Abstract
Background
Rapid spread of the severe acute respiratory syndrome‐coronavirus‐2 (SARS‐CoV‐2) virus has left many health systems around the world overwhelmed, forcing triaging of scarce medical resources. Identifying indicators of hospital admission for coronavirus disease 2019 (COVID‐19) patients early in the disease course could aid the efficient allocation of medical interventions. Self‐reported olfactory impairment has recently been recognized as a hallmark of COVID‐19 and may be an important predictor of clinical outcome.
Methods
A retrospective review of all patients presenting to a San Diego Hospital system with laboratory‐confirmed positive COVID‐19 infection was conducted with evaluation of olfactory and gustatory function and clinical disease course. Univariable and multivariable logistic regression were performed to identify risk factors for hospital admission and anosmia.
Results
A total of 169 patients tested positive for COVID‐19 disease between March 3 and April 8, 2020. Olfactory and gustatory data were obtained for 128 (75.7%) of 169 subjects, of which 26 (20.1%) of 128 required hospitalization. Admission for COVID‐19 was associated with intact sense of smell and taste, increased age, diabetes, and subjective and objective parameters associated with respiratory failure. On adjusted analysis, anosmia was strongly and independently associated with outpatient care (adjusted odds ratio [aOR] 0.09; 95% CI, 0.01‐0.74), whereas positive findings of pulmonary infiltrates and/or pleural effusion on chest radiograph (aOR 8.01; 95% CI, 1.12‐57.49) was strongly and independently associated with admission.
Conclusion
Normosmia is an independent predictor of admission in COVID‐19 cases. Smell loss in COVID‐19 may be associated with a milder clinical course.
Keywords: COVID‐19, smell loss, taste loss, patient outcomes, admission, hospitalization
The rapid worldwide spread of the coronavirus disease 2019 (COVID‐19) pandemic has placed an unprecedented strain on hospitals and healthcare systems. 1 Severe acute respiratory syndrome‐coronavirus‐2 (SARS‐CoV‐2), the virus mediating COVID‐19, continues to affect individuals of all ages, ranging from asymptomatic to fatal infection. 2 , 3 , 4 , 5 , 6 , 7 Recent examples of overwhelmed healthcare systems underline the urgent need for developing strategies to predict early symptoms and disease trajectory with biomarkers and clinical prognosticators. 8 A cost‐effective method of early risk stratification of disease severity would enable improved medical decision‐making, rapid and severity‐appropriate intervention, and facilitate the allocation of limited medical resources. Under such a paradigm, patients found to carry markers associated with severe manifestations of COVID‐19 would be considered high‐risk and monitored closely for further escalation of care. Meanwhile, those displaying markers associated with low‐risk disease could be recommended to self‐monitor under quarantine conditions, allowing health systems to conserve scarce resources for impending COVID‐19 case surges or the care of non–COVID‐19 patients.
Olfactory and gustatory dysfunction have recently been found to be associated with COVID‐19 infection. 9 , 10 , 11 , 12 The growing number of Internet searches inquiring about loss of smell strongly correlates with the increased prevalence of COVID‐19. 13 In ambulatory populations, patients who present with influenza‐like symptoms and anosmia are 6 to 10 times more likely to test positive for COVID‐19 infection. 9 , 10 Indeed, 59% to 86% of outpatient COVID‐19 positive patients self‐reported olfactory loss. 9 , 10 , 12 Notably, self‐reported anosmia in COVID‐19–positive hospitalized patients has also been identified as a common symptom, but is consistently reported at lower rates in this population (5‐35%). 11 , 14 , 15 Although limited quantitative olfactory data exist in either outpatient or inpatient contexts, early findings in inpatients with COVID‐19 suggest that only 28% self‐reported smell loss and a minority (25%) demonstrated complete anosmia, despite some degree of measurable olfactory dysfunction in almost all subjects. 11 In contrast, in an outpatient managed cohort of COVID‐19 subjects, the self‐reported severity and incidence of olfactory dysfunction was high, with most patients reporting a profound and complete loss of smell (0/10). 9 Comparing the prevalence of self‐reported smell loss between mild or ambulatory cases and moderate to severe inpatient cases of COVID‐19 may provide insights into an individual's disease prognosis. This study sought to investigate the association between self‐reported anosmia and hospital admission during the course of COVID‐19.
Subjects and methods
Study design and population
A retrospective analysis of all adult subjects presenting to the UC San Diego Health System (Jacobs and Hillcrest Medical Centers) with confirmed polymerase chain reaction (PCR)‐positive testing for the SARS‐CoV‐2 viral nucleic acid from nasopharyngeal swabs was done. Demographic data (Table 1) along with subjective and objective clinical data (Table 2) were obtained from review of electronic medical records (EMR), specifically encounters pertaining to COVID‐19 diagnosis. Self‐reported sense of smell and taste during time of illness were compared to premorbid levels as dichotomous variables (loss of smell/taste vs normal/baseline). Specifically, we first retrospectively queried the EMR for assessments on olfactory/gustatory function in the encounters related to COVID‐19. If self‐reported olfactory/gustatory function data were not available in the EMR, we emailed or called patients to inquire about the status of olfactory and gustatory function (“Have you had any smell loss during this period of illness compared to before?” and “Have you had any taste loss during this illness compared to before?”). Chest radiograph findings were categorized as “negative” if no findings were present and defined as “positive” if presence of pulmonary infiltrates and/or pleural effusions were reported on final read by the attending radiologist. This study was approved by the Institutional Review Board of University of California San Diego (IRB #200485).
