Abstract
Coronavirus disease 2019 (COVID‐19), the worst pandemic in more than a century, has claimed >125,000 lives worldwide to date. Emerging predictors for poor outcomes include advanced age, male sex, preexisting cardiovascular disease, and risk factors including hypertension, diabetes, and, more recently, obesity. This article posits new obesity‐driven predictors of poor COVID‐19 outcomes, over and above the more obvious extant risks associated with obesity, including cardiometabolic disease and hypoventilation syndrome in intensive care patients. This article also outlines a theoretical mechanistic framework whereby adipose tissue in individuals with obesity may act as a reservoir for more extensive viral spread, with increased shedding, immune activation, and cytokine amplification. This paper proposes studies to test this reservoir concept with a focus on specific cytokine pathways that might be amplified in individuals with obesity and COVID‐19. Finally, this paper underscores emerging therapeutic strategies that might benefit subsets of patients in which cytokine amplification is excessive and potentially fatal.
Introduction
Coronavirus disease 2019 (COVID‐19) has now infected over 2 million people worldwide, with a death toll of more than 125,000 people. Emerging risk factors for poor outcomes in this disease include age, male sex, and cardiovascular comorbidities, including hypertension, prior cardiovascular disease, diabetes, and, more recently, obesity (1). The Centers for Disease Control and Prevention (Atlanta, Georgia) has now reported a threefold increase in death in New Orleans compared with New York, and speculation has grown as to whether these worrying mortality statistics might in part be attributable to higher levels of morbid obesity (2). In this paper, we develop a theoretical framework that describes why individuals with obesity may be at increased risk of poor outcomes compared with counterparts without obesity (3). We propose a mechanism for adverse consequences of virus seeding to adipose tissue (AT), with potential for prolonged viral shedding and extended cytokine activation in a voluminous and richly vascularized organ that is already perturbed in a metabolic and inflammatory sense in human individuals with obesity (4). We present a rationale for testing this concept in patients with COVID‐19 through prospective studies of individuals with and without obesity with accessible AT and plasma to determine whether or not an inflammatory and cytokine signature presages a systemic cytokine storm and clinical decline.
Evidence for Association of Obesity with Worse COVID‐19 Outcome
Obesity was not specifically reported in the initial cohort studies of COVID‐19 from Wuhan, China (5), but regional epidemiological data from the United States suggests that at least 25% of patients who die of this disease have obesity, which is similar to reported rates of cardiovascular disease in the same high‐risk group (21%) (6). More recently, a small retrospective study of 85 individuals with COVID‐19 identified obesity as a risk factor for admission to the intensive care unit, with patients requiring increased medical attention (3). Moreover, in the influenza A subtype H1N1 pandemic, obesity was also strongly associated with a worse disease outcome and death (7). Together, these data raise the questions of whether there is a mechanistic link between obesity and disease survival and whether obesity over and above its endocrine or cardiometabolic associations might independently contribute to COVID‐19 risk.
Likely Mechanisms Involved in Poor Outcomes in Individuals with Obesity
It is clear that obesity could contribute to both diabetic and cardiovascular COVID‐19 risk, and these elements of risk, in addition to thrombosis more recently, have been well described in the scientific literature (8, 9, 10, 11, 12). Moreover, obesity is an independent risk factor for hypoventilation syndrome in patients in the intensive care unit (13) and could thus contribute to respiratory failure in patients with acute respiratory distress syndrome (14). Here, we propose additional unheralded pathophysiologic aspects of increased AT burden in morbid obesity that may amplify the pro‐inflammatory response to extensive viral infection. AT should be viewed as a highly active organ interfacing immune, endocrine, and metabolic homeostasis throughout the body (15). In individuals with obesity, there is marked dysregulation of myeloid and lymphoid responses within AT, with associated dysregulation of cytokine profiles (15). Intrinsically bound to this are endocrine and metabolic derangements, including insulin resistance and adipokine dysregulation with dysfunctional lipid and fatty acid metabolism (16). In highly vascularized AT, endothelial and smooth‐muscle cells, as well as resident macrophages, exhibit additional perturbations in response to an activated renin angiotensin system at a local level, with attendant depletion and dysfunction of the counterregulatory angiotensin converting enzyme 2 Mas receptor system (17, 18). This makes AT, particularly in visceral distributions, pro‐immunogenic, metabolically active, and highly integrated into the cardiovascular system, with the capability to drive acute disease through augmented inflammation at an organ level in the heart, vasculature, pancreas, liver, and kidneys (19). This “preactivation state” of AT in obesity makes this organ a potential target for further immune amplification by external pathogens such as viruses.
