Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is rapidly infecting people worldwide, resulting in the infectious disease coronavirus disease 19 (COVID-19) that has been declared a pandemic. Much remains unknown about COVID-19, including its effects on solid organ transplant (SOT) recipients. Given their immunosuppressed state, SOT recipients are presumed to be at high risk of complications with viral infections such as SARS-CoV-2. Limited case reports in single SOT recipients, however, have not suggested a particularly severe course in this population. In this report, we present a dual-organ (heart/kidney) transplant recipient who was found to have COVID-19 and, despite the presence of a number of risk factors for poor outcomes, had a relatively mild clinical course.
KEYWORDS: biomarker, clinical research/practice, diabetes: type 2, graft survival, heart transplantation/cardiology, immunosuppressive regimens - minimization/withdrawal, infection and infectious agents - viral, infectious disease, kidney transplantation/nephrology
Abbreviations: ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; BNP, B-type natriuretic peptide; COVID-19, Coronavirus disease 19; CRP, C-reactive protein; CXR, chest x-ray; LDH, lactate dehydrogenase; LVEF, left ventricular ejection fraction; MMF, mycophenolate mofetil; O2 sat, oxygen saturation; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOT, solid organ transplant; URI, upper respiratory infection
1. BACKGROUND
Infection with the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), results in a newly identified disease called coronavirus disease 19 (COVID-19).1 In March 2020, the World Health Organization declared COVID-19 a pandemic, and as of April 2, 2020, over one million cases and 50 000 deaths worldwide have been reported.2 Early Chinese data have identified risk factors for adverse clinical outcomes with COVID-19, including older age and comorbidities such as cardiovascular disease, diabetes, and obesity.3 Based on prior studies of respiratory viral infections in solid organ transplant (SOT) recipients,4 it has also been assumed that this population, with its associated immunosuppressed state, is at higher risk of morbidity and mortality with COVID-19. However, the limited case reports published thus far have shown relatively mild clinical courses in heart5 and kidney transplant recipients.6 , 7 Here we present a case of SARS-CoV-2 infection in a diabetic dual-organ (heart/kidney) recipient, whose course was unexpectedly mild despite the presence of numerous risk factors.
2. CASE
A 39-year-old man with a history of idiopathic dilated cardiomyopathy and chronic kidney disease who underwent combined heart/kidney transplant in 2017 presented to our Emergency Department for COVID-19 testing via nasopharyngeal swab after experiencing 1 day of rapid onset symptoms that included fevers (up to 101.8°F), headache, sore throat, dry cough, dyspnea, fatigue, and myalgias. The next day (Symptom Day 3), his real-time reverse transcriptase polymerase chain reaction test for SARS-CoV-2 resulted positive. He was contacted by telephone, but as he was minimally symptomatic, a shared decision was made for him to isolate at home. However, the following day, he was found to have worsening fever, cough, and dyspnea, and he was advised to return to the hospital for further evaluation.
His past medical history was significant for antibody-mediated cardiac graft rejection causing moderately reduced biventricular function in the year prior, which was treated with high-dose steroids (methylprednisolone 500 mg intravenously daily ×3 doses), plasmapheresis, intravenous immunoglobulin (Gamunex 1 g/kg ×2 doses), and rabbit anti-thymocyte globulin (1.5 mg/kg/d ×3 days). He completed this treatment 8 months prior to his current presentation, and his myocardial function subsequently recovered. Additionally, his history was significant for poorly controlled insulin-dependent diabetes mellitus (hemoglobin A1c 10.5%), hypertension, morbid obesity (body mass index 38.4 kg/m2), and chronic diabetic foot ulcers. He lived with his wife, who had an upper respiratory infection (URI) 3 weeks earlier, and his son, who developed URI symptoms at the same time.
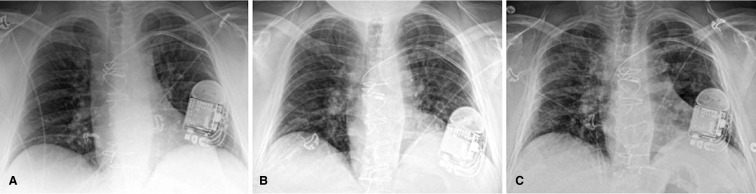
Upon his repeat presentation to the hospital, he endorsed headache, fatigue, and dyspnea, but felt subjectively better than the day prior. He was afebrile, normotensive, mildly tachycardic at baseline heart rate of 100 bpm, and comfortably breathing with an oxygen saturation (O2 sat) of 97% on room air. His exam revealed mild inspiratory rales at the lung bases bilaterally, but he did not have other signs of volume overload. Another nasopharyngeal swab was obtained and was again positive for SARS-CoV-2. His laboratory tests revealed a low white blood cell count (2.4 × 103/μL), with associated lymphopenia (0.23 × 103/μL). His C-reactive protein (CRP) was elevated (6.7 mg/dL), as were his serum lactate (27 mg/dL) and lactate dehydrogenase (LDH, 361 U/L). His procalcitonin was <0.1 μg/mL. His troponin I was mildly elevated at 0.17 ng/mL, but his B-type natriuretic peptide (BNP) was normal at 43 pg/mL. D-dimer was elevated at 1124 ng/mL. His creatinine remained at his baseline. A bedside ultrasound revealed grossly normal biventricular function. Electrocardiogram was unchanged from his baseline with no ischemic changes. Chest x-ray (CXR) revealed mild pulmonary vascular congestion ( Figure 1A). Due to his immunocompromised state and the presence of elevated biomarkers, he was admitted for continued monitoring and supportive care.