TABLE 1.
Baseline characteristics: comparison of demographic and baseline clinical characteristics in admitted and ambulatory COVID‐19–positive subjects
| Characteristic |
COVID‐19–positive Admitted (n = 26) |
COVID‐19–positive Ambulatory (n = 102) |
p a |
|---|---|---|---|
| Baseline characteristics | |||
| Age (years), median (IQR) b | 53.5 (40–65) | 43 (34–54) | 0.0093 |
| Gender, n (%) | |||
| Male | 9 (34.6) | 52 (51) | 0.14 |
| Female | 17 (65.4) | 50 (49) | |
| Race, n (%) | |||
| White | 8 (30.8) | 50 (49) | 0.29 |
| Black | 3 (11.5) | 5 (4.9) | |
| Hispanic | 7 (26.9) | 21 (20.6) | |
| Asian | 4 (15.4) | 7 (6.9) | |
| Other/mixed | 4 (15.4) | 13 (12.8) | |
| Unknown/missing | 0 | 6 (5.9) | |
| BMI (kg/m2), median (IQR) b | 28.4 (25.7–31.2) | 25.9 (23.1–29.7) | 0.11 |
| Tobacco use, n (%) | |||
| Never smoker | 22 (84.6) | 79 (77.5) | 0.87 |
| Current/recent smoker | 4 (15.4) | 13 (12.8) | |
| Unknown/missing | 0 | 10 (9.8) | |
| Past medical history, n (%) | |||
| Hypertension | |||
| No | 19 (73.1) | 81 (79.4) | 0.18 |
| Yes | 7 (26.9) | 15 (14.7) | |
| Unknown/missing | 0 | 6 (5.9) | |
| Diabetes mellitus | |||
| No | 18 (69.2) | 90 (88.2) | 0.001 |
| Yes | 8 (30.8) | 6 (5.9) | |
| Unknown/missing | 0 | 6 (5.9) | |
| COPD | |||
| No | 26 (100) | 92 (90.2) | 0.29 |
| Yes | 0 | 4 (3.9) | |
| Unknown/missing | 0 | 6 (5.9) | |
| Asthma | |||
| No | 23 (88.5) | 86 (84.3) | 0.87 |
| Yes | 3 (11.5) | 10 (9.8) | |
| Unknown/missing | 0 | 6 (5.9) | |
| Sinusitis c | |||
| No | 26 (100) | 91 (89.2) | 0.24 |
| Yes | 0 | 5 (4.9) | |
| Unknown/missing | 0 | 6 (5.9) | |
| Cardiovascular disease | |||
| No | 23 (88.5) | 91 (89.2) | 0.25 |
| Yes | 3 (11.5) | 5 (4.9) | |
| Unknown/missing | 0 | 6 (5.9) | |
| Chronic kidney disease | |||
| No | 24 (92.3) | 94 (92.2) | 0.15 |
| Yes | 2 (7.7) | 2(2.0) | |
| Unknown/missing | 0 | 6 (5.9) | |
| Cancer | |||
| No | 25 (96.2) | 91 (89.2) | 0.78 |
| Yes | 1 (3.8) | 5 (4.9) | |
| Unknown/missing | 0 | 6 (5.9) | |
| HIV/other immunosuppression | |||
| No | 24 (92.3) | 82 (80.4) | 0.41 |
| Yes | 2 (7.7) | 13 (12.8) | |
| Unknown/missing | 0 | 7 (6.9) | |
| Obstructive sleep apnea | |||
| No | 25 (96.2) | 93 (91.2) | 0.86 |
| Yes | 1 (3.8) | 3 (2.9) | |
| Unknown/missing | 0 | 6 (5.9) | |
| Stroke | |||
| No | 23 (88.5) | 96 (94.1) | 0.001 |
| Yes | 3 (11.5) | 0 | |
| Unknown/missing | 0 | 6 (5.9) |
Values of p determined by chi‐square test unless otherwise specified, unknowns excluded in statistical testing.
Student 2‐way t test.
Chronic rhinosinusitis or currently experiencing acute episode of rhinosinusitis.
BMI = body mass index; COVID‐19 = coronavirus 2019; COPD = chronic obstructive pulmonary disease; HIV = human immunodeficiency virus; IQR = interquartile range.
TABLE 2.
