Abstract
The rapid spread of SARS‐CoV‐2 in 2019 and 2020 has resulted in a worldwide pandemic characterized by severe pulmonary inflammation, effusions, and rapid respiratory compromise. The result of this pandemic is a large and increasing number of patients requiring endotracheal intubation and prolonged ventilator support. The rapid rise in endotracheal intubations coupled with prolonged ventilation requirements will certainly lead to an increase in tracheostomy procedures in the coming weeks and months. Performing tracheostomy in the setting of active SARS‐CoV‐2, when necessary, poses a unique situation, with unique risks and benefits for both the patient and the health care providers. The New York Head and Neck Society has collaborated on this document to provide guidance on the performance of tracheostomies during the SARS‐CoV‐2 pandemic.
Keywords: airway, COVID19, SARS‐CoV‐2, tracheal stenosis, tracheostomy
1. BACKGROUND
The rapid spread of SARS‐CoV‐2 in 2019 and 2020 has resulted in a worldwide pandemic.1, 2, 3, 4 The dramatic proinflammatory effects of SARS‐CoV‐2 result in a wide variety of clinical presentations; however, severe pulmonary inflammation, effusions and rapid respiratory compromise are a hallmark of this disease.5, 6, 7 Subsequent pneumonia, acute respiratory distress syndrome and death have been reported not infrequently. The result of this pandemic is a large and increasing number of patients requiring endotracheal intubation and prolonged ventilator support.8, 9, 10, 11, 12, 13 Certainly, the rapid rise in endotracheal intubations coupled with prolonged ventilation requirements will lead to an increase in tracheostomy procedures in the coming weeks and months.14, 15
Although a generally well‐tolerated and safe procedure, the risks and benefits of tracheostomy in terms of outcomes, pulmonary care and risks to the health care team remain unknown.16, 17 Fortunately, although not perfect, rapid testing protocols have allowed us to be able to detect active infection in patients who are affected by SARS‐CoV‐2.18, 19, 20, 21 What is clear is that the upper aerodigestive tract, the nasopharynx and the trachea harbor a high viral load during the acute stages of the infection.22, 23, 24 Therefore, performing a tracheostomy in the setting of active SARS‐CoV‐2, when necessary, poses a unique situation, with unique risks and benefits for both the patient and the health care providers. The risk of this procedure has to be balanced with the known risks of prolonged intubation, primarily tracheal and subglottic stenosis, the management of which can be problematic if significant mucosal injury and subsequent stenosis occur.
The New York Head and Neck Society is a nonprofit organization founded in 1979, which encourages the exchange and advancement of scientific knowledge relative to the management of head and neck cancer and includes several member institutions, including Columbia University Medical Center, Cornell Medical College, Icahn School of Medicine at Mount Sinai, Memorial Sloan Kettering Cancer Center, New York University Medical Center and Montefiore Medical Center Albert Einstein College of Medicine, and has several other affiliate institutions in the greater New York City area. The New York Head and Neck Society has collaborated on this document to provide guidance on the performance of tracheostomy during the SARS‐CoV‐2 pandemic.
2. RECOMMENDATIONS
2.1. Monitor endotracheal and tracheostomy tube cuff pressures every Q4 hours
In patients who are intubated, especially in prolonged intubations >72 hours, the risk of tracheal stenosis increases over time. Teams managing these patients should stress that all intubated patients have a Q4‐hour cuff pressure check with a goal of approximately 30 cm H2O if feasible, given the vent parameter requirements, as pressures higher than 30 cm H2O may result in pressure necrosis. Certainly, adequate pressure to avoid cuff leakage and aerosolization is critical when managing SARS‐CoV‐2 patients, but it should be recognized that unnecessarily high cuff pressures are also problematic. The minimum cuff pressure required to create an adequate seal should be individualized for each patient and verified frequently by care providers. Certainly, high peak pressures, or issues with ventilation may make the appropriate cuff pressure a moving target. This is a dynamic process, and frequent adjustments may be indicated depending on ventilation parameters. Prevention of tracheal mucosal pressure necrosis, resulting tracheal and cricoid chondritis and subsequent stenosis is critical in the SARS‐CoV‐2 population.25, 26
SARS‐CoV‐2 testing via the reverse transcription‐ polymerase chain reaction (rt‐PCR) detection platform for SARS‐CoV‐2 and pan‐sarbecovirus detection is recommended for all patients who are being considered for tracheostomy. It should be remembered that data surrounding accuracy of the test during the pandemic is forthcoming, and false negatives are a real possibility. 27 In addition, rt‐PCR may not be reliable when determining infectivity/active virus vs the mere presence of viral DNA and therefore while levels detected by rt‐PCR do tend to correlate with the active viral load, robust data are lacking to support the utilization of a testing protocol for viral load and decision making in SARS‐CoV‐2 positive patients. The test may be performed a second time if clinical suspicion or institutional policy warrants repeat testing for the presence of SARS‐CoV‐2 prior to high‐risk procedures.