Viral Spread to AT and Potential for Activation of Resident Inflammation and Cytokine Pathways
Currently, there is no evidence for direct severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection of AT, although angiotensin converting enzyme 2 receptor expression represents a basis for viral tropism in several cells within this tissue (20), including adipocytes, smooth‐muscle cells, and endothelial cells (21). Moreover, many AT‐resident cells are proven targets for multiple viruses, including adipocytes [H1N1, type A influenza, and adenovirus 36 (7, 22, 23)], adipo‐stromal cells [adenovirus 36 (24), cytomegalovirus (25)], endothelial cells [SARS‐CoV (26)], macrophages [influenza A, SARS‐CoV, adenovirus36, human immunodeficiency virus (26, 27)], and lymphocytes [SARS‐CoV, human immunodeficiency virus (25, 26)]. Although SARS‐CoV‐2 was detected only at low levels in blood in a small human study (28), we cannot exclude hematogenous spread to AT, given the very high virus affinity for its target cell receptor. Alternative routes of SARS‐CoV‐2 spread to AT include local egress of the virus from organs known to be infected to adjacent visceral fat deposits, such as intrathoracic fat (lungs), epicardial fat (heart), perirenal fat (kidneys), and mesenteric fat (intestines). Finally, shared viral tropism for lung epithelium and AT has already been shown for H5N1 virus infection (29), and AT significantly prolongs the duration of viral shedding in humans with obesity infected with influenza (22). Were similar tropism of SARS‐CoV‐2 to occur within AT of individuals with COVID‐19 and obesity, there exists the potential for prolonged viral shedding in this organ, with extended activation of local “preactivated” immune systems and resident cytokine signaling pathways.
Resident myeloid and lymphoid cells are plentiful in AT, and obesity is associated with macrophage (30) and lymphocyte dysfunction (31). Expansion of distinct memory T lymphocytes within AT can also activate aberrant immune responses with wider tissue damage on viral challenge (31). A recent report from Wuhan suggests that SARS‐CoV‐2 induces a dysregulated immune response in severely ill individuals with COVID‐19 (32), characterized by reduced numbers of circulating memory T lymphocytes, as well as reduced helper/suppressor and regulatory T‐cell subtypes. It is tempting to speculate whether already dysfunctional immune responses in individuals with obesity may accentuate this SARS‐CoV‐2 effect on T‐cell function.
Specific inflammatory cytokine programs such tumor necrosis factor (TNF)‐α, interleukin (IL)‐1, and IL‐6 are known to be preactivated in AT in the context of obesity (33), and, thus, viral infection may similarly amplify the already primed organ cytokine response in AT. The cytokine storm identified in multiple respiratory viral infections, including COVID‐19, exhibits diverse interferon, IL, chemokine, TNF, and colony‐stimulating factor responses, which are beyond the scope of this paper but are comprehensively reviewed elsewhere (34). The intensity of inflammatory lung responses reflect the imbalance between pro‐inflammatory cytokines (such as TNF and Il1β) and their soluble cognate receptors that inhibit cytokine effects in aqueous phase (35). IL‐10 produced by macrophages and T lymphocytes (T helper 2 and regulatory T cells) acts as a negative regulator of inflammation, whereas IL‐6 and its soluble receptor enhance activity of IL‐6 on target cells, providing a mechanism for enhancement of TNF and IL‐1β activity when these soluble cognate receptors are particularly high (35). Thus, a balance between pro‐ and anti‐inflammatory mechanisms is critical in maintaining lung‐tissue homeostasis. It is conceivable that if one or more of these regulatory elements were absent or dysfunctional, then it might contribute to a cytokine storm in the lung or in other tissues such as AT, where aberrant cytokine activation exists. Temporal studies of cytokine dynamics in human “cytokine storm” models show that IL‐6 sustains activation of multiple cytokine pathways for many days after the initial immune insult (36). Interestingly, in early COVID‐19 studies, IL‐6 was a strong independent predictor of mortality (37). Human AT is a major source of IL‐6 and its receptor IL‐6R (38), and, thus, AT may provide a reservoir for IL‐6 activation and cascade signaling in viral infection (Figure 1). Viral spread from affected organs to adjacent AT may take days, with subsequent prolonged viral shedding contributing to the delayed cytokine storm and consequences for tissue injury in patients with COVID‐19.
