Abstract
The Middle East respiratory syndrome coronavirus (MERS‐CoV) is an emerging virus that causes infection with a potentially fatal outcome. Dendrimers are highly branched molecules that can be added to antiviral preparations to improve their delivery, as well as their intrinsic antiviral activity. Studies on identifying anti‐MERS‐CoV agents are few. Three types of polyanionic dendrimers comprising the terminal groups sodium carboxylate (generations 1.5, 2.5, 3.5, and 4.5), hydroxyl (generations 2, 3, 4, and 5), and succinamic acid (generations 2, 3, 4, and 5) and polycationic dendrimers containing primary amine (generations 2, 3, 4, and 5) were used to assess their antiviral activity with the MERS‐CoV plaque inhibition assay. The hydroxyl polyanionic set showed a 17.36% to 29.75% decrease in MERS‐CoV plaque formation. The most potent inhibition of MERS‐CoV plaque formation was seen by G(1.5)‐16COONa (40.5% inhibition), followed by G(5)‐128SA (39.77% inhibition). In contrast, the cationic dendrimers were cytotoxic to Vero cells. Polyanionic dendrimers can be added to antiviral preparations to improve the delivery of antivirals, as well as the intrinsic antiviral activity.
Keywords: antiviral agents, cell cultures, coronavirus, research and analysis methods, virus classification
Highlights
MERS CoV is an emerging viral disease with fatal consequences.
Anti‐MERS CoV studies are very limited.
The anti‐MERS CoV activity of several generations polyvalent charge dendrimers was investigated.
PAMAM dendrimers bears intrinsic anti‐MESR CoV activity.
Polyanionic carboxylate PAMAM dendrimers were the most effective agents.
1. INTRODUCTION
The Middle East respiratory syndrome coronavirus (MERS‐CoV) is a major health hazard in several countries. 1 Like the severe acute respiratory system (SARS)‐CoV, the MERS‐CoV is transferred to humans from animal sources. 2 The MERS‐CoV was found to be transferred within human families, so that it caused a communicable disease. 3 The disease was initially found on the Arabian peninsula and it then spread to several countries around the world. 4
Dendrimers are highly branched structures with repetitive sequences of monomers called dendrons. Dendrimers have three main components: (a) a core moiety, (b) branching units, and (c) surface groups. 5 The diameter of a dendrimer is nanosized, similar to certain globular proteins. For instance, the G4 polyamidoamine (PAMAM) dendrimer has a diameter of 4 nm, which is identical to the diameter of cytochrome c. The diameter of the G5 PAMAM is 5 nm, like that of hemoglobin. Therefore, dendrimers are considered to be biomimetics of synthesized proteins, but they have significantly better stability (protease resistance); more lack of complex beta‐sheets, coils, and loops of proteins; and a better intrinsic ability to bind drugs through their well‐defined internal cavities and surface functions. 6
Dendrimers have unique structural features 7 : (a) their sizes vary from less than 2 nm to more than 10 nm, according to the number of dendrimeric generations. (b) Their monodispersity results from the formation of a uniform molecular structure. (c) They have a modifiable surface functionality because of their various chemical compositions or drug conjugates. (d) They have water solubility owing to the coating of their hydrophobic cores with charged molecules. (e) Their core compositions vary, especially their hydrophobic cores, and this attracts hydrophobic drugs. The mixed hydrophobic cores and charged surfaces can allow for the solubilization of hydrophobic drugs and modulation of their absorption, distribution, and other pharmacokinetic and pharmacodynamic properties.
Dendrimers have been shown to have unique intrinsic antimicrobial properties, including antiviral activities. 8 Dendrimers have been shown to have antiviral activity against the influenza virus, 9 human immunodeficiency virus, 10 and respiratory syncytial virus. 11 Dendrimers have different functional groups on their surfaces and can block the entry of a virus into cells either by cellular protection or by their direct effects on virus particles. 12 Previous studies revealed that the antiviral mechanism against the herpes simplex virus occurred during the early stages of infection, possibly during the adsorption of the virus to the cell. 13 This was shown by the poor efficiency of the dendrimers when they were added after the exposure of cells to the virus.
This study was carried out to interpolate the effect of the dendrimer size and variable terminal charge on the ability of MERS‐CoV to produce viral plaques in infected Vero cells. To the best of our knowledge, this is the first study to test the effect of dendrimers on this newly emerged fatal virus.