2.2. Delay timing of tracheostomy until 21 days after the onset of symptoms if feasible
When determining the appropriate time of tracheostomy in the SARS‐CoV‐2 patient, several factors are considered, and individual cases may certainly have mitigating circumstances that lead to the decision to perform tracheostomy. However, for the majority of patients, health care teams should seek to capitalize on the intersection of the risk of contamination/infection and decreasing viral load in the upper and lower airways over time with the risks of prolonged intubation (ie, tracheal stenosis, weaning issues, pulmonary toilet, etc.). Although the overall risk of tracheal stenosis secondary to prolonged intubation depends on a variety of factors, reported rates of severe, symptomatic stenosis are generally in the 1% to 2% range when modern low‐pressure cuffs are utilized.28, 29, 30, 31, 32 Therefore, in light of the relatively low risk of clinically relevant stenosis, and despite the traditional 10‐day cutoff for increased stenosis risk used by many practitioners in the general population, when dealing with a SARS‐CoV‐2 patient, the risk for symptomatic or severe tracheal stenosis is acceptable in light of the significant risks of tracheostomy in the acute phase of the infection during periods of the highest viral loads. The decreasing viral load, although logarithmic in nature, is somewhat variable, and high viral loads have been observed somewhat late in the course of the infection in critically ill patients (Figures 1 and 2).23, 24, 33 Therefore, when feasible, waiting until approximately 21 days after the onset of symptoms is recommended prior to consideration of tracheostomy for the majority of cases in order to avoid exposing health care teams to increased risk. Earlier tracheostomy may certainly be medically indicated in some situations depending on the clinical situation, or health care system issues during a pandemic situation, and we recognize the potential need to perform tracheostomy more urgently. Tracheostomy should not be delayed regardless of SARS‐CoV‐2 status in life‐saving situations or in situations in which the tracheostomy would significantly improve the prognosis of the patient. Certainly the timing of tracheostomy remains a controversial issue and data is limited related to SARS‐CoV‐2. 53 Alternative emerging strategies in the management of SARS‐CoV‐2 critically ill patients, such as extracorporeal membrane oxygenation, antiviral therapy and convalescent plasma therapy, may also be considered by the team, but the available data and decision making regarding this is beyond the scope of these recommendations.34, 35, 36, 37, 38 Clearly, these are multidisciplinary decisions that will be individualized depending on the patient and institutional expertise.