FIGURE 1.
Chest x-rays (CXR). A, Initial CXR on first admission (Symptom Day 4) shows mild pulmonary vascular congestion. B, CXR on second admission (Symptom Day 8) demonstrates mildly increased bilateral interstitial opacification. C, CXR on Symptom Day 12 shows slightly more conspicuous peribronchial airspace opacities. Note: The implantable cardioverter defibrillator (ICD) device shown had its leads transected at the time of heart transplantation
On admission, his tacrolimus was continued, but his mycophenolate mofetil (MMF) was held due to his pronounced lymphopenia. His prednisone was continued at his home dose of 9 mg daily. His home losartan was continued at 25 mg twice daily. The next day (Symptom Day 5), he remained afebrile with minimal dyspnea on exertion, but he did not require oxygen supplementation (O2 sat 97% on room air). Despite recommendations to remain in the hospital for observation, he insisted on being discharged so he could tend to personal matters, and he was discharged with strict isolation precautions. He was instructed to continue holding MMF until further notice.
Three days later (Symptom Day 8), during a Telehealth Visit, he was found to again have worsening fever, dyspnea, cough, myalgias, fatigue, and dizziness with ambulation. He noted new hypoxia on his home pulse oximeter. He was advised to return to the hospital for re-admission. He was afebrile (after acetaminophen), normotensive, mildly tachycardic and tachypneic, with an O2 sat 95% on room air initially. His laboratory tests were significant for rising D-dimer, CRP, LDH, and BNP; his troponin I remained mildly elevated ( Table 1). Our institution’s target tacrolimus trough level is 5-8 ng/mL when a patient is >1 year posttransplant, and his tacrolimus doses were adjusted accordingly. Notably, his immune function study (ImmuKnow; Cylex) revealed a severely low immune cell response (ATP Level 6 ng/mL), whereas his most recent test 2 months prior had shown a moderate immune cell response (433 ng/mL). Non-invasive molecular testing for graft rejection revealed stable AlloMap score and low AlloSure result (CareDx), suggesting low risk of graft rejection. His CXR revealed increased bilateral interstitial opacification (Figure 1B). His tacrolimus and prednisone were continued, while his MMF remained held. Losartan was held, and he was started on hydroxychloroquine (400 mg twice daily).
TABLE 1.
Laboratory values over the COVID-19 clinical course
| Symptom day | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 wk prior | Day 4 | Day 5 | Day 8 | Day 9 | Day 10 | Day 11 | Day 12 | Day 13 | Day 14 | Day 15 | |
| WBC (×103/μL) | 8.2 | 2.4 | 2.59 | 2.77 | 3.39 | 3.14 | 4.47 | 5.17 | 5.13 | 6.01 | |
| ALC (×103/μL) | 0.23 | 0.28 | 0.24 | 0.36 | 0.26 | 0.37 | 0.36 | 0.39 | 0.33 | ||
| Platelets (×103/μL) | 209 | 143 | 183 | 169 | 228 | 223 | 301 | 347 | 397 | 454 | |
| Lactate (mg/dL) | 27 | 15 | 13 | 8 | 11 | ||||||
| D-dimer (ng/mL) | 1124 | 2417 | 2563 | 2543 | 3204 | 2650 | 2232 | ||||
| INR | 1.0 | 1.0 | 1.0 | 1.0 | 1.2 | 1.2 | |||||
| LDH (U/L) | 361 | 404 | 518 | 462 | 434 | 395 | 442 | 381 | 445 | ||
| AST (U/L) | 44 | 39 | 60 | 44 | 38 | 26 | 30 | 28 | 43 | ||
| ALT (U/L) | 54 | 54 | 53 | 47 | 39 | 36 | 33 | 33 | 44 | ||
| CRP (mg/dL) | 6.7 | 11.1 | 10.9 | 13.9 | 9.8 | 8.4 | 8.3 | 5.9 | |||
| ESR (mm/h) | 69 | 85 | 67 | 82 | 70 | 64 | |||||
| IL-6 (pg/mL) | 15 | 30 | |||||||||
| Ferritin | 75 | 191 | 206 | 217 | 234 | 213 | 179 | 146 | |||
| Procalcitonin (μg/mL) | <0.1 | 0.1 | |||||||||
| Troponin (ng/mL) | 0.17 | 0.15 | 0.17 | 0.18 | 0.12 | 0.14 | 0.14 | 0.08 | |||
| CK total (U/L) | 155 | 99 | 72 | 79 | |||||||
| BNP (pg/mL) | 43 | 70 | 136 | 227 | 173 | ||||||
| Creatinine (mg/dL) | 1.02 | 0.85 | 1.02 | 1.05 | 1.01 | 1.03 | 1.2 | 1 | 0.88 | 0.92 | |
| Tacrolimus (ng/mL) | 14.2 | 8.5 | 13.2 | 17.4 | 11.5 | 8.6 | 8.8 | 8.7 | |||
Abbreviations: ALC, absolute lymphocyte count; ALT, alanine aminotransferase; AST, aspartate transaminase; BNP, B-type natriuretic peptide; CK, creatine kinase; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; FK-506, tacrolimus IL-6, interleukin-6; INR, international normalized ratio; LDH, lactate dehydrogenase; WBC, white blood cell.