2. MATERIALS AND METHODS
2.1. Dendrimers
All dendrimers were synthesized by Dendritech, Inc (Midland, MI). The dendrimer set included three different polyanionic dendrimers and one polycationic dendrimer. The polyanionic dendrimer sets comprised one and one‐half to five generations and three different terminal functional groups, the hydroxyl, carboxyl, and succinamic acid terminated PAMAMs. The polycationic dendrimers comprised primary amine terminal groups (Figure 1). All dendrimers were prepared in dimethyl sulfoxide with 1‐mM stock. In this study, dendrimers with negative or positive charges were studied to evaluate their deleterious effects on the MERS‐CoV outer membrane. The negative‐charge, or polyanionic, dendrimers bore either sodium carboxylate (generations 1.5, 2.5, 3.5, and 4.5), hydroxyl (generations 2, 3, 4, and 5), or succinamic acid (generations 2, 3, 4, and 5). The positive‐charge, or polycationic, dendrimers contained primary amine (generations 2, 3, 4, and 5).
Figure 1.

The structure of G1 and G2 PAMAMs, showing the terminal 8 and 16 primary amine terminal groups, respectively. In this study, the PAMAM dendrimer terminal groups used were sodium carboxylate, primary amine, hydroxyl, and succinamic acid. PAMAM, polyamidoamine
2.2. Cell line and virus
Vero cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM, Thermo Fisher Scientific, Waltham, MA) supplemented with 10% fetal bovine serum (FBS, Thermo Fisher Scientific), 25 mM HEPES, 100 U/mL penicillin, and 100 µg/mL streptomycin in a CO2 incubator at 37°C. MERS‐CoV/KOR/KNIH/002_05_2015 was provided by the Korea Centers for Disease Control and Prevention (permission no. 1‐001‐MER‐IS‐2015001).
2.3. Virus amplification and quantification
Vero cells (2 × 105/well) were cultured in six‐well plates using DMEM media containing 10% FBS and incubated at 37°C overnight in a CO2 incubator. After rinsing with phosphate buffered saline (PBS), MERS‐CoV at multiplicity of infection 0.01 in 500 μL of PBS was added to each well and then incubated at 37°C in a CO2 incubator. After incubation for 1 hour, the supernatants were removed, 2 mL of DMEM/F12 medium (Thermo Fisher Scientific) was added, and the substance was incubated at 37°C in a CO2 incubator for 3 days. The virus culture supernatants were harvested and centrifuged at 2000 rpm for 10 minutes at 4°C. The amplified viruses were quantified by plaque assay. Vero cells (6 × 105/well) were cultured in six‐well plates. The cells were cultured until a monolayer was formed at 37°C in a CO2 incubator, and then the cells were washed with PBS and infected with the amplified MERS‐CoV culture supernatants after a 10‐fold serial dilution. After incubation for 1 hour, the supernatants were removed and an overlay of DMEM/F12 medium (Thermo Fisher Scientific) containing 0.6% oxoid agar was placed over the cell monolayer. Plaques were allowed to develop for 72 hours at 37°C. Plates were stained with 0.1% crystal violet in 20% methanol for 1 hour, before enumeration. The resultant virus supernatants (5 × 106 pfu/mL) were aliquoted at 400 µL per Eppendorf tube and stored in a deep freezer.
2.4. Plaque formation assay
The plaque reduction assay was performed as reported previously. 14 Briefly, Vero cells (6 × 105)/well were cultured on six‐well plates (Thermo Fisher Scientific) for 12 hours. MERS‐CoV (600 pfu/well) was mixed with each dendrimer at a final concentration of 10 µM for 30 minutes at 37°C. The mixtures of MERS‐CoV and each dendrimer were treated to Vero cells in each well and then incubated for 1 hour. After incubation, the supernatants were removed, and a DMEM/F12 medium (Thermo Fisher Scientific) containing 0.6% oxoid agar was transferred to each well. Four days after infection, plaque formation was observed by staining with crystal violet, and the plaque numbers were counted.
3. RESULTS AND DISCUSSION
In this study, we focused on the first structure‐activity relationships of the dendrimers of various generations and their surface charges in the infectivity of MERS‐CoV.
G(1.5)‐16COONa was the most effective carboxylate polyanionic dendrimer, with a 40.5% decrease in MERS‐CoV plaque formation (Table 1; Figure 2). G(2.5)‐32COONa was inactive, and G(3.5)‐64COONa had 19.83% inhibition and G(4.5)‐128COONa had 29.17% inhibition.
Table 1.