COVID‐19–associated clinical findings: comparison of subjective and objective clinical findings in admitted and ambulatory COVID‐19–positive subjects
| Parameter | COVID‐19–positive Admitted (n = 26) | COVID‐19–positive Ambulatory (n = 102) | p a |
|---|---|---|---|
| Subjective clinical findings | |||
| Days symptomatic prior to COVID‐19 test, median (IQR) b | 4 (2–7) | 5.5 (3–8) | 0.39 |
| Anosmia/hyposmia, n (%) | <0.001 | ||
| No | 19 (73.1) | 34 (33.3) | |
| Yes | 7 (26.9) | 68 (66.7) | |
| Dysgeusia, n (%) | <0.001 | ||
| No | 19 (73.1) | 31 (30.4) | |
| Yes | 6 (23.1) | 64 (62.7) | |
| Unknown/missing | 1 (3.8) | 7 (6.9) | |
| Fatigue, n (%) | 0.19 | ||
| No | 5 (19.2) | 33 (32.4) | |
| Yes | 21 (80.8) | 69 (67.6) | |
| Diarrhea, n (%) | |||
| No | 12 (46.2) | 68 (66.7) | 0.054 |
| Yes | 14 (53.8) | 34 (33.3) | |
| Fever, n (%) | 0.074 | ||
| No | 4 (15.4) | 34 (33.3) | |
| Yes | 22 (84.6) | 68 (66.7) | |
| Cough, n (%) | 0.14 | ||
| No | 1 (3.8) | 15 (14.7) | |
| Yes | 25 (96.2) | 87 (85.3) | |
| Dyspnea, n (%) | 0.002 | ||
| No | 6 (23.1) | 58 (56.9) | |
| Yes | 20 (76.9) | 44 (43.1) | |
| Sputum production, n (%) | 0.03 | ||
| No | 11 (42.3) | 53 (51.9) | |
| Yes | 8 (30.8) | 12 (11.8) | |
| Unknown/missing | 7 (26.9) | 37 (36.3) | |
| Sore throat, n (%) | 0.34 | ||
| No | 17 (65.4) | 56 (54.9) | |
| Yes | 9 (34.6) | 46 (45.1) | |
| Headache, n (%) | 0.86 | ||
| No | 13 (50.0) | 53 (51.9) | |
| Yes | 13 (50.0) | 49 (48.1) | |
| Rhinorrhea, n (%) | 0.11 | ||
| No | 25 (96.2) | 86 (84.3) | |
| Yes | 1 (3.8) | 16 (15.7) | |
| Nasal obstruction/thick discharge, n (%) | 0.13 | ||
| No | 22 (84.6) | 71 (69.6) | |
| Yes | 4 (15.4) | 31 (30.4) | |
| Objective clinical findings | |||
| Temperature, median (IQR) b | 99.8 (99–101.4) | 98.6 (98.3–99) | <0.001 |
| Heart rate, median (IQR) b | 95.5 (83–106) | 83 (75–96) | 0.004 |
| Respiratory rate, median (IQR) b | 19 (18–23) | 18 (16–18) | <0.001 |
| Blood oxygen saturation, median (IQR) b | 96 (94–98) | 98 (95–100) | 0.77 |
| Total leukocyte count, median (IQR) b | 6.5 (5.7–9.4) | 4.9 (3–5.4) | 0.012 |
| Lymphocyte count, median (IQR) b | 1.1 (0.6–13) | 1.8 (0.9–24) | 0.41 |
| Serum creatinine, median (IQR) b | 0.8 (0.6–1.0) | 0.9 (0.8–0.9) | 0.34 |
| Serum AST, median (IQR) b | 44 (28–68) | 34 (27–51) | 0.14 |
| Serum ALT, median (IQR) b | 32 (20–82) | 31 (23–44) | 0.15 |
| Serum lactate, median (IQR) b | 1.9 (1.5–2.2) | 1.4 (1.1–1.9) | 0.43 |
| Chest radiograph performed, n (%) | <0.001 | ||
| No | 2 (7.7) | 64 (62.7) | |
| Yes | 24 (92.3) | 35 (34.3) | |
| Unknown/missing | 0 | 3 (2.9) | |
| Chest radiograph findings, n (%) c | <0.001 | ||
| Negative | 2 (7.7) | 20 (19.6) | |
| Positive | 23 (88.5) | 15 (14.7) | |
| No chest radiograph | 1 (3.8) | 67 (65.7) |
Value of p determined by chi‐square test, unless otherwise specified, unknowns excluded in statistical test.
Value of p determined by t test.
Positive findings include pulmonary infiltrates and/or pleural effusion.
ALT = alanine aminotransferase; AST = aspartate aminotransferase; COVID‐19 = coronavirus 2019; IQR = interquartile range.
Statistical analysis
Statistical analysis was conducted with Stata 15.1 software (StataCorp, College Station, TX). The primary outcome was hospital admission. The secondary outcome was anosmia. Descriptive comparisons stratified by hospital admission and anosmia were conducted using a chi‐square (χ2) test for categorical data and 2‐way Student t test for continuous variables. The threshold for statistical significance was set at p < 0.05. Multivariable logistic regression models were built to identify independent patient characteristics associated with hospital admission as the dependent variable. Logistic regression was also performed with anosmia as the dependent variable. Inclusion criteria for factor inclusion in multivariable models were set a priori and included variables with the strongest magnitudes of association and lowest probability of type I error on univariable logistic regression. A maximum of 8 variables were included to minimize potential model overfitting. Given the known colinear relationship between anosmia and dysgeusia, analyses focused on anosmia alone. Similarly, analyses involving decision to admit and decision to obtain a chest radiograph focused on decision to admit; these variables were thus also considered colinear. Although anosmia is not plausibly dependent upon hospital admission, hospital admission was kept in the multivariable model investigating associations with anosmia as a marker for overall disease severity. Distribution medians with interquartile ranges (IQRs), odds ratios (ORs; univariable logistic regression), adjusted odds ratios (aORs; multivariable logistic regression), and 95% confidence intervals (CIs) were reported with corresponding probabilities of type‐I error (p value).
Results
Clinical characteristics
A total of 169 patients tested positive for COVID‐19 infection between March 3, 2020, and April 8, 2020. Smell and taste data were available for 128 subjects (75.7%) who were included in the final study cohort. Patients without smell and taste data included those who were deceased (3/169; 1.7%) or intubated (6/169; 3.5%) at the close of data collection, were recently hospitalized with incomplete admission data (4/169; 2.4%), or could not be reached or declined to participate in the study and had no smell or taste information recorded in the medical record (28/169; 16.6%). The demographics and baseline clinical characteristics of the study participants are described in Table 1. Table 2 reports the cohort's subjective and objective clinical findings at presentation.