Figure 1.
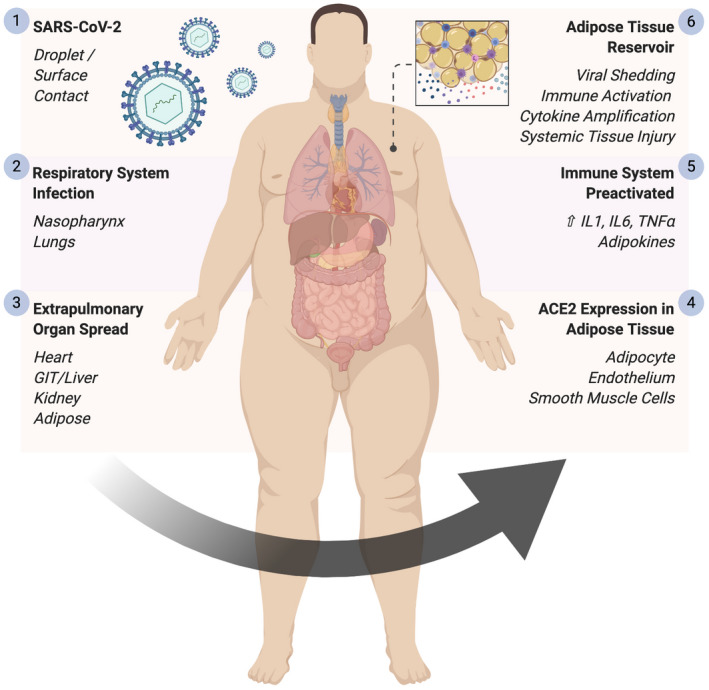
Adipose tissue as a reservoir for SARS‐CoV‐2 spread, viral shedding, immune activation, and cytokine amplification. Schematic demonstrating the proposed centrality of adipose tissue in the dissemination of SARS‐CoV‐2 and the ensuing systemic immune activation. Created with Biorender.com. GIT, gastrointestinal tract; IL, interleukin; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; TNF, tumor necrosis factor. [Color figure can be viewed at wileyonlinelibrary.com]
Testing AT Inflammatory Cytokine Reservoir Concept in COVID‐19
Initial studies should aim to detect SARS‐CoV‐2 in AT on autopsy of individuals who have died of COVID‐19. Focus should be on analysis of specific cells that have evidence of infection by immunocytopathic and in situ viral detection techniques. Parallel studies should identify whether specific cell types in AT can support SARS‐CoV‐2 infection and replication ex vivo. With respect to the cytokine storm, an integrated‐systems biology approach would enable multiple pathways to be assessed simultaneously. In this regard, cytokine and chemokine genomic data analysis in blood and AT would be an important first step. Moreover a weighted gene correlation network analysis of SARS‐CoV‐2–mediated transcriptional response in infected cells could also be used as a model for human AT analysis downstream (39). Interesting aspects of transcriptional network analysis could then be tested in appropriate animal models to determine pivotal components of the cytokine storm, including key cytokine and chemokine genes that are conserved across species (40). These insights may allow rational diagnostics and therapeutic strategies to be developed. In line with this, IL‐6 inhibition has already been proposed as a treatment in COVID‐19, and the results of trials of tocilizumab are awaited (41). It would be interesting to examine whether individuals with obesity, who are expected to have higher circulating IL‐6 levels compared with lean counterparts, respond more favorably to IL‐6 inhibition strategies in COVID‐19 in a post hoc analysis of this randomized controlled trial. Similarly, tissue and systemic analysis of cytokine dynamics may identify likely responders and nonresponders to such therapy.