Inhibition of MERS‐CoV infection by the dendrimers
| Total plaque no. | % Plaque no. | |
|---|---|---|
| Control (DMSO) | 484 | 100.00 |
| G(1.5)‐16COONa | 288 | 59.50 |
| G(2.5)‐32COONa | 480 | 99.17 |
| G(3.5)‐64COONa | 388 | 80.17 |
| G(4.5)‐128COONa | 340 | 70.25 |
| G(2)‐16OH | 400 | 82.64 |
| G(3)‐32OH | 348 | 71.90 |
| G(4)‐64OH | 340 | 70.25 |
| G(5)‐128OH | 380 | 78.51 |
| G(2)‐16SA | 356 | 73.55 |
| G(3)‐32SA | 416 | 85.95 |
| G(4)‐64SA | 400 | 82.64 |
| G(5)‐128SA | 292 | 60.33 |
| G(2)‐16NH2 | 376 | 77.69 |
| G(3)‐32NH2 | 316 | 65.29 |
| G(4)‐64NH2 | ND | ND |
| G(5)‐128NH2 | ND | ND |
Note: Before MERS‐CoV infection in Vero cells, MERS‐CoV was incubated with one of 12 candidate dendrimers (10 µM) for 30 minute at 37°C and then the plaque formation assay was performed. The numbers of plaques on the quadrant of the plates were counted and then multiplied by four. The percentage of the MERS‐CoV infection in the samples pretreated with each dendrimer was compared with 1% DMSO treatment control.
Abbreviations: DMSO, dimethyl sulfoxide; MERS‐CoV, Middle East respiratory syndrome coronavirus; ND, not determined.
Figure 2.
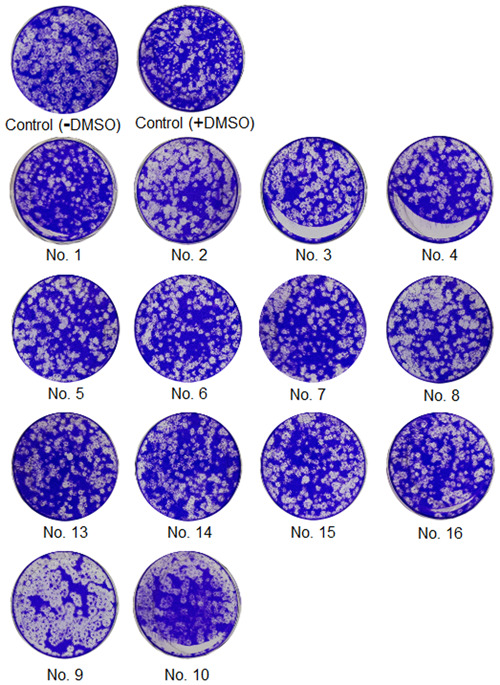
Screening of the dendrimers against MERS‐CoV infection. The plaque formation assay was performed with 12 dendrimers. Before MERS‐CoV infection, MERS‐CoV (600 pfu/well) was incubated with each dendrimer (10 µM) for 30 minutes at 37°C and then added to Vero cells to infect them with MERS‐CoV. After 4 days of incubation in DMEM/F12 containing 0.6% oxoid agar, the plaques were revealed by staining with crystal violet and counted. +DMSO indicates treatment with 1% DMSO. DMSO, dimethyl sulfoxide; MERS‐CoV, Middle East respiratory syndrome coronavirus
The hydroxyl polyanionic set was weaker than the sodium carboxylates, showing 17.36% to 29.75% in the MERS‐CoV plaque formation. Succinamic acid generations 2 and 5 produced 26.45% and 39.77% inhibition, respectively.
Primary amine‐terminated PAMAM dendrimers were severely cytotoxic. G(2)‐16NH2 and G(3)‐32NH2 produced a 22% and 44% decrease, respectively, in MERS‐CoV plaque formation. However, G(4)‐64NH2 and G(5)‐128NH2 were severely cytotoxic.