Twenty‐six (20.1%) of 128 patients were admitted for management of COVID‐19. Patients who were admitted were significantly less likely to report anosmia/hyposmia (26.9% vs 66.7%, p < 0.001) and dysgeusia (23.1% vs 62.7%, p < 0.001) than those who were managed as outpatients. Beyond the symptoms of anosmia/hyposmia and dysgeusia, age (median 53.5 years [IQR, 40‐65 years] vs 43.0 years [IQR, 34‐54 years], p = 0.009) and diabetes (30.1% vs 5.9%, p = 0.001) were associated with admission. Clinical subjective and objective characteristics associated with respiratory failure at the time of presentation were also associated with admission (Table 2). Specifically, admitted patients more frequently reported dyspnea (76.9% vs 43.1%, p = 0.002) and sputum production (30.8% vs 11.8%, p = 0.03), and exhibited elevated respiratory rate (median 19 [IQR, 18‐23] vs 18 [IQR, 16‐18], p < 0.001) and body temperature (median 99.8°C [IQR, 99.0°C to 101.4°C] vs 98.6°C [IQR, 98.3°C to 99.0°C], p < 0.001). Both the decision to obtain a chest radiograph (92.3% vs 34.3%, p < 0.001) as well as the presence of positive findings (88.5% vs 14.7%, p < 0.001) were more commonly found in the patients who were ultimately admitted. Further data profiling the admitted cohort, but not applicable to comparisons with an outpatient cohort, is included in Supporting Table 1.
Regression analysis
On univariable logistic regression anosmia/hyposmia was found to be inversely related to hospital admission in COVID‐19 patients; patients reporting anosmia/hyposmia were 5‐fold more likely to be managed in the outpatient setting (OR 0.20; 95% CI, 0.06‐0.64). Other factors associated with hospital admission included age (OR 1.04; 95% CI, 1.01‐1.07), diabetes mellitus (OR 6.67; 95% CI, 2.06‐21.55), dyspnea (OR 4.39; 95% CI, 1.63‐11.86), sputum production (OR 3.21; 95% CI, 1.81‐9.70), temperature (OR 2.33; 95% CI, 1.52‐3.59), heart rate (OR 1.04; 95% CI, 1.01‐1.07), respiratory rate (OR 1.04; 95% CI, 1.01‐1.07), whether chest radiograph was performed (OR 21.94; 95% CI, 4.90‐98.36), and chest radiograph findings positive for infiltrates and/or pleural effusion (OR 20.91; 95% CI, 4.13‐105.81; Table 3).
TABLE 3.
Clinical characteristics associated with admission for COVID‐19 *
| Characteristic | Univariable regression OR (95% CI) | p | Multivariable regression aOR (95% CI) | p |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, years | 1.04 (1.01–1.07) | 0.012 | 0.97 (0.90–1.04) | 0.39 |
| Sex, male (Ref: female) | 1.96 (0.80–4.81) | 0.14 | ||
| Race (Ref: white) | 1.17 (0.92–1.48) | 0.2 | ||
| BMI (kg/m2) | 1.07 (0.98–1.17) | 0.11 | ||
| Tobacco use | 1.10 (0.33–3.73) | 0.87 | ||
| Past medical history | ||||
| Hypertension | 1.99 (0.71–5.56) | 0.19 | ||
| Diabetes mellitus | 6.67 (2.06–21.55) | 0.002 | 4.23 (0.34–52.52) | 0.26 |
| COPD | – | – | ||
| Asthma | 1.12 (0.29–4.41) | 0.87 | ||
| Sinusitis | – | – | ||
| Cardiovascular disease | 2.37 (0.53–10.67) | 0.26 | ||
| Chronic kidney disease | 3.92 (0.52–29.25) | 0.18 | ||
| Cancer | 0.73 (0.08–6.52) | 0.78 | ||
| HIV/other immunosuppression | 0.53 (0.11–2.49) | 0.42 | ||
| Obstructive sleep apnea | 1.24 (0.12–12.44) | 0.86 | ||
| Stroke | – | – | ||
| Subjective clinical findings | ||||
| Anosmia/hyposmia | 0.20 (0.06–0.64) | 0.007 | 0.09 (0.01–0.74) | 0.025 |
| Fatigue | 2.01 (0.70–5.80) | 0.19 | ||
| Diarrhea | 2.33 (0.97–5.59) | 0.057 | ||
| Fever | 2.75 (0.88–8.62) | 0.083 | ||
| Cough | 4.31 (0.54–34.25) | 0.17 | ||
| Dyspnea | 4.39 (1.63–11.86) | 0.003 | 0.49 (0.08–3.13) | 0.45 |
| Sputum production | 3.21 (1.81–9.70) | 0.039 | ||
| Sore throat | 0.64 (0.26–1.58) | 0.34 | ||
| Headache | 1.08 (0.46–2.56) | 0.86 | ||
| Rhinorrhea | 0.22 (0.03–1.70) | 0.15 | ||
| Nasal obstruction/thick discharge | 0.42 (0.13–1.30) | 0.13 | ||
| Objective clinical findings | ||||
| Temperature | 2.33 (1.52–3.59) | <0.001 | 2.40 (0.95–6.05) | 0.063 |
| Heart rate | 1.04 (1.01–1.07) | 0.007 | 0.96 (0.90–1.04) | 0.35 |
| Respiratory rate | 1.46 (1.16–1.86) | 0.002 | 1.34 (0.95–1.88) | 0.09 |
| Blood oxygen saturation | 0.99 (0.95–1.04) | 0.77 | ||
| Total leukocyte count | 1.50 (1.06–2.14) | 0.023 | ||
| Lymphocyte count | 0.98 (0.93–1.03) | 0.41 | ||
| Serum creatinine | 0.45 (0.09–2.33) | 0.34 | ||
| Serum AST | 1.02 (0.99–1.05) | 0.16 | ||
| Serum ALT | 1.02 (0.99–1.04) | 0.18 | ||
| Serum lactate | 5.41 (0.26–111.36) | 0.27 | ||
| Chest radiograph performed | 21.94 (4.90–98.36) | <0.001 | ||
| Chest radiograph findings | 20.91 (4.13–105.81) | <0.001 | 8.01 (1.12–57.49) | 0.039 |
*Associations of baseline demographics and clinical findings of COVID‐19 individuals with hospital admission were determined using univariable (reporting ORs) and multivariable (reporting aORs) logistic regression models. Dependent variable = admission.