FIGURE 1.
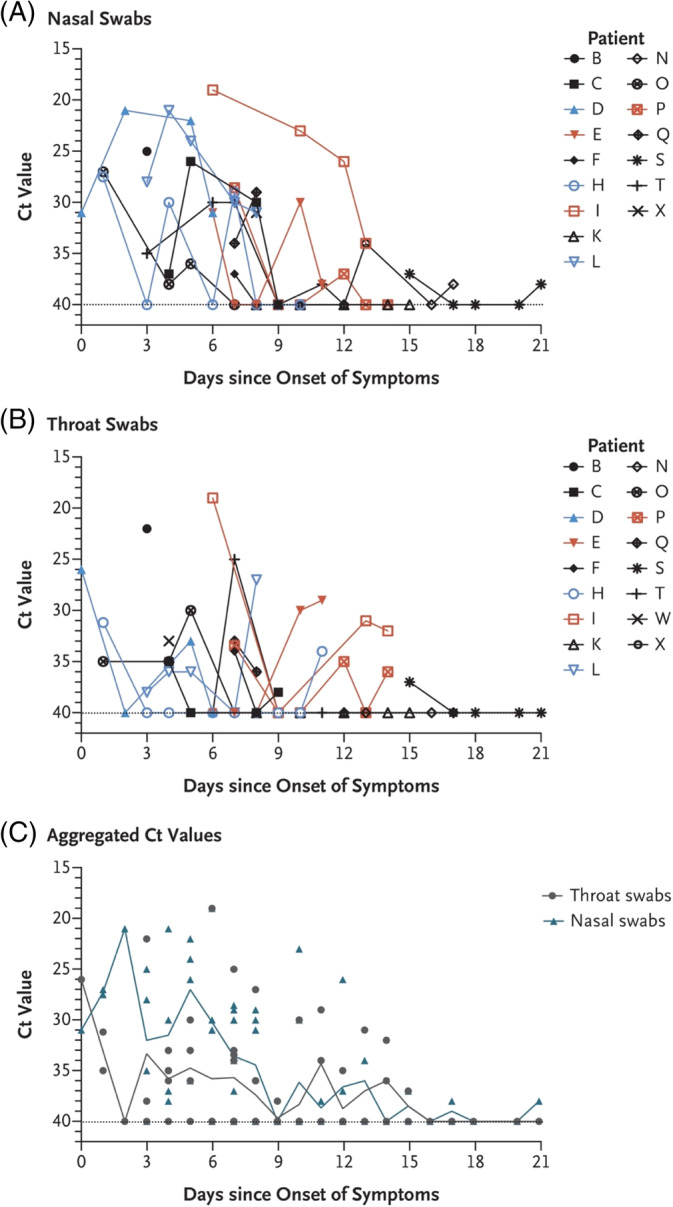
Viral load detected in nasal and throat swabs obtained from patients infected with SARS‐CoV‐2 24 [Color figure can be viewed at http://wileyonlinelibrary.com]
FIGURE 2.
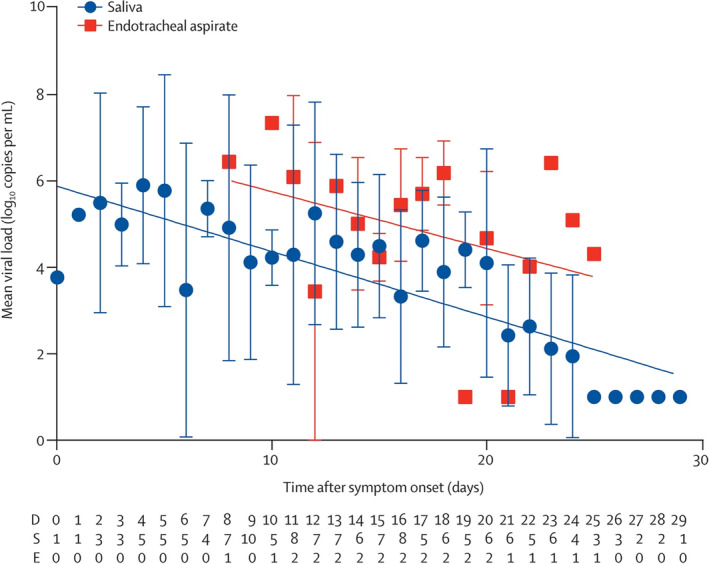
Temporal profile of serial viral load from all patients (n = 23) 23 [Color figure can be viewed at http://wileyonlinelibrary.com]
In addition, it should be noted that avoiding tracheostomy in patients at high risk of mortality is critical. If the primary team managing the patient determines that there is an extremely high risk of mortality in the near future or that the patient has a high likelihood of withdrawal of care, the risks of tracheostomy should be avoided in this situation. Patients with significant medical comorbidities, acute respiratory distress syndrome/severe respiratory failure and a low chance of recovery who are infected with SARS‐CoV‐2 should be carefully evaluated, and discussions with family members, consultants, institutional ethics committees and the treating team should focus on overall prognosis and goals of care prior to performing tracheostomy as a routine matter of care. These decisions are highly individualized and rely on solid communication among team members managing these high‐risk patients.
2.3. Tracheostomy technical considerations and recommendations
Although the exact technical details regarding tracheostomy will depend on the situation and procedural protocols and technical expertise, there are some specific technical aspects related to the SARS‐CoV‐2 (and other viral pandemics) that should be considered. Ideally, the procedure should be performed at bedside in the intensive care unit in a negative pressure room or using a portable high efficiency particulate air (HEPA) filtration system to avoid patient transportation and contamination of other areas in the medical center. If it is necessary to perform the procedure in the operating room (OR), a specific OR cluster should be designated to avoid contamination of additional OR resources for noninfected patients. In addition to standard airborne and droplet precautions, techniques to minimize aerosolization of the virus during the procedure include the following: paralysis to prevent coughing; consider glycopyrrolate to reduce secretions; preoxygenation and cessation of ventilation during the tracheostomy procedure; utilization of closed suctioning systems; avoiding monopolar electrocautery, or harmonic technology, and using cold instrumentation when feasible; minimizing suctioning and bronchoscopy during the procedure; and ensuring the cuff is inflated prior to resuming ventilation, so the circuit is closed.
