Gaining insight into exclusive and common transcriptomic features linked with biotic stress responses in Malus. Front Plant Sci. 2018;8:1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Benny J, Pisciotta A, Caruso T, et al. Identification of key genes and its chromosome regions linked to drought responses in leaves across different crops thorugh meta‐analysis of RNA‐ seq data. BMC Plant Biol. 2019;19:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martinelli F, Scalenghe R, Giovino A, et al. Proposal of a citrus translational genomic approach for early and infield detection of Flavescence dorée in Vitis. Plant Biosyst. 2016;150:43‐53. [Google Scholar]
- 13. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alqahtani SM, Alamri MA, et al.Structural basis of SARS‐CoV‐2 3Cpro and Anti‐COVID‐19. Preprints 2020;2020020193. Doi: 10.20944/preprints202002.0193.v1 [DOI] [PMC free article] [PubMed]
- 15. Ahmed SF, Quadeer AA, McKay MR.Preliminary identification of potential vaccine targets for SARS‐CoV‐2 based on SARS‐CoV immunological studies. BioRxiv preprint. doi: 10.1101/2020.02.03.933226 [DOI] [PMC free article] [PubMed]
- 16. Hoffmann M, Kleine‐Weber H, Kruger N, et al.The novel coronavirus 2019 (SARS‐CoV‐2) uses the SARS‐coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. BioRxiv 2020;2020.01.31.929042.
- 17. Letko M, Munster V. Functional assessment of cell entry and receptor usage for lineage B β‐ coronaviruses, including SARS‐CoV‐2. BioRxiv 2020;2020.01.22.915660. doi: 10.1101/2020.01.22.915660 [DOI] [PMC free article] [PubMed]
- 18. Li CK‐F, Wu H, Yan H, et al. T cell responses to whole SARS coronavirus in humans. J Immunol. 2008;181:5490‐5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Channappanavar R, Fett C, Zhao J, et al. Virus‐specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection. J Virol. 2014;88:11034‐11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ahmed SF, Ahmed AQ, McKay MR.Preliminary identification of potential vaccine targets for SARS‐CoV‐2 based on SARS‐CoV immunological studies. BioRxiv 2020; 04 February. 10.1101/2020.02.03.933226 [DOI] [PMC free article] [PubMed]
- 21. Fan Y‐Y, Huang Z‐T, Li L, et al. Characterization of SARS‐ CoV‐specific memory T cells from recovered individuals 4 years after infection. Arch Virol. 2009;154:1093‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]