ALT = alanine aminotransferase; aOR = adjusted odds ratio; AST = aspartate aminotransferase; BMI = body mass index; CI = confidence interval; COPD = chronic obstructive pulmonary disease; COVID‐19 = coronavirus 2019; HIV = human immunodeficiency virus; OR = odds ratio.
Multivariable logistic regression adjusting for factors associated with admission on univariable analysis showed that self‐reported intact olfactory function and positive chest radiograph findings were the only factors independently associated with hospital admission. Notably, both smell loss and positive findings on chest radiograph showed clinically significant association strengths. Patients with anosmic/hyposmic COVID‐19 were more than 10‐fold less likely to be admitted than those with normosmic COVID‐19 (aOR 0.09; 95% CI, 0.01‐0.74). Patients with positive findings on chest radiograph were 8 times more likely to be admitted (aOR 8.01; 95% CI, 1.12‐57.49), consistent with an expected clinical indicator of pulmonary disease and potentially impending respiratory failure requiring hospital admission (Table 3).
A multivariable analysis of independent predictors of anosmia revealed that anosmia was negatively associated with sputum production (aOR 0.26; 95% CI, 0.08‐0.91). Hospital admission status was included in the model as a surrogate control for disease severity and, as was demonstrated in the primary analysis, showed a strong inverse relationship to anosmia/hyposmia (aOR 0.26; 95% CI, 0.07‐0.96) (Table 4).
TABLE 4.
Clinical characteristics associated with COVID‐19–associated anosmia *
| Characteristic | Univariable regression OR (95% CI) | p | Multivariable regression aOR (95% CI) | p |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age (years) | 0.97 (0.95–0.99) | 0.017 | 0.99 (0.96–1.03) | 0.75 |
| Sex (Ref: female) | 0.75 (0.37–1.51) | 0.42 | ||
| Race (Ref: white) | 0.93 (0.76–1.14) | 0.47 | ||
| BMI (kg/m2) | 1.03 (0.95–1.11) | 0.52 | ||
| Tobacco use | 1.06 (0.37–3.01) | 0.91 | ||
| Admission | 0.18 (0.07–0.48) | 0.001 | 0.26 (0.07–0.96) | 0.043 |
| Past medical history | ||||
| Hypertension | 0.44 (0.17–1.13) | 0.09 | ||
| Diabetes mellitus | 0.52 (0.17–1.59) | 0.25 | ||
| COPD | 0.24 (0.02–2.34) | 0.22 | ||
| Asthma | 1.21 (0.37–3.95) | 0.75 | ||
| Sinusitis | 3.09 (0.34–25.50) | 0.32 | ||
| Cardiovascular disease | 1.26 (0.29–5.51) | 0.76 | ||
| Chronic kidney disease | 0.24 (0.02–2.34) | 0.22 | ||
| Cancer | 1.52 (0.27–8.60) | 0.78 | ||
| HIV/other immunosuppression | 0.62 (0.21–1.84) | 0.39 | ||
| Obstructive sleep apnea | 0.74 (0.10–5.39) | 0.76 | ||
| Stroke | 0.36 (0.03–4.11) | 0.41 | ||
| Subjective clinical findings | ||||
| Fatigue | 0.55 (0.25–1.23) | 0.15 | ||
| Diarrhea | 0.65 (0.32–1.35) | 0.25 | ||
| Fever | 0.55 (0.25–1.23) | 0.15 | ||
| Cough | 0.83 (0.28–2.44) | 0.74 | ||
| Dyspnea | 0.82 (0.41–1.67) | 0.59 | ||
| Sputum production | 0.26 (0.08–0.80) | 0.019 | 0.26 (0.08–0.91) | 0.034 |
| Sore throat | 1.11 (0.54–2.26) | 0.78 | ||
| Headache | 1.43 (0.70–2.87) | 0.34 | ||
| Rhinorrhea | 1.01 (0.36–2.85) | 0.98 | ||
| Nasal obstruction/thick discharge | 2.59 (1.10–6.13) | 0.03 | 2.44 (0.16–7.89) | 0.14 |
| Objective clinical findings | ||||
| Temperature | 0.73 (0.52–1.04) | 0.08 | ||
| Heart rate | 0.98 (0.96–1.01) | 0.15 | ||
| Respiratory rate | 0.88 (0.75–1.02) | 0.08 | ||
| Blood oxygen saturation | 0.99 (0.94–1.04) | 0.62 | ||
| Total leukocyte count | 0.87 (0.69–1.10) | 0.24 | ||
| Lymphocyte count | 0.99 (0.94–1.04) | 0.75 | ||
| Serum creatinine | 0.34 (0.04–2.62) | 0.30 | ||
| Serum AST | 1.01 (0.98–1.03) | 0.63 | ||
| Serum ALT | 1.00 (0.99–1.01) | 0.98 | ||
| Serum lactate | 0.42 (0.08–2.07) | 0.29 | ||
| Chest radiograph performed | 0.45 (0.22–0.93) | 0.03 | ||
| Chest radiograph findings | 0.38 (0.13–1.14) | 0.08 |
*Associations of baseline demographics and clinical findings of COVID‐19 subjects with anosmia were determined using univariable (reporting ORs) and multivariable (reporting aORs) logistic regression models. Dependent variable = anosmia.