The next morning, he developed a low-grade fever (T 100.2°F) and worsening hypoxia (O2 sat 90% on room air), now requiring up to 4 lpm of oxygen via nasal cannula. Due to concern for potential rapid respiratory decompensation, he was transferred to the intensive care unit (ICU). He was enrolled in the Adaptive COVID-19 Treatment Trial (NCT04280705) evaluating remdesivir versus placebo and started on the study drug. Due to the criteria of the study, his hydroxychloroquine was discontinued.
Over the next 2 days, he experienced recurrent mild fevers (up to 100.7°F) and chills, which were controlled with aspirin (acetaminophen not permitted in clinical trial). He remained normotensive with mild tachycardia, and telemetry monitoring did not reveal any arrhythmias. He did not require non-invasive positive pressure ventilation or intubation, as his oxygen requirements steadily decreased. Transthoracic echocardiography revealed a normal left ventricular ejection fraction (LVEF). His laboratory trends are shown in Table 1.
As his symptoms and oxygen requirements continued to improve, he was transferred out of the ICU. On Symptom Day 12, his CXR remained stable compared to his prior images (Figure 1C). He was steadily weaned off oxygen supplementation entirely, and on Symptom Day 15, he was discharged home with an O2 sat of 96% on room air.
3. DISCUSSION
Despite the rapidly growing number of cases of COVID-19 worldwide, there remains a limited number of reports of the disease’s impact on a specifically vulnerable, immunosuppressed population: SOT recipients. In the sole report of heart transplant recipients with COVID-19 published thus far, the two patients from Wuhan survived and were successfully discharged home.6 Neither of the patients required mechanical ventilatory support. Additionally, COVID-19 was described in a kidney transplant recipients in Spain,6 China,7 and Italy.8
While the immunocompromised status of SOT recipients raises the concern for atypical presentations of viral infection, our patient presented with the classic triad of fever, fatigue, and dry cough, which were the most common clinical features of infected patients in an early study from Wuhan.9 Interestingly, he noted a rather distinct onset of his symptoms on the afternoon of Symptom Day 1, and had a mild early infection stage, or “Stage 1” in the three-stage system proposed by Siddiqi and Mehra, of his disease course.10 Similar to the other reported cases in heart transplant recipients,5 the pulmonary phase (“Stage 2”) was also rather mild, requiring minimal oxygen support. Lastly, despite his persistently elevated inflammatory markers and troponin I levels, he did not develop a clinically significant hyperinflammation phase (“Stage 3”), with no evidence of significant myocardial dysfunction. Notably, rare cases of fulminant myocarditis in COVID-19 have been described.11 , 12
Currently, the optimal medical management of the immunocompetent, let alone immunosuppressed, patient with COVID-19 is unknown. Based on our institution’s protocol in heart transplant recipients with suspected viral infection, as well as the patient’s significant lymphopenia on presentation (which was likely due to SARS-CoV-2 infection), MMF was held on his initial admission and throughout his course, while his tacrolimus and prednisone were continued. Interestingly, ImmuKnow testing revealed a markedly depressed immune cell response, though interpretation of the results of this test in the setting of COVID-19 is unclear and has not previously been described. The decision to hold MMF was weighed against the patient’s recent history of significant cardiac graft rejection within the previous year; reassuringly, echocardiography showed normal graft function and his renal graft function remained relatively normal as well. The presence of de novo donor specific antibodies will be assessed during his outpatient follow-up to help guide his immunosuppression management.