In addition to standard open tracheostomy with an apneic technique, percutaneous/dilational tracheostomy techniques have been evaluated extensively in the literature and have been shown to be a safe alternative to traditional open surgical tracheostomy.39, 40, 41 Understandably, the techniques utilized when performing tracheostomy will vary based on patient characteristics, provider expertise and institutional experience. Although data are limited, techniques that avoid opening the airway and are closed, such as a percutaneous dilational technique, may be preferential in the setting of active SARS‐CoV‐2 infection.42, 43 Therefore, if there are no anatomical or other contraindications, percutaneous dilational tracheostomy may be considered if the expertise is available. It should be remembered that the decrease in aerosolization during percutaneous tracheostomy only holds true if airway manipulation (ie, bronchoscopy with cuff deflation or concurrent ventilation) is not performed, and although there have been some associated higher complication rates with blind percutaneous tracheostomy compared to bronchoscopic technique, ultrasound‐guided techniques have been shown to be noninferior to bronchoscopic techniques.44, 45, 46 Therefore, when considering a percutaneous tracheostomy, a closed ultrasound‐guided or “cuff‐up” apneic technique is recommended for SARS‐CoV‐2 patients.
2.4. Use of appropriate PPE during tracheostomy procedures for active SARS‐CoV‐2
Although there are limited data on the current pandemic to fully inform personal protective equipment (PPE) recommendations, performing tracheostomy in an actively infected SARS‐CoV‐2 patient is certainly a high‐risk procedure for health care workers. 47 Health care personnel performing the tracheostomy should wear at minimum: waterproof cap, goggles with an antimist screen, impermeable operating room surgeon's gown and gloves and a transparent plastic facial shield worn outside the goggles and N95 that is effective in filtering 99.5% particles larger than 0.75 μm and 95% effective in the .1 to .3 μm range. 48 The minimum number of health care workers required to perform the procedure should be present to prevent unnecessary exposures.
The effectiveness of the N95 mask in the prevention of SARS‐CoV‐2 infection during tracheostomy procedures remains unknown, but given the high risk and the reported size of thevirus ranging from 70‐90 nm, consideration for power air‐purifying respirator (PAPR) systems for personnel performing tracheostomy should be entertained. 54 These systems should be used when available in situations of acute active infection, or suspicion of high viral loads, as there is some evidence of superior protection (PAPR provides 2.5‐100 times greater protection than the N95 when staff are appropriately trained).47, 49, 50 The effectiveness of N95 and PAPR in this situation has certainly not been compared in a head‐to‐head trial, and therefore, the use of PAPR vs N95 will depend on institutional resources and policy and the clinical situation until further data is available.
2.5. Avoid emergent tracheostomy if possible
Techniques to manage the acute airway with endotracheal intubation, video laryngoscope for example, should be utilized if possible to avoid emergent tracheostomy in SARS‐CoV‐2 patients due to the high risk of unsafe conditions and health care worker contaminations. 48 Similarly, intubation techniques (ie, rapid sequence intubation) that avoid mask ventilation, such as prolonged open airway manipulation, are recommended when appropriate. When life‐threatening airway obstruction occurs in a setting in which intubation is not possible, health care workers should perform the tracheostomy with the above‐noted PPE, keeping in mind that PAPR respirator use is often not feasible or available in emergent situations. In situations in which cardiopulmonary resuscitation is being performed, chest compressions should be avoided at the time the airway is entered until the airway is secured and the cuff is inflated on the device to minimize health care worker exposure.
2.6. Appropriate posttracheostomy management
Posttracheostomy management should also be mentioned as, in addition to routine tracheostomy care, there are some considerations for SARS‐CoV‐2 patients. Securing circuits properly and avoiding unnecessary humidification systems may reduce the risk of unexpected circuit disconnection and aerosolization leading to exposure. The circuit should remain closed as much as possible, and closed‐line suctioning should be used. Heat moister exchangers with viral filters and HEPA filtration should be used when possible. Tracheostomy tube changes should be avoided and should only be performed in cases of cuff failure or emergent situations.