ALT = alanine aminotransferase; aOR = adjusted odds ratio; AST = aspartate aminotransferase; BMI = body mass index; CI = confidence interval; COPD = chronic obstructive pulmonary disease; COVID‐19 = coronavirus 2019; HIV = human immunodeficiency virus; OR = odds ratio.
Discussion
Given the rapidly increasing number of COVID‐19 cases that threaten to overwhelm healthcare systems, strategies to risk stratify patients and early determination of disease severity are urgently needed. Clinical characteristics associated with critical illness and mortality from COVID‐19 have been reported. A meta‐analysis of clinical characteristics of over 40,000 COVID‐19 patients from Wuhan found that patients with severe disease requiring intensive care were more likely to have comorbidities of hypertension (OR 2.36), respiratory system disease (OR 2.46), and cardiovascular disease (OR 3.42) compared to patient not requiring intensive care. 16 Similar observations were made in Italy, with hypertension and coronary artery disease being more prevalent in the severely ill COVID‐19 patients. 17 Of critically ill patients, risk factors for mortality included increased age as a predictor of inpatient death (OR 1.1, or 10% increase with each additional year of age) 18 along with markers of end‐organ failure. 17 , 18
In addition to investigating markers of critical illness, it is important to consider characteristics that may help stratify mild and moderate infections in early‐stage COVID‐19. Here we present a novel clinical characteristic to help stratify mild from moderate disease early in SARS‐CoV‐2 infection. Patients reporting loss of smell were 10 times less likely to be admitted for COVID‐19 (OR 0.09; 95% CI, 0.01‐0.74) compared to those without loss of smell. Furthermore, anosmia/hyposmia was not associated with any other measures typically related to the decision to admit, suggesting that smell loss is truly an independent correlate and may serve as a marker for milder manifestations of COVID‐19.
Our study's findings are consistent with those of other studies evaluating both inpatient and outpatient self‐reported olfactory dysfunction. 9 , 10 , 11 , 12 , 14 , 15 Moein et al. 11 reported very high rates of measured olfactory dysfunction (98%) on quantitative analysis of COVID‐19 inpatients, but only 25% showed complete anosmia and 35% self‐reported smell loss. This discrepancy between quantitative and self‐reported olfactory dysfunction is thought to be related to a general unawareness or underreporting of hyposmia. 19 In the COVID‐19 inpatient population, decreased awareness of chemosensory dysfunction may also be influenced by the presence of more severe symptoms such as respiratory distress. Despite this mismatch, we believe that our study's findings differentiating the incidence of smell loss between COVID‐19 inpatients and outpatients are valuable, because self‐reported loss may more closely reflect profound or functional anosmia. Although the present study did not specifically investigate degree of smell loss, our previous study demonstrated that those who reported smell loss typically suffered from its complete absence (0 out of 10 on a 0‐10 scale). 9 Although quantitative testing is more sensitive in detecting olfactory dysfunction, self‐reporting of anosmia is relatively accurate (90%). 19 Thus, our study suggests that milder cases of COVID‐19 may be heralded by profound anosmia and higher self‐reporting, compared to the undetected or mild hyposmia associated with moderate to severe COVID‐19 cases. Further objective olfactory testing of both outpatient and inpatient cohorts is required to clarify if quantitative differences in the severity of olfactory dysfunction correlate with differences in self‐reported loss.
Assessment of smell may not only aid in the diagnosis of COVID‐19 infection during pretest screening, but also help guide the posttest triaging of patients at all levels. Patients with influenza‐like symptoms concerning for COVID‐19 infection but without anosmia should be more vigilant and present earlier for evaluation and management. Although in this particular study we did not ask subjects the timing of smell loss onset in relationship to their other symptoms, it has previously been shown that 33% to 60% of anosmic COVID‐19 patients reported smell loss either before or at the same time of other symptoms. 9 , 12 , 15 Of the self‐reported anosmic/hyposmic inpatients, 12 of 20 (60%) noted onset of smell loss prior to admission whereas 91% experienced taste loss before admission, which we suspect may be a profound anosmia impairing the odorant component of flavor. 15 Furthermore, epidemiologic studies found that the average time from symptom onset to admission was 11 days. 18 These data, taken together, suggest that profound anosmia is a relatively early manifestation, whereas admission tends to occur relatively late in the disease process. Although smell loss may not yet be manifested for all patients at the time of their evaluation by a healthcare professional, it still may be a useful early indicator in the majority of patients. If the findings reported here remain consistent in independent cohorts, anosmia/hyposmia could be considered as a clinical marker inversely related to disease severity. As such, anosmia/hyposmia could be included in the clinical assessment of disease severity and potentially aid in the allocation of limited medical interventions. Just as Apgar scores are an effective means to rapidly assess and triage at‐risk newborns, 20 a future COVID‐19 risk stratification model may have a potential to improve resource allocation and thereby save lives. Further research is needed to shed light on clinical findings, upon which a model based on disease severity as shown in Figure 1 could be built. Although the presented hypothetical model is not intended to substitute for nuanced, patient‐driven clinical decision making, it may in the future serve as a general model to organize decision making and risk stratification. Further study and validation of all risk factors will evolve as the pandemic progresses, but anosmia is emerging early as an important clinical component of both COVID‐19 diagnosis and potentially outcome as well.