In summary, we present a rationale for studying the relationship between obesity and COVID‐19 disease severity. We provide a theoretical framework whereby viral systemic spread, entry, and prolonged viral shedding in already “inflamed” AT may augment immune responses with consequences for cytokine cascade amplification. We highlight AT as an abundant source for local and systemic enrichment of cytokines, some already independently associated with increased COVID‐19 mortality. Finally, we suggest a series of research studies to identify whether a mechanistic link exists among AT, SARS‐CoV‐2 infection, organ seeding of infection, immune activation, and the delayed cytokine storm known to presage rapid clinical decline in high‐risk patients with COVID‐19.
Disclosure
The authors declared no conflict of interest.
References
- 1. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin‐angiotensin‐aldosterone system inhibitors in patients with COVID‐19. N Engl J Med 2020;382:1653‐1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . Coronavirus Disease 2019 (COVID‐19): groups at higher risk for severe illness. Published 2020. Accessed April 4, 2020. https://www.cdc.gov/coronavirus/2019‐ncov/need‐extra‐precautions/groups‐at‐higher‐risk.html
- 3. Simonnet A, Chetboun M, Poissy J, et al; Lille Intensive Care COVID‐19 and Obesity Study Group . High prevalence of obesity in severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28:1195‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O'Rourke RW. Inflammation in obesity‐related diseases. Surgery 2009;145:255‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention [Published online February 24, 2020]. JAMA. doi: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 6. Louisiana Department of Health . Louisiana Department of Health Updates for 3/31/2020. Accessed April 6, 2020. http://ldh.la.gov/index.cfm/newsroom/detail/5522
- 7. Tsatsanis C, Margioris AN, Kontoyiannis DP. Association between H1N1 infection severity and obesity‐adiponectin as a potential etiologic factor. J Infect Dis 2010;202:459‐460. [DOI] [PubMed] [Google Scholar]
- 8. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with novel coronavirus‐infected pneumonia in Wuhan, China. JAMA 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China [Published online March 25, 2020]. JAMA Cardiol. doi: 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klok FA, Kruip M, van der Meer N, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res 2020;191:145‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marik PE, Chen C. The clinical characteristics and hospital and post‐hospital survival of patients with the obesity hypoventilation syndrome: analysis of a large cohort. Obes Sci Pract 2016;2:40‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gong MN, Bajwa EK, Thompson BT, Christiani DC. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax 2010;65:44‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mraz M, Haluzik M. The role of adipose tissue immune cells in obesity and low‐grade inflammation . J Endocrinol 2014;222:R113‐R127. [DOI] [PubMed] [Google Scholar]
- 16. Trim W, Turner JE, Thompson D. Parallels in immunometabolic adipose tissue dysfunction with ageing and obesity. Front Immunol 2018;9:169. doi: 10.3389/fimmu.2018.00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patel VB, Mori J, McLean BA, et al. ACE2 deficiency worsens epicardial adipose tissue inflammation and cardiac dysfunction in response to diet‐induced obesity. Diabetes 2016;65:85‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pahlavani M, Kalupahana NS, Ramalingam L, Moustaid‐Moussa N. Regulation and functions of the renin‐angiotensin system in white and brown adipose tissue. Compr Physiol 2017;7:1137‐1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chait A, den Hartigh LJ. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med 2020;7:22. doi: 10.3389/fcvm.2020.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uhlen M, Oksvold P, Fagerberg L, et al. Towards a knowledge‐based Human Protein Atlas. Nat Biotechnol 2010;28:1248‐1250. [DOI] [PubMed] [Google Scholar]