2.7. Organize an appropriate team
Although the members of the health care team performing tracheostomy vary across institutions, team members may include surgeons, medicine/intensivists, anesthesiologists, respiratory therapists, nurses and other ancillary staff required during these procedures. The importance of appropriate PPE/PAPR training and usage cannot be overstated in the setting of active SARS‐CoV‐2 infection. Teams that perform the procedure regularly will be more efficient and less likely to be unfamiliar with the procedure or appropriate health care protective measures and infection control. The inclusion of trainees such as residents and fellows during these procedures requires careful consideration and will vary based on institutional policies.
Currently, there are limited data on the host innate immune status of SARS‐CoV‐2 infected patients. 51 Consideration of the inclusion of health care workers who have previously been exposed and subsequently recovered from documented SARS‐CoV‐2 infection may be warranted. Although the exact timing of immunity and subsequent safety for the return of health care providers infected with SARS‐CoV‐2 remains unknown, sufficient antibody responses have been documented to occur between 15 and 20 days, or approximately 2 weeks, after the onset of symptoms (Figure 3). 23 Inclusion of these individuals on these teams may allow for high‐risk procedures to be performed by health care workers who have mounted an immune response to the virus depending on institutional quarantine policies. Similarly, these individuals should not be involved in tracheostomy procedures or other airway procedures in noninfected patients due to the risk of iatrogenic infection with SARS‐CoV‐2 due to limited available data about the risks. 52
FIGURE 3.
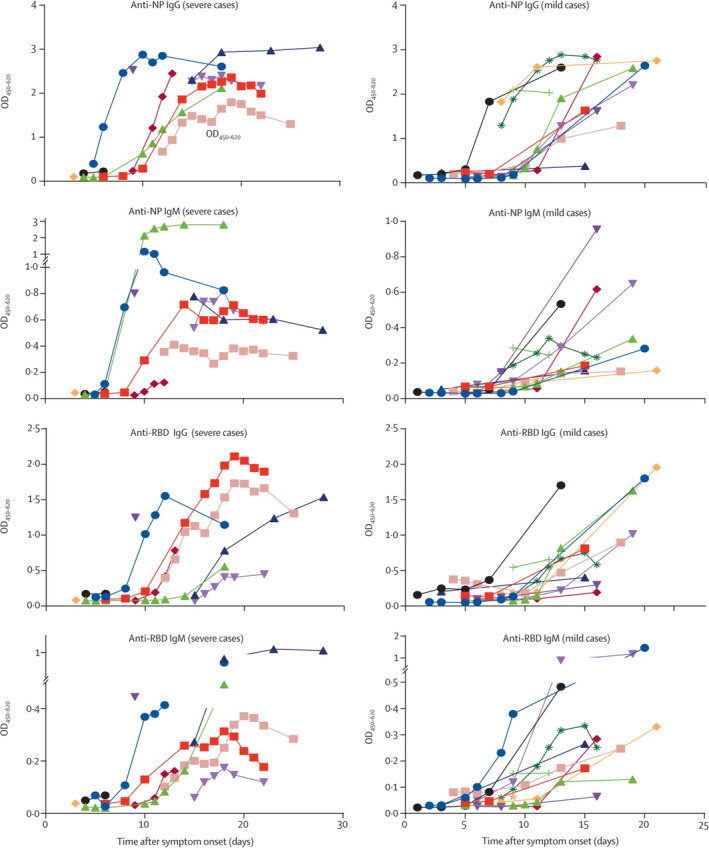
Temporal profiles of serum IgM and IgG against NP and spike protein RBD, as ascertained by EIA 23 . EIA, enzyme immunoassay; IgG, immunoglobulin G; IgM, immunoglobulin M; NP, nucleprotein; RBD, receptor‐binding domain [Color figure can be viewed at http://wileyonlinelibrary.com]
3. CONCLUSION
Tracheostomy in the SARS‐CoV‐2 infected patient represents a unique situation, with a unique set of risks and implications. When compared to traditional tracheostomy procedures in the setting of prolonged ventilation, SARS‐CoV‐2 represents a unique entity in terms of timing, indications and infection control considerations that must be remembered when performing these procedures and managing patient's posttracheostomy. Additional resources are listed below.
4. SUMMARY POINTS
Careful consideration of “who” and “when” when tracheostomy is planned is recommended.
Careful consideration of the location and technique to avoid unnecessary risks to health care providers is recommended.
When clinically appropriate, delay of tracheostomy procedures is recommended to allow for reduced viral load and to decrease the risk of nosocomial infection to critical health care providers.
Selecting appropriately sized endotracheal tube (ETT), and careful monitoring of ETT cuff pressures to maintain appropriate seal to avoid aerosolization when mitigating the risk of long‐term tracheal complications is recommended.