FIGURE 1.
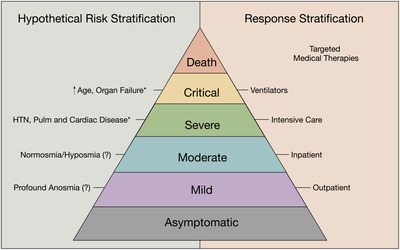
Presentation of a hypothetical model suggesting that effective risk and response stratification of all levels of COVID‐19 disease severity may assist in efficient allocation of limited resources. *Risk factors for severe and critical disease based on early reports on the COVID‐19 pandemic. 16 , 17 , 18 COVID‐19 = coronavirus 2019.
Beyond the immediate practical applications of anosmia in addressing the pandemic, these findings potentially hint at some characteristics of the pathophysiology of the infection. The site and dosage of the initial viral burden, along with the effectiveness of the host immune response are all potentially important variables in determining the spread of the virus within an individual and ultimately the clinical course of infection. A focused, small upper airway SARS‐CoV‐2 viral load may be associated with a less fulminant infection, decreasing the risk of overwhelming the host immune response and subsequently, decreasing the risk of respiratory failure and hospitalization. This hypothesis, is in essence the concept underlying live vaccinations, where low dosage and distant site of inoculation generates an immune response without provoking a severe infection. 21
Similarly, anosmia may be a biomarker of the magnitude of a host's innate immune response to SARS‐CoV‐2 infection. Although the mechanism of olfactory loss remains unclear, radiographic imaging of a single case of isolated anosmia and COVID‐19 infection shows bilateral olfactory cleft obstruction consistent with local inflammation. 22 This finding may be consistent with a greater local immune response in the infection of patients presenting with anosmia leading to an olfactory loss secondary to local infection and edema and perhaps a milder overall clinical course. Indeed, early, pre–peer review analyses of transcriptome data suggest that the candidate receptors mediating cellular entry of SARS‐CoV‐2, angiotensin converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2), are expressed on olfactory epithelial support cells and not on olfactory sensory neurons. 23 , 24 Taken together, data showing that SARS‐CoV2 infects olfactory epithelia and causes highly localized inflammation of the olfactory cleft suggest that COVID‐19–related olfactory dysfunction may result in a conductive olfactory loss. Such a phenomenon would be consistent with relatively rapid recovery of olfactory function with the resolution of viral infection in most patients, consistent with previously reported clinical findings. 9 In addition, these preliminary findings may be consistent with a greater local immune response in patients presenting with anosmia that may be indicative of a milder overall clinical course. The inverse association of anosmia and sputum production (OR 0.26) in the present study suggest that anosmic COVID‐19 are less likely to have a symptom associated with more severe lower airway inflammatory response. Although these are tantalizing hypotheses of the underlying pathophysiology currently only supported by piecemeal, circumstantial molecular and clinical findings, significant investigations into SARS‐CoV‐2 infection of airway mucosa and the host immune response are required to begin elucidating the underlying pathophysiology of the present study's clinical observations.
Additionally, further studies are warranted to validate our clinical findings. Limitations include the use of self‐reported anosmia/hyposmia, particularly in those who reported anosmia after being informed of COVID‐19 diagnosis. This group is most susceptible to recall bias. Therefore, findings herein and elsewhere based on self‐reported anosmia after COVID‐19 diagnosis should be validated in future studies with quantitative testing of olfaction. In other disease processes there is discrepancy between self‐reported anosmia and measured anosmia. 25 Furthermore, the design of the questions posed to the subjects in this particular study did not specifically assess the severity of smell loss, but only if there was a loss of smell during their illness. Thus, in this analysis, we cannot draw a distinction between hyposmia and anosmia. However, our prior study did assess severity of smell loss and those who reported smell loss typically recalled complete functional anosmia. 9 Our study has also achieved a higher response rate from ambulatory COVID‐19 patients, some of whom were unable to be evaluated for olfactory function due to their clinical status. As a result, our data does not inform any potential relationship between anosmia and critical illness requiring intensive care monitoring and intubation, or mortality. Notwithstanding these limitations, in the absence of objective olfactory testing, self‐reported anosmia retains robust associations with specific COVID‐19–related disease features.
Our report is among the minority of reports focusing on mild to moderate COVID‐19 in which we attempt to elucidate clinical features to differentiate between patients with mild disease who could be managed at home and “moderately” sick patients who require admission and may be at risk of further clinical deterioration. Prospective studies are required to better determine the extent to which anosmia informs overall disease trajectory. Most early data have focused on severely ill patients, 16 , 17 , 18 but as the pandemic approaches plateau and eventually passes its peak, the clinical characteristics of the moderately sick patient are important to identify and administer early intervention before a subset succumbs to critical, potentially fatal infection. Further research is required to validate the association between olfactory function and hospitalization risk in patients with COVID‐19, which may include prospective, longitudinal, and larger multi‐institutional studies.
