Abstract
Neurologic sequelae can be devastating complications of respiratory viral infections. We report the presence of virus in neural and capillary endothelial cells in frontal lobe tissue obtained at postmortem examination from a patient infected with severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2). Our observations of virus in neural tissue, in conjunction with clinical correlates of worsening neurologic symptoms, pave the way to a closer understanding of the pathogenic mechanisms underlying central nervous system involvement by SARS‐CoV‐2.
Keywords: CNS infection, endothelium, neuroinvasion, neurotropism, SARS‐CoV‐2
Highlights
The clinical spectrum of COVID-19 has expanded to include neurologic manifestations such as anosmia, ageusia, ataxia and seizures, suggesting that SARS-CoV-2 may also be neurotropic.
Ultrastructural analysis of tissue from this case revealed the presence of viral-like particles in brain and capillary endothelium, which was further confirmed by molecular testing for SARS-CoV-2.
This case provides first evidence for the potential direct propagation and presence of SARS-CoV-2 in human brain tissue.
These findings have direct implications for neurologic clinical practice and should raise awareness amongst physicians managing SARS-CoV-2-infected patients with CNS symptoms.
1. INTRODUCTION
The emerging viral pathogen, severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) 1 has been reported in association with upper and lower respiratory tract infections ranging from asymptomatic to severe disease with fatal outcomes. 2 Recent reports have noted that anosmia, ageusia, ataxia, and seizures may be early signs and symptoms in SARS‐CoV‐2‐infected patients, suggesting that this virus may also be neurotropic. 3 Herein, we confirm the likely presence of the virus in brain tissue from postmortem examination of a SARS‐CoV‐2‐infected patient.
2. CASE REPORT
A 74‐year‐old Hispanic male with resting tremors and gait impairment due to Parkinson's disease was admitted because of fever, confusion, and a positive nasopharyngeal swab test for SARS‐CoV‐2 by real‐time reverse transcription‐polymerase chainreaction amplification (RT‐PCR, cobas 6800 system, Roche Diagnostics). He was brought to the emergency department by his family following two falls at home. There was no history of fever, cough, nausea, vomiting, diarrhea, recent sick contacts, or travel. At admission, he was confused but oriented to person and place, consistent with his baseline. Other findings included fever to 38.9°C, thrombocytopenia (122 000/µL, reference range 150 000‐450 000/µL), and blood oxygen saturation (SpO2) = 94% on room air. Chest radiography showed an enlarged cardiac silhouette and tortuous aorta, but no infiltrates. Findings on computerized tomography of the head were nonspecific with patchy subcortical and periventricular hypodensities unchanged from a scan 6 months earlier. Treatment with oral hydroxychloroquine and subcutaneous enoxaparin was initiated.
Over the next 4 days, he was intermittently alert and at times agitated and combative. He was persistently febrile, with episodes of hypotension and progressively worsening SpO2. On day 5, he developed new onset atrial fibrillation with a rapid ventricular rate and QTc prolongation to 515 millisecond. His blood pressure improved after a bolus of intravenous fluid and initiation of amiodarone, with reversion to normal sinus rhythm. Hydroxychloroquine was discontinued, and intravenous tocilizumab and metoprolol were started. Repeat chest radiography showed new left basilar densities consistent with left lower lobe consolidation and a component of pleural fluid, right basilar densities, and patchy densities in the right mid‐lung. He continued to decompensate clinically and expired on day 11.
During the hospital course, the inflammatory markers C‐reactive protein (CRP) and ferritin rose to peak on day 7 at 183.5 mg/L (CRP reference range, 0.0‐5.0 mg/L) and 2837 ng/mL (ferritin reference range, 30‐400 ng/mL), respectively, then declined to 10.7 mg/L and 1063 ng/mL by day 11. The D‐dimer level rose from 0.74 µg/mL FEU (reference range, 0.00‐0.50 µg/mL FEU) on day 3 to 5.85 µg/mL FEU at day 10.