Most of the toxic effects of the dendrimers came from their size or surface charge, which interacted with the lipids of cell membranes. Cationic dendrimers were more toxic to human and animal cells, whereas anionic dendrimers had safe profiles. 15 Cell membranes bore a negative charge, which by electrostatic interactions could attract cationic dendrimers to their surfaces. Therefore, larger generations of charged dendrimers had higher degrees of toxic effects on cell membranes. Higher‐numbered dendrimer generations with a more positive surface charge could attract and lyse the lipids in cell membranes, resulting in cytotoxicity. 16 Lower‐numbered generations of cationic dendrimers could distribute readily to the inside of cells, allowing the rapid intracellular delivery of drugs by their attraction to cell membranes. Besides, the cell penetration capacity of noncharged dendrimers depended on the polarity of their terminal moieties, so that nonpolar substitutions could readily penetrate cell membranes, and vice versa. 17 Fortunately, the in vivo toxicity in whole animals was lower compared with that in isolated cell culture experiments. 18 For example, the PAMAM dendrimer was nontoxic up to G5, and toxicity was observed in up to G7 in mice. 19 Based on these previously estimated data, G(1.5)‐16COONa with terminal 16 negative groups can be recommended for further anti‐MERS‐CoV studies and for application in antiviral preparations owing to its safety and intrinsic antiviral activity. This agrees with previous findings on the effect of anionic citrate‐PEG‐citrate dendrimers, which showed the strongest anti‐HIV activity by G2 dendrimers. 20 Furthermore, G3 sulphated and G2 naphthylsulphonated carbosilane dendrimers were the most effective antivirals against HIV. 21
There were several trials for the development of new therapeutics against MERS‐CoV. Three new small‐molecule fusion inhibitors were developed by targeting the fusion core protein. 22 The natural phenolic derivative resveratrol showed potent anti‐MERS‐CoV actions accompanied by a lower expression of nucleocapsid and a reduced viral RNA expression and MERS‐CoV yield. 23 Several short peptides were effective fusion inhibitors against MERS‐CoV in the low nanomolar range. 24 Small‐molecule inhibitors were also found for MERS‐CoV papain‐like protease, including the main protease and the proteases miscellaneously acting on host proteins or interfering with virus entry and replication. 24 Future studies are required on the combination of several dendrimers for checking the potential synergistic actions. Because dendrimers interact with lipid membranes, further studies are needed of them in combination with other anti‐MERS‐CoV agents that act on the extracellular or intracellular stages of the virus replication cycle.
4. CONCLUSIONS
In establishing a structure‐activity relationship of cationic and anionic dendrimers against MERS‐CoV, 16 dendrimers with various generations and terminal charges were used. The dendrimers at a final concentration of 10 µM were mixed with the MERS‐CoV before the infection of Vero cells. Most dendrimers produced a variable degree of inhibitory activity on plaque formation. Among them, G(1.5)‐16COONa and G(5)‐128SA were the strongest, with ∼40% decrease in MERS‐CoV plaque formation. These dendrimers can be a base for further modifications and inclusions in antiviral therapy.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for the studies that are basis of this study.
CONFLICT OF INTERESTS
All the authors declare that there are no conflict of interests.
Supporting information
Supplementary information
ACKNOWLEDGMENTS
The authors acknowledge the Deanship of Scientific Research at King Faisal University for the financial support under Strategic projects track (grant no 171001). Hyung‐Joo Kwon was supported by grants from the National Research Foundation (2016M3A9B6916708) funded by the Ministry of Science and ICT in the Republic of Korea. We thank the central labs at King Faisal University for facilities.
Kandeel M, Al‐Taher A, Park BK, Kwon H‐J, Al‐Nazawi M. A pilot study of the antiviral activity of anionic and cationic polyamidoamine dendrimers against the Middle East respiratory syndrome coronavirus. J Med Virol. 2020;92:1665–1670. 10.1002/jmv.25928
Contributor Information
Mahmoud Kandeel, Email: mkandeel@kfu.edu.sa.
Hyung‐Joo Kwon, Email: hjookwon@hallym.ac.kr.
REFERENCES
- 1. Rahman A, Sarkar A. Risk factors for fatal middle east respiratory syndrome coronavirus infections in Saudi Arabia: analysis of the WHO line list, 2013–2018. Am J Public Health. 2019;109(9):1288‐1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan JF, Lau SK, To KK, Cheng VC, Woo PC, Yuen K‐Y. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS‐like disease. Clin Microbiol Rev. 2015;28(2):465‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Memish ZA, Zumla AI, Al‐Hakeem RF, Al‐Rabeeah AA, Stephens GM. Family cluster of Middle East respiratory syndrome coronavirus infections. New Engl J Med. 2013;368(26):2487‐2494. [DOI] [PubMed] [Google Scholar]