Despite our patient’s immunosuppressed status with three immunosuppressive agents and two solid organ grafts, he had a rather mild disease course with no evidence of rejection of either of his transplanted grafts. His creatinine remained near his baseline throughout his course, and his echocardiogram showed a normal LVEF. In addition to his risk factors of hypertension, diabetes mellitus, and morbid obesity, his laboratory tests on presentation were significant for lymphopenia, elevated inflammatory markers (CRP, interleukin-6), LDH, D-dimer, and troponin I, all of which were associated with increased disease severity and poorer outcomes in prior COVID-19 studies.3 , 9 , 13 , 14 This overall profile heightened our concern for potential decompensation and contributed to his brief ICU stay, but ultimately he required minimal supportive care.
The relative contributions of the medical therapy he was given and his immunosuppression regimen to his clinical course is currently unclear. Importantly, a significant limitation is the fact that we do not know whether he received remdesivir or placebo as part of the clinical trial. Further, we cannot entirely exclude the possibility that his AMR treatment 8 months prior had an effect on his outcome. However, his overall course aligns with the limited prior reports of COVID-19 positive heart transplant recipients with similarly benign courses.5 Additionally, a recent report followed 87 heart transplant recipients in Hubei, finding a low rate of SARS-CoV-2 infection with no severe cases of COVID-19 among them.15 Interestingly, SOT recipients similarly did not appear to be at particularly increased risk of adverse outcomes in past novel coronaviruses pandemics caused by SARS-CoV in 2003 and the Middle East respiratory syndrome-related coronavirus (MERS-CoV) in 2012.16 Nonetheless, more evidence is needed to determine the true risk profile of SOT recipients in the current COVID-19 pandemic. The question of whether immunosuppression of SOT patients attenuates the hyper-inflammatory Stage 310 of the COVID-19 clinical course associated with multiorgan failure is a proposed theory. This hypothesis will be critical to delineate as we continue to investigate the pathophysiology of the disease and construct predictive algorithms of which patients benefit from immunomodulatory therapies and when these therapies should be administered to most effectively suppress a hyper-inflammatory response.
Acknowledgments
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to patient privacy or ethical restrictions.
REFERENCES
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Coronavirus Disease (COVID-19) Pandemic. https://who.int. Accessed April 1, 2020.
- 3.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weigt SS, Gregson AL, Deng JC, Lynch JP, Belperio JA. Respiratory viral infections in hematopoietic stem cell and solid organ transplant recipients. Semin Respir Crit Care Med. 2011;32(4):471–493. doi: 10.1055/s-0031-1283286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li F, Cai J, Dong N. First cases of COVID-19 in heart transplantation from China. J Heart Lung Transplant. 2020;39(5):496–497. doi: 10.1016/j.healun.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guillen E, Pineiro GJ, Revuelta I, et al. Case report of COVID-19 in a kidney transplant recipient: does immunosuppression alter the clinical presentation? [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.15874 [DOI] [PMC free article] [PubMed]
- 7.Zhu L, Xu X, Ma K, et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.15869 [DOI] [PMC free article] [PubMed]
- 8.Gandolfini I, Delsante M, Fiaccadori E, et al. COVID-19 in kidney transplant recipients [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.15891 [DOI] [PMC free article] [PubMed]
- 9.Wang D, Hu BO, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. [published online ahead of print 2020]. 10.1093/eurheartj/ehaa190 [DOI] [PMC free article] [PubMed]
- 12.Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) [published online ahead of print 2020]. JAMA Cardiol. 10.1001/jamacardio.2020.1096 [DOI] [PMC free article] [PubMed]
- 13.Shi S, Qin MU, Shen BO, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China [published online ahead of print 2020]. JAMA Cardiol. 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed]
- 14.Lippi G, Plebani M. The critical role of laboratory medicine during coronavirus disease 2019 (COVID-19) and other viral outbreaks [published online ahead of print 2020]. Clin Chem Lab Med. 10.1515/cclm-2020-0240 [DOI] [PubMed]
- 15.Ren Z-L, Hu R, Wang Z-W, et al. coronavirus outbreak in Wuhan, China: a descriptive survey report [published online ahead of print 2020]. J Heart Lung Transplant. 2020 10.1016/j.healun.2020.03.008 [DOI] [PMC free article] [PubMed]
- 16.D’Antiga L. Coronaviruses and immunosuppressed patients. The facts during the third epidemic. Liver Transpl. 2020;26:832–834. doi: 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to patient privacy or ethical restrictions.