Transmission electron microscopy of sections obtained at postmortem examination revealed the presence of 80 to 110 nm viral particles in frontal lobe brain sections (Figure 1A‐D). Pleomorphic spherical viral‐like particles were observed individually and in small vesicles of endothelial cells (Figure 1A, arrow). Blebbing of viral‐like particles coming in/out of the endothelial wall (Figure 1B, arrows) pointing to presumed active pathogen entry‐transit (transcellular penetration) across the brain microvascular endothelial cells into the neural niche was recorded. Neural cell bodies exhibited distended cytoplasmic vacuoles (Figure 1C,D) containing enveloped viral particle exhibiting electron dense centers with distinct stalk‐like peplomeric projections (Figure 1D, arrows/insert).
Figure 1.
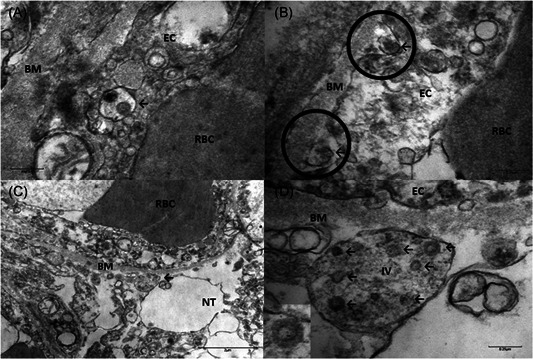
A, Brain capillary endothelial cells showing virus particles within cytoplasmic vacuoles (← arrow) B, Blebbing of viral particles coming in/out of the endothelial cell wall (circles) The relationship of virus particles (arrows←) to the endothelial cells (virus ingress/egress) is depicted. Note the dense inner core and densely stained periphery of viral particles. C, Endothelial neural cell interface showing a cytoplasmic vacuole filled with viral particles in various stages of bud formation (arrow←) adjacent to the basement membrane within the neural cell (frontal lobe). D, Neural intracytoplasmic vesicle showing viral‐like particles. Insert: Detail on viral particle exhibiting electron dense centers with distinct stalk‐like peplomeric projections. Scale bars are shown at the bottom left/right of each figure. BM, basement membrane; EC, endothelial cells; IV, intracytoplasmic vesicles; NT, neural tissue; RBC, red blood cell
The presence of SARS‐Cov‐2 in the brain was confirmed by testing frozen tissue that was minced and resuspended in BD universal viral transport media, and running in parallel in four RT‐PCR assays that target different regions of the viral genome, ORF1/a and E‐gene, N1, N2, N3, N2, and E‐gene, and ORF1ab and S genes. SARS‐CoV‐2 was detected in the brain tissue by all four systems. RT‐PCR testing did not detect SARS‐CoV‐2 in a postmortem cerebrospinal fluid (CSF) sample.
3. DISCUSSION
Central nervous system (CNS) involvement can complicate infections both by viruses that primarily affect humans, and by some animal viruses that can cross species barriers to infect humans, particularly in vulnerable populations. 4 Examples of respiratory viruses with neuroinvasive and neurotropic potential include the influenza virus, 5 human metapneumovirus, 5 members of the Enterovirus/rhinovirus genus, echoviruses, coxsackieviruses, 6 respiratory syncytial virus, 5 and the highly pathogenic zoonotic members of the Henipavirus genus, Hendra, and Nipah viruses. 7
Notably, coronaviruses have also been shown to have neurotropic potential in a range of animal hosts, as well as in humans. In animals, the neuroinvasive coronaviruses have been linked to encephalitis (feline coronavirus, 8 porcine hemaggluttinating encephalitis virus, 9 and mouse hepatitis virus 10 ) and paralytic poliomyelitis (mouse hepatitis virus). 4 Human coronaviruses that have been associated with neurological involvement include the endemic circulating strains OC43 and 229E, and the emerging SARS‐CoV and Middle East respiratory syndrome‐ coronavirus (MERS‐CoV). 4
Neurologic and psychiatric symptoms have been described in patients with SARS‐CoV‐2 infection. Yin et al 11 reported the case of a 64‐year‐old male patient from Wuhan with SARS‐CoV‐2 infection confirmed by an initial nasopharyngeal RT‐PCR test, who presented with febrile respiratory illness accompanied by altered mental status and psychiatric symptoms. Their patient developed lethargy, irritability, meningismus, and signs of pyramidal tract impairment, although RT‐PCR testing of CSF remained negative. The patient recovered uneventfully 17 days after admission. 11 More recently, a report from Moriguchi et al 12 from Japan describes a case of CNS involvement in a SARS‐CoV‐2‐infected patient, with meningitis/encephalitis confirmed by SARS‐CoV‐2 RT‐PCR detection in CSF.