- 4. Cho SY, Kang J‐M, Ha YE, et al. MERS‐CoV outbreak following a single patient exposure in an emergency room in South Korea: an epidemiological outbreak study. Lancet. 2016;388(10048):994‐1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nanjwade BK, Bechra HM, Derkar GK, Manvi FV, Nanjwade VK. Dendrimers: emerging polymers for drug‐delivery systems. Eur J Pharm Sci. 2009;38(3):185‐196. [DOI] [PubMed] [Google Scholar]
- 6. Esfand R, Tomalia DA. Poly(amidoamine) (PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discov Today. 2001;6(8):427‐436. [DOI] [PubMed] [Google Scholar]
- 7. Talanov VS, Regino CA, Kobayashi H, Bernardo M, Choyke PL, Brechbiel MW. Dendrimer‐based nanoprobe for dual modality magnetic resonance and fluorescence imaging. Nano Lett. 2006;6(7):1459‐1463. [DOI] [PubMed] [Google Scholar]
- 8. Bourne N, Stanberry LR, Kern ER, Holan G, Matthews B, Bernstein DI. Dendrimers, a new class of candidate topical microbicides with activity against herpes simplex virus infection. Antimicrob Agents Chemother. 2000;44(9):2471‐2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oka H, Onaga T, Koyama T, et al. Sialyl α (2→ 3) lactose clusters using carbosilane dendrimer core scaffolds as influenza hemagglutinin blockers. Bioorg Med Chem Lett. 2008;18(15):4405‐4408. [DOI] [PubMed] [Google Scholar]
- 10. Witvrouw M, Pannecouque C, Matthews B, et al. Dendrimers inhibit the replication of human immunodeficiency virus (HIV) by a dual mechanism of action. Antiviral Res. 1999;41(2):25. [Google Scholar]
- 11. Barnard D, Sidwell R, Gage T, Okleberry K, Matthews B, Holan G. Anti‐respiratory syncytial virus activity of dendrimer polyanions. Antiviral Res. 1997;34(2):A88. [Google Scholar]
- 12. Rodríguez‐Izquierdo I, Natalia C, García F, Los Ángeles Muñoz‐Fernandez M. G2‐S16 sulfonate dendrimer as new therapy for treatment failure in HIV‐1 entry inhibitors. Nanomedicine. 2019;14(9):1095‐1107. [DOI] [PubMed] [Google Scholar]
- 13. Gajbhiye V, Palanirajan VK, Tekade RK, Jain NK. Dendrimers as therapeutic agents: a systematic review. J Pharm Pharmacol. 2009;61(8):989‐1003. [DOI] [PubMed] [Google Scholar]
- 14. Park BK, Maharjan S, Lee SI, et al. Generation and characterization of a monoclonal antibody against MERS‐CoV targeting the spike protein using a synthetic peptide epitope‐CpG‐DNA‐liposome complex. BMB Rep. 2019;52(6):397‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ziemba B, Matuszko G, Bryszewska M, Klajnert B. Influence of dendrimers on red blood cells. Cell Mol Biol Lett. 2012;17(1):21‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sebestik J, Niederhafner P, Jezek J. Peptide and glycopeptide dendrimers and analogous dendrimeric structures and their biomedical applications. Amino Acids. 2011;40(2):301‐370. [DOI] [PubMed] [Google Scholar]
- 17. Mishra V, Gupta U, Jain N. Surface‐engineered dendrimers: a solution for toxicity issues. J Biomater Sci Polym Ed. 2009;20(2):141‐166. [DOI] [PubMed] [Google Scholar]
- 18. Pryor JB, Harper BJ, Harper SL. Comparative toxicological assessment of PAMAM and thiophosphoryl dendrimers using embryonic zebrafish. Int J Nanomed. 2014;9:1947‐1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roberts JC, Bhalgat MK, Zera RT. Preliminary biological evaluation of polyamidoamine (PAMAM) StarburstTM dendrimers. J Biomed Mater Res. 1996;30(1):53‐65. [DOI] [PubMed] [Google Scholar]
- 20. Kandi MR, Mohammadnejad J, Shafiee Ardestani M, et al. Inherent anti‐HIV activity of biocompatible anionic citrate‐PEG‐citrate dendrimer. Mol Biol Rep. 2019;46(1):143‐149. [DOI] [PubMed] [Google Scholar]
- 21. Vacas Córdoba E, Arnaiz E, Relloso M, et al. Development of sulphated and naphthylsulphonated carbosilane dendrimers as topical microbicides to prevent HIV‐1 sexual transmission. AIDS. 2013;27(8):1219‐1229. [DOI] [PubMed] [Google Scholar]
- 22. Kandeel M, Yamamoto M, Al‐Taher A, et al. Small molecule inhibitors of Middle East respiratory syndrome coronavirus fusion by targeting cavities on heptad repeat trimers [published online ahead of print March 4, 2020]. Biomol Ther. 2020. 10.4062/biomolther.2019.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin S‐C, Ho C‐T, Chuo W‐H, Li S, Wang TT, Lin C‐C. Effective inhibition of MERS‐CoV infection by resveratrol. BMC Infect Dis. 2017;17(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liang R, Wang L, Zhang N, et al. Development of small‐molecule MERS‐CoV inhibitors. Viruses. 2018;10(12):721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