Appropriate PPE training and utilization, including N95 or PAPR when indicated, is recommended for all patients undergoing tracheostomy, regardless of SARS‐CoV‐2 status, during the pandemic.
Avoidance of unnecessary airway manipulation, such as ventilation during bronchoscopy; routine trach changes, with a focus on a closed‐circuit ventilation; and utilizing apneic open tracheotomy/bronchoscopy or ultrasound guidance for percutaneous tracheostomy, is recommended.
5. RESOURCES
American Academy of Otolaryngology‐Head and Neck Surgery.
Tracheostomy Recommendations during COVID‐19 Pandemic.
https://www.entnet.org/content/tracheotomy-recommendations-during-covid-19-pandemic
American Head and Neck Society Guidance for Tracheostomy during COVID‐19 Pandemic.
Percutaneous Tracheostomy Technique:
https://www.hopkinsmedicine.org/tracheostomy/about/how.html
Open Tracheostomy Technique:
https://www.mayoclinic.org/tests-procedures/tracheostomy/about/pac-20384673
https://my.clevelandclinic.org/health/treatments/17568-tracheostomy-care
https://medicine.uiowa.edu/iowaprotocols/tracheotomy-tracheostomy
Miles BA, Schiff B, Ganly I, et al. Tracheostomy during SARS‐CoV‐2 pandemic: Recommendations from the New York Head and Neck Society. Head & Neck. 2020;42:1282–1290. 10.1002/hed.26166
REFERENCES
- 1. Amodio E, Vitale F, Cimino L, et al. Outbreak of novel coronavirus (SARS‐Cov‐2): first evidences from international scientific literature and pending questions. Healthcare (Basel). 2020;8(1), 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ashour HM, Elkhatib WF, Rahman MM, Elshabrawy HA. Insights into the recent 2019 novel coronavirus (SARS‐CoV‐2) in light of past human coronavirus outbreaks. Pathogens. 2020;2020:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dilcher M, Werno A, Jennings LC. SARS‐CoV‐2: a novel deadly virus in a globalised world. N Z Med J. 2020;133:6‐11. [PubMed] [Google Scholar]
- 4. Hsu LY, Chia PY, Lim JF. The novel coronavirus (SARS‐CoV‐2) epidemic. Ann Acad Med Singapore. 2020;49:1‐3. [PubMed] [Google Scholar]
- 5. Conti P, Ronconi G, Caraffa A, et al. Induction of pro‐inflammatory cytokines (IL‐1 and IL‐6) and lung inflammation by Coronavirus‐19 (COVI‐19 or SARS‐CoV‐2): anti‐inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2). 10.23812/CONTI-E [DOI] [PubMed] [Google Scholar]
- 6. Coronaviridae Study Group of the International Committee on Taxonomy of V . The species severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nat Microbiol. 2020;5:536‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fu Y, Cheng Y, Wu Y. Understanding SARS‐CoV‐2‐mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. 2020. 10.1007/s12250-020-00207-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Giani M, Seminati D, Lucchini A, Foti G, Pagni F. Exuberant plasmocytosis in bronchoalveolar lavage specimen of the first patient requiring extracorporeal membrane oxygenation for SARS‐CoV‐2 in Europe. J Thorac Oncol. 2020. 10.1016/j.jtho.2020.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kamel Boulos MN, Geraghty EM. Geographical tracking and mapping of coronavirus disease COVID‐19/severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) epidemic and associated events around the world: how 21st century GIS technologies are supporting the global fight against outbreaks and epidemics. Int J Health Geogr. 2020;19:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lai CC, Liu YH, Wang CY, et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2): facts and myths. J Microbiol Immunol Infect. 2020. 10.1016/j.jmii.2020.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and coronavirus disease‐2019 (COVID‐19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li T. Diagnosis and clinical management of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection: an operational recommendation of Peking Union Medical College Hospital (V2.0). Emerg Microbes Infect. 2020;9:582‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sun P, Qie S, Liu Z, et al. Clinical characteristics of hospitalized patients with SARS‐CoV‐2 infection: a single arm meta‐analysis. J Med Virol. 2020. 10.1002/jmv.25735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lombardi A, Bozzi G, Mangioni D, et al. Duration of quarantine in hospitalized patients with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection: a question needing an answer. J Hosp Infect. 2020. 10.1016/j.jhin.