- 21. Gupte M, Thatcher SE, Boustany‐Kari CM, et al. Angiotensin converting enzyme 2 contributes to sex differences in the development of obesity hypertension in C57BL/6 mice. Arterioscler Thromb Vasc Biol 2012;32:1392‐1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maier HE, Lopez R, Sanchez N, et al. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis 2018;218:1378‐1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bouwman JJ, Visseren FL, Bouter KP, Diepersloot RJ. Infection‐induced inflammatory response of adipocytes in vitro. Int J Obes (Lond) 2008;32:892‐901. [DOI] [PubMed] [Google Scholar]
- 24. Ponterio E, Cangemi R, Mariani S, et al. Adenovirus 36 DNA in human adipose tissue. Int J Obes (Lond) 2015;39:1761‐1764. [DOI] [PubMed] [Google Scholar]
- 25. Zwezdaryk KJ, Ferris MB, Strong AL, et al. Human cytomegalovirus infection of human adipose‐derived stromal/stem cells restricts differentiation along the adipogenic lineage. Adipocyte 2016;5:53‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gu J, Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am J Pathol 2007;170:1136‐1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bouwman JJ, Diepersloot RJ, Visseren FL. Intracellular infections enhance interleukin‐6 and plasminogen activator inhibitor 1 production by cocultivated human adipocytes and THP‐1 monocytes. Clin Vaccine Immunol 2009;16:1222‐1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang W, Xu Y, Gao R, et al. Detection of SARS‐CoV‐2 in different types of clinical specimens. JAMA 2020;323:1843‐1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nishimura H, Itamura S, Iwasaki T, Kurata T, Tashiro M. Characterization of human influenza A (H5N1) virus infection in mice: neuro‐, pneumo‐ and adipotropic infection. J Gen Virol 2000;81:2503‐2510. [DOI] [PubMed] [Google Scholar]
- 30. Boutens L, Stienstra R. Adipose tissue macrophages: going off track during obesity. Diabetologia 2016;59:879‐894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Misumi I, Starmer J, Uchimura T, Beck MA, Magnuson T, Whitmire JK. Obesity expands a distinct population of T cells in adipose tissue and increases vulnerability to infection. Cell Rep 2019;27:514‐524.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis 2020:ciaa248. doi: 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kern L, Mittenbuhler MJ, Vesting AJ, Ostermann AL, Wunderlich CM, Wunderlich FT. Obesity‐induced TNFalpha and IL‐6 signaling: the missing link between obesity and inflammation‐driven liver and colorectal cancers. Cancers (Basel) 2018;11:E24. doi: 10.3390/cancers11010024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev 2012;76:16‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Park WY, Goodman RB, Steinberg KP, et al. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;164:1896‐1903. [DOI] [PubMed] [Google Scholar]
- 36. Yiu HH, Graham AL, Stengel RF. Dynamics of a cytokine storm. PLoS One 2012;7:e45027. doi: 10.1371/journal.pone.0045027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46:846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sindhu S, Thomas R, Shihab P, Sriraman D, Behbehani K, Ahmad R. Obesity is a positive modulator of IL‐6R and IL‐6 expression in the subcutaneous adipose tissue: significance for metabolic inflammation. PLoS One 2015;10:e0133494. doi: 10.1371/journal.pone.0133494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li C, Bankhead A 3rd, Eisfeld AJ, et al. Host regulatory network response to infection with highly pathogenic H5N1 avian influenza virus. J Virol 2011;85:10955‐10967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McDermott JE, Shankaran H, Eisfeld AJ, et al. Conserved host response to highly pathogenic avian influenza virus infection in human cell culture, mouse and macaque model systems. BMC Syst Biol 2011;5:190. doi: 10.1186/1752-0509-5-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chinese Clinical Trial Registry . ChiCTR2000029765 ‐ a multicenter, randomized controlled trial for the efficacy and safety of tocilizumab in the treatment of new coronavirus pneumonia (COVID‐19). Posted 2020. Accessed April 6, 2020. http://www.chictr.org.cn/showprojen.aspx?proj=49409