Conclusion
The current study has identified a strong inverse association between COVID‐19–related anosmia and a critical branch point in management of COVID‐19: the decision to commit to hospital admission. Patients admitted for COVID‐19 were 10 times less likely to report anosmia. These findings have important immediate practical applications to the lay public as well as healthcare workers and healthcare systems looking to efficiently risk‐stratify patients to efficiently provide appropriate medical and nonmedical interventions. The association between olfactory dysfunction and clinical outcomes also carries important implications for future investigations seeking to understand the ability of SARS‐CoV‐2 virus to overwhelm the host immune response.
Supporting information
Supporting Material
How to Cite this Article:Yan CH, Faraji F, Prajapati DP, Ostrander BT, DeConde AS. Self‐reported olfactory loss associates with outpatient clinical course in COVID‐19. Int Forum Allergy Rhinol. 2020;10:821–831.
Funding sources for the study: National Institutes of Health (Clinical and Translational Science Awards [CTSA], UL1TR001442).
Potential conflict of interest: A.S.D. is a consultant for Stryker Endoscopy, Olympus, IntersectENT, Sanofi, and Optinose. The other authors have no financial disclosures.
References
- 1. Phua J, Weng L, Ling L, et al. Intensive care management of coronavirus disease 2019 (COVID‐19): challenges and recommendations. Lancet Respir Med. 2020;8:506‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. (in press). Epub 24 February 2020. 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3. Xie J, Tong Z, Guan X, Du B, Qiu H. Clinical characteristics of patients who died of coronavirus disease 2019 in China. JAMA Netw Open. 2020;3:e205619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid‐19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382:2012‐2022. 10.1056/nejmoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sutton D, Fuchs K, D'Alton M, Goffman D. Universal screening for SARS‐CoV‐2 in women admitted for delivery. N Engl J Med. 2020;382:2163‐2164. 10.1056/nejmc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID‐19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:689‐696. 10.1016/s1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xie J, Tong Z, Guan X, Du B, Qiu H, Slutsky AS. Critical care crisis and some recommendations during the COVID‐19 epidemic in China. Intensive Care Med. 2020;46:837‐840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and Covid‐19 in patients presenting with influenza‐like symptoms. Int Forum of Allergy Rhinol. (806‐813). Epub 12 April 2020. 10.1002/alr.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Menni C, Valdes A, Freydin MB, et al. Real‐time tracking of self‐reported symptoms to predict potential COVID‐19. Nat Med. 2020. 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moein ST, Hashemian SMR, Mansourafshar B, Khorram‐Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID‐19. Int Forum Allergy Rhinol. (in press). Epub 17 April 2020. 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lechien JR, Chiesa‐Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease (COVID‐19): a multicenter European study. Eur Arch Otorhinolaryngol. (in press). Epub 06 April 2020. 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walker A, Hopkins C, Surda P. The use of google trends to investigate the loss of smell related searches during COVID‐19 outbreak. Int Forum Allergy Rhinol. (in press). Epub 11 April 2020. 10.1002/alr.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. (in press). Epub 10 April 2020. 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giacomelli A, Pezzati L, Conti F, et al. Self‐reported olfactory and taste disorders in SARS‐CoV‐2 patients: a cross‐sectional study. Clin Infect Dis. (in press). Epub 26 March 2020. 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID‐19) infection: a systematic review and meta‐analysis. Int J Infect Dis. 2020;94:91‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wehling E, Nordin S, Espeseth T, Reinvang I, Lundervold AJ. Unawareness of olfactory dysfunction and its association with cognitive functioning in middle aged and old adults. Arch Clin Neuropsychol. 2011;26:260‐269. [DOI] [PubMed] [Google Scholar]
- 20. Iliodromiti S, Mackay DF, Smith GCS, Pell JP, Nelson SM. Apgar score and the risk of cause‐specific infant mortality: a population‐based cohort study. Lancet. 2014;384:1749‐1755. [DOI] [PubMed] [Google Scholar]
- 21. Roestenberg M, Hoogerwerf M‐A, Ferreira DM, Mordmüller B, Yazdanbakhsh M. Experimental infection of human volunteers. Lancet Infect Dis. 2018;18:e312‐e322. [DOI] [PubMed] [Google Scholar]
- 22. Eliezer M, Hautefort C, Hamel A‐L, et al. Sudden and complete olfactory loss function as a possible symptom of COVID‐19. JAMA Otolaryngol Head Neck Surg. (in press). Epub 08 April 2020. 10.1001/jamaoto.2020.0832. [DOI] [PubMed] [Google Scholar]
- 23. Brann D, Tsukahara T, Weinreb C, et al. Non‐neural expression of SARS‐CoV‐2 entry genes in the olfactory epithelium suggests mechanisms underlying anosmia in COVID‐19 patients. bioRxiv. (in press). Epub 09 April 2020. 10.1101/2020.03.25.009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gupta K, Mohanty SK, Kalra S, et al. The molecular basis of loss of smell in 2019‐NCoV infected individuals. Research Square Preprint. Epub 01 April 2020. 10.21203/rs.3.rs-19884/v1. [DOI] [Google Scholar]
- 25. Boesveldt S, Postma EM, Boak D, et al. Anosmia—a clinical review. Chem Senses. 2017;42:513‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Material