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marraro GA, Spada C. Consideration of the respiratory support strategy of severe acute respiratory failure caused by SARS‐CoV‐2 infection in children. Zhongguo Dang Dai Er Ke Za Zhi. 2020;22:183‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Niederman MS, Richeldi L, Chotirmall SH, Bai C. Rising to the challenge of the novel SARS‐coronavirus‐2 (SARS‐CoV‐2): advice for pulmonary and critical care and an agenda for research. Am J Respir Crit Care Med. 2020. 10.1164/rccm.202003-0741ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ong SWX, Tan YK, Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) from a symptomatic patient. JAMA. 2020. 10.1001/jama.2020.3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu R, Han H, Liu F, et al. Positive rate of RT‐PCR detection of SARS‐CoV‐2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin Chim Acta. 2020;505:172‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patel R, Babady E, Theel ES, et al. Report from the American Society for Microbiology COVID‐19 International Summit, March 23, 2020: value of diagnostic testing for SARS‐CoV‐2/COVID‐19. mBio. 2020;11 10.1128/mBio.00722-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pfefferle S, Reucher S, Norz D, Lutgehetmann M. Evaluation of a quantitative RT‐PCR assay for the detection of the emerging coronavirus SARS‐CoV‐2 using a high throughput system. Euro Surveill. 2020;25(9). 10.2807/1560*7917.ES.2020.25.9.2000152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Y, Kang H, Liu X, Tong Z. Combination of RT‐qPCR testing and clinical features for diagnosis of COVID‐19 facilitates management of SARS‐CoV‐2 outbreak. J Med Virol. 2020. 10.1002/jmv.25721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pan Y, Zhang D, Yang P, Poon LLM, Wang Q. Viral load of SARS‐CoV‐2 in clinical samples. Lancet Infect Dis. 2020;20:411‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. To KK , Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS‐CoV‐2: an observational cohort study. Lancet Infect Dis. 2020. 10.1016/S1473-3009(20)30196-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zou L, Ruan F, Huang M, et al. SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177‐1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kumar CM, Seet E, Van Zundert T. Measuring endotracheal tube intracuff pressure: no room for complacency. J Clin Monit Comput. 2020. 10.1007/s10877-020-005001-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mehel DM, Ozdemir D, Celebi M, et al. Classification of laryngeal injury in patients with prolonged intubation and to determine the factors that cause the injury. Am J Otolaryngol. 2020;102432 10.1016/j.amjoto.2020.102432 [DOI] [PubMed] [Google Scholar]
- 27. Li D, Wang D, Dong J, et al. False‐negative results of real‐time reverse‐transcriptase polymerase chain reaction for severe acute respiratory syndrome coronavirus 2: role of deep‐learning‐based CT diagnosis and insights from two cases. Korean J Radiol. 2020;21:505‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zias N, Chroneou A, Tabba MK, et al. Post tracheostomy and post intubation tracheal stenosis: report of 31 cases and review of the literature. BMC Pulm Med. 2008;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stauffer JL, Olson DE, Petty TL. Complications and consequences of endotracheal intubation and tracheotomy. A prospective study of 150 critically ill adult patients. Am J Med. 1981;70:65‐76. [DOI] [PubMed] [Google Scholar]
- 30. Nouraei SA, Ma E, Patel A, et al. Estimating the population incidence of adult post‐intubation laryngotracheal stenosis. Clin Otolaryngol. 2007;32:411‐412. [DOI] [PubMed] [Google Scholar]
- 31. Nesek‐Adam V, Mrsic V, Oberhofer D, et al. Post‐intubation long‐segment tracheal stenosis of the posterior wall: a case report and review of the literature. J Anesth. 2010;24:621‐625. [DOI] [PubMed] [Google Scholar]
- 32. Storm B, Dybwik K, Nielsen EW. Late complications after percutaneous tracheostomy and oral intubation: evaluation of 1,628 procedures. Laryngoscope. 2016;126:1077‐1082. [DOI] [PubMed] [Google Scholar]