Failure to detect virus in the CSF in our case and others 11 , 13 could have several explanations. The first potential explanation is that the virus is mainly cell‐bound, spreading from cell‐to‐cell. Another possibility is that the virus may have been at concentrations below the level of detection of the testing method. A third possibility is the presence of low concentrations of endonucleases/exonucleases and proteins acting as inhibitors in the CSF. 14
SARS‐CoV, which is taxonomically very similar to SARS‐CoV‐2, has been demonstrated in brain tissue from autopsies. 15 Tissue edema and neuronal degeneration were prominent findings in hematoxylin and eosin‐stained sections, and the presence of viral particles was detected by electron microscopy. 15 Similarly, ultrastructural analysis of tissue from our patient revealed the presence of viral‐like particles ranging from 80 to 110 nm and characteristic club shaped projections 16 with two morphologically distinct types of spike protein structures, typical of betacoronaviruses. 16 The viral particles were located within dilated vesicles in the cytoplasm of a neuron cell body. The evidence of virus in frontal lobe sections provides an alternative explanation for the behavioral changes seen during this patient's hospital course.
Our observation of viral particles in endothelial cells may have implications for the route of entry of SARS‐CoV‐2 into the CNS. Two major pathways, the hematogenous and neuronal retrograde routes, have been proposed for entry of neurotropic respiratory viruses into the CNS. 4 In the neuronal retrograde route, viruses undergo retrograde axonal transport to reach the neuron cell bodies in the peripheral and or central nervous system. 4 In the hematogenous route, viruses gain access by infecting endothelial cells of the blood‐brain‐barrier, epithelial cells of the blood‐cerebrospinal fluid barrier in the choroid plexus, or use inflammatory cells as Trojan horses to gain access into the CNS. 4 Investigations into MERS‐CoV tissue pantropism have indicated that virus can enter the bloodstream followed by endothelial infection in vivo. 17
Our observation of viral‐like particles in brain capillary endothelium and actively budding across endothelial cells strongly suggests a similar role of the endothelial bed and the hematogenous route as the most likely pathway for SARS‐CoV‐2 to the brain. Expression of the human receptor angiotensin‐converting enzyme 2 the binding target receptor for the trimeric spike protein of SARS‐CoV‐2, by the vascular endothelium 18 also supports this interpretation.
However, other routes of CNS entry, such as retrograde axonal transport from the olfactory bulb cannot be ruled out. Experimental evidence has shown that intranasal infection with HCoV‐OC43 and SARS‐CoV in mice can lead to neuroinvasion via disruption of the nasal epithelium and subsequent neuronal dissemination. 4 This may explain the onset of early signs as anosmia as an antecedent to other neurological symptoms. Moreover, it has been suggested that the neuroinvasive potential of SARS‐CoV‐2 may influence respiratory failure of patients with coronavirus disease‐2019, 19 based on previous observations of dysfunction of the cardiorespiratory center that can be induced by other viruses such as SARS‐CoV in the brainstem. 19
The clinical spectrum of SARS‐CoV‐2 infection continues to broaden, raising crucial fundamental questions regarding its cellular tropism and pathogenic mechanisms. Our observations provide first evidence for the direct propagation and presence of SARS‐CoV‐2 in human brain tissue; hematogenous dissemination and brain capillary endothelial cells of the brain‐blood‐barrier in SARS‐CoV–2‐infected individuals are hypothesized to enable pathogen entry into the CNS, and consequent virus‐induced neuropathic effects. In addition, our observations of virus in neural tissue, in conjunction with clinical correlates of worsening neurologic symptoms, pave the way to a closer understanding of the pathogenic mechanisms underlying CNS involvement. However, these results should be interpreted with caution. Further immunohistochemistry, in situ hybridization and ultrastructural studies including immunolabeling will enable better assessment of SARS‐CoV‐2 distribution and help to elucidate the early events driving its potential neurovirulence and neuroinvasiveness.
CONFLICT OF INTERESTS
All the authors declare that there are no conflict of interests.
Paniz‐Mondolfi A, Bryce C, Grimes Z, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2). J Med Virol. 2020;92:699:699–702. 10.1002/jmv.25915
Alberto Paniz‐Mondolfi and Clare Bryce contributed equally to this work.
REFERENCES
- 1. Gorbalenya AE, Baker SC, Baric RS, et al. The species severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nat Microbiol. 2020;5:536‐544. 10.1038/s41564-020-0695-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baig AM. Neurological manifestations in COVID‐19 caused by SARS‐CoV‐2. CNS Neurosci Ther. 2020;00:1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Desforges M, Le Coupanec A, Dubeau P, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bohmwald K, Gálvez N, Ríos M, Kalergis AM. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 2018;12:386. 10.3389/fncel.2018.00386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rhoades RE, Tabor‐Godwin JM, Tsueng G, Feuer R. Enterovirus infections of the central nervous system. Virology. 2011;411(2):288‐305. 10.1016/j.virol.2010.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dawes BE, Freiberg AN. Henipavirus infection of the central nervous system. Pathog Dis. 2019;77(2):ftz023. 10.1093/femspd/ftz023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Foley JE, Rand C, Leutenegger C. Inflammation and changes in cytokine levels in neurological feline infectious peritonitis. J Feline Med Surgery. 2003;5:313‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greig AS, Mitchell D, Corner AH, Bannister GL, Meads EB, Julian RJ. A hemagglutinating virus producing encephalomyelitis in baby pigs. Can J Comp Med Vet Sci. 1962;26:49‐56. [PMC free article] [PubMed] [Google Scholar]
- 10. Cowley TJ, Weiss SR. Murine coronavirus neuropathogenesis: determinants of virulence. J Neurovirol. 2010;16:427‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yin R, Feng W, Wang T, et al. Concomitant neurological symptoms observed in a patient diagnosed with coronavirus disease 2019 [published online ahead of print April 15, 2020]. J Med Virol. 2020. 10.1002/jmv.25888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS‐coronavirus‐2. Int J Infect Dis. 2020;94:55‐58. 10.1016/j.ijid.2020.03.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS‐CoV‐2 infection [published online ahead of print April 15, 2020]. N Engl J Med. 2020. 10.1056/NEJMc2008597 [DOI] [Google Scholar]
- 14. DeBiasi RL, Tyler KL. Polymerase chain reaction in the diagnosis and management of central nervous system infections. Arch Neurol. 1999;10:1215‐1219. [DOI] [PubMed] [Google Scholar]
- 15. Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oshiro LS, Schieble JH, Lennette EH. Electron microscopic studies of coronavirus. J Gen Virol. 1971;12:161‐168. [DOI] [PubMed] [Google Scholar]
- 17. Hocke AC, Becher A, Knepper J, et al. Emerging human Middle East respiratory syndrome coronavirus causes widespread infection and alveolar damage in human lungs. Am J Resp Crit Care Med. 2013;188:882‐886. [DOI] [PubMed] [Google Scholar]
- 18. Hamming I, Timens W, Bulthuis MLC, et al. Neurologic features in severe SARS‐CoV‐2 infection [published online ahead of print April 15, 2020]. N Engl J Med. 2020. 10.1056/NEJMc2008597 [DOI] [Google Scholar]
- 19. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients [published online ahead of print February 27, 2020]. J Med Virol. 2020. 10.1002/jmv.25728 [DOI] [PMC free article] [PubMed] [Google Scholar]