- 33. Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS‐CoV‐2 in infected patients. Clin Infect Dis. 2020. 10.1093/cid/ciaa345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ramanathan K, Antognini D, Combes A, et al. Planning and provision of ECMO services for severe ARDS during the COVID‐19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir Med. 2020. 10.1016/S2213-2600(20)302121-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Henry BM. COVID‐19, ECMO, and lymphopenia: a word of caution. Lancet Respir Med. 2020;8:e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. MacLaren G, Fisher D, Brodie D. Preparing for the most critically ill patients with COVID‐19: the potential role of extracorporeal membrane oxygenation. JAMA. 2020;323:1245. [DOI] [PubMed] [Google Scholar]
- 37. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID‐19 with convalescent plasma. JAMA. 2020. 10.1001/jama.2020.4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu J, Li W, Shi X, et al. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID‐19). J Intern Med. 2020. 10.1111/joim.13063 [DOI] [PubMed] [Google Scholar]
- 39. Brass P, Hellmich M, Ladra A, et al. Percutaneous techniques versus surgical techniques for tracheostomy. Cochrane Database Syst Rev. 2016;7:CD008045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boran OF, Bilal B, Bilal N, et al. Comparison of the efficacy of surgical tracheostomy and percutaneous dilatational tracheostomy with flexible lightwand and ultrasonography in geriatric intensive care patients. Geriatr Gerontol Int. 2020;20:201‐205. [DOI] [PubMed] [Google Scholar]
- 41. Iftikhar IH, Teng S, Schimmel M, Duran C, Sardi A, Islam S. A network comparative meta‐analysis of percutaneous dilatational tracheostomies using anatomic landmarks, bronchoscopic, and ultrasound guidance versus open surgical tracheostomy. Lung. 2019;197:267‐275. [DOI] [PubMed] [Google Scholar]
- 42. Flum DR, Steinberg SD, Adams PX, Wallack MK. Bedside percutaneous tracheostomy in acquired immunodeficiency syndrome. Am Surg. 1998;64:444‐446. [PubMed] [Google Scholar]
- 43. Ahmed N, Hare GM, Merkley J, et al. Open tracheostomy in a suspect severe acute respiratory syndrome (SARS) patient: brief technical communication. Can J Surg. 2005;48:68‐71. [PMC free article] [PubMed] [Google Scholar]
- 44. Gobatto AL, Besen BA, Tierno PF, et al. Comparison between ultrasound‐ and bronchoscopy‐guided percutaneous dilational tracheostomy in critically ill patients: a retrospective cohort study. J Crit Care. 2015;30(220):e213–227. [DOI] [PubMed] [Google Scholar]
- 45. Song J, Xuan L, Wu W, Zhu D, Zheng Y. Comparison of percutaneous dilatational tracheostomy guided by ultrasound and bronchoscopy in critically ill obese patients. J Ultrasound Med. 2018;37:1061‐1069. [DOI] [PubMed] [Google Scholar]
- 46. Gobatto ALN, Besen B, Tierno P, et al. Ultrasound‐guided percutaneous dilational tracheostomy versus bronchoscopy‐guided percutaneous dilational tracheostomy in critically ill patients (TRACHUS): a randomized noninferiority controlled trial. Intensive Care Med. 2016;42:342‐351. [DOI] [PubMed] [Google Scholar]
- 47. Kwan A, Fok WG, Law KI, Lam SH. Tracheostomy in a patient with severe acute respiratory syndrome. Br J Anaesth. 2004;92:280‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wei WI, Tuen HH, Ng RW, Lam LK. Safe tracheostomy for patients with severe acute respiratory syndrome. Laryngoscope. 2003;113:1777‐1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zamora JE, Murdoch J, Simchison B, Day AG. Contamination: a comparison of 2 personal protective systems. CMAJ. 2006;175:249‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tompkins BM, Kerchberger JP. Special article: personal protective equipment for care of pandemic influenza patients: a training workshop for the powered air purifying respirator. Anesth Analg. 2010;111:933‐945. [DOI] [PubMed] [Google Scholar]
- 51. Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID‐19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1‐9. [DOI] [PubMed] [Google Scholar]
- 52. Xing Y, Mo P, Xiao Y, et al. Post‐discharge surveillance and positive virus detection in two medical staff recovered from coronavirus disease 2019 (COVID‐19), China, January to February 2020. Euro Surveill. 2020;25(10). 10.2807/1560-7917.ES.2020.25.10.2000191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andriolo BN, Andriolo RB, Saconato H, Atallah ÁN, Valente O. Early versus late tracheostomy for critically ill patients. Cochrane Database Syst Rev. 2015;1:1‐55. 10.1002/14651858.CD007271.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jeong‐Min K, Yoon‐Seok C, Jo Hye Jun, et al. Identification of Coronavirus Isolated from a Patient in Korea with COVID‐19. Osong Public Health Res Perspect. 2020;11(1):3‐7. 10.24171/j.phrp.2020.11.1.02 [DOI] [PMC free article] [PubMed] [Google Scholar]


