Abstract
Early life stress (ELS) may accelerate frontoamygdala development related to socioemotional processing, serving as a potential source of resilience. Whether this circuit is associated with other proposed measures of accelerated development is unknown. In a sample of young adolescents, we examined the relations among ELS, frontoamygdala circuitry during viewing of emotional faces, cellular aging as measured by telomere shortening, and pubertal tempo. We found that greater cumulative severity of ELS was associated with stronger negative coupling between bilateral centromedial amygdala and the ventromedial prefrontal cortex, a pattern that may reflect more mature connectivity. More negative frontoamygdala coupling (for distinct amygdala subdivisions) was associated with slower telomere shortening and pubertal tempo over 2 years. These potentially protective associations of negative frontoamygdala connectivity were most pronounced in adolescents who had been exposed to higher ELS. Our findings provide support for the formulation that ELS accelerates maturation of frontoamygdala connectivity and provide novel evidence that this neural circuitry confers protection against accelerated biological aging, particularly for adolescents who have experienced higher ELS. Although negative frontoamygdala connectivity may be an adaptation to ELS, frontoamygdala connectivity, cellular aging, and pubertal tempo do not appear to be measures of the same developmental process.
Keywords: amygdala, early life stress, prefrontal cortex, puberty, telomere length
Introduction
Exposure to early life stress (ELS) during childhood and early adolescence—including family violence, poverty, and emotional abuse—has significant implications for brain development (McLaughlin et al. 2019). Connections between the amygdala and the prefrontal cortex (PFC), including the ventromedial region (vmPFC), are sensitive to a wide range of experiences of ELS and to developmental stages (Gee et al. 2013b; VanTieghem and Tottenham 2018) and constitute a critical circuit for socioemotional processing (Banks et al. 2007; Ochsner et al. 2012). Inverse functional coupling between these regions may reflect top-down regulation of amygdala activity by the vmPFC (Kim et al. 2003; Urry et al. 2006; Ganella et al. 2017) and has generally been associated with better outcomes such as effective emotion regulation and lower levels of internalizing symptoms (Banks et al. 2007; Kim et al. 2011; Gee et al. 2013a, 2013b).
There is evidence that ELS is associated with earlier emergence of inverse frontoamygdala connectivity. Gee et al. (2013a, 2013b) reported that whereas children typically show positive connectivity as they process socioemotional stimuli, adolescents and adults exhibit negative connectivity. In a study comparing previously institutionalized (PI) youth with a history of early psychosocial deprivation and family-reared children, PI youth demonstrated more negative (i.e., more mature) frontoamygdala connectivity at an earlier age (Gee et al. 2013b). Furthermore, in PI youth, positive and negative connectivities were associated with higher and lower levels of anxiety, respectively (Gee et al. 2013b). Thus, there is evidence that ELS is associated with negative frontoamygdala connectivity and that this may confer some positive adaptation in the context of stressful environments—at least in the short term (Callaghan and Tottenham 2016; Teicher et al. 2016). Some neuroimaging studies, however, have found that ELS is associated with positive frontoamygdala connectivity during socioemotional processing. For example, Marusak et al. (2015) found that adolescents who had experienced various types of trauma exhibited positive coupling between the amygdala and perigenual anterior cingulate cortex (pgACC) during an emotional conflict task; in contrast, a comparison group showed negative amygdala–pgACC connectivity. Furthermore, less negative amygdala–pgACC connectivity was associated with reduced behavioral performance in the emotional conflict task. Thus, although there is accumulating evidence that ELS is associated with altered frontoamygdala connectivity, there are inconsistent findings regarding whether ELS is associated with positive or negative coupling between these regions; moreover, it is unclear whether this connectivity strictly confers risk or resilience following ELS.
To date, research examining frontoamygdala connectivity during socioemotional processing in adolescents has primarily focused on psychopathology and on socioemotional skills (Hare et al. 2008; Gee et al. 2013b; Wolf and Herringa 2016; Fowler et al. 2017). These psychological measures of adolescent well-being are also associated with proposed indices of biological aging, including telomeres and pubertal development. Telomeres are protein caps on chromosome ends that serve as a marker of cellular aging (Blackburn 2000). Pubertal tempo is the pace at which adolescents mature (Mendle 2014). Shorter telomere length and accelerated pubertal tempo have been associated both with stress and with a range of mental health problems (Mendle 2014; Epel and Prather 2018). There are at least two reasons to examine the associations between frontoamygdala connectivity and measures of biological aging. The first focuses on the potential of frontoamygdala connectivity as a measure of neurobiological maturation. To the extent that negative frontoamygdala connectivity, greater telomere shortening, and accelerated pubertal maturation are similar indicators of overall biological aging or development in adolescence, these measures should be associated with each other (Belsky 2019). The second reason focuses on frontoamygdala connectivity as a neural-level measure of socioemotional processing. Given that frontoamygdala connectivity plays a central role in emotion and regulation, the functioning of this circuit may have implications for related biological processes, including biological aging. From this perspective, negative frontoamygdala connectivity may be associated with less telomere shortening and slower pubertal maturation. While a growing body of studies have examined telomere length and brain morphology (Henje Blom et al. 2015; Wolkowitz et al. 2015; Staffaroni et al. 2018), only one fMRI study—a cross-sectional investigation conducted with adults—has examined the relation between amygdala reactivity and telomere length (Powell et al. 2019). Cross-sectional studies with adolescents have linked pubertal stage with increased amygdala reactivity during socioemotional processing, including viewing emotional faces and experiencing peer rejection (Moore et al. 2012; Silk et al 2014). To date, however, researchers have not examined frontoamygdala connectivity and subsequent changes in cellular and pubertal measures of biological aging in adolescence.
In the current longitudinal study, we used fMRI and an emotional face task to assess associations among ELS, frontoamygdala connectivity, and telomere shortening and pubertal tempo in a sample of adolescents. We conducted analyses aimed at three goals. First, we assessed whether and how ELS was associated with frontoamygdala connectivity. The stress acceleration hypothesis would predict that higher ELS will be associated with more negative frontoamygdala connectivity, representing an adaptation to early adversity. We operationalized risk following exposure to greater severity of ELS as accelerated telomere shortening and pubertal tempo over approximately 2 years. Thus, our second set of analyses evaluated two different hypotheses regarding whether and how frontoamygdala connectivity is associated with changes in telomere length and puberty. If negative frontoamygdala connectivity in early adolescence, cellular aging, and pubertal maturation are measures of the same developmental process, then negative frontoamygdala connectivity should be associated with more rapid telomere shortening and pubertal tempo. Conversely, from the perspective that frontoamygdala connectivity underlies effective emotion regulation, we would predict that negative frontoamygdala connectivity would be protective against telomere shortening and accelerated pubertal tempo. Lastly, the stress acceleration hypothesis suggests that negative frontoamygdala connectivity confers adaptive outcomes, particularly in the context of exposure to more adversity. Thus, our third set of analyses examined whether severity of ELS moderated associations between frontoamygdala connectivity and both telomere shortening and pubertal tempo.
Materials and Methods
Participants and Procedures
Participants were 214 youth (121 girls, 93 boys) ages 9–13 years (mean age = 11.40 years, SD = 1.0), recruited from the community to participate in a longitudinal study of the effects of early life stress on neurodevelopment across the transition to puberty. Families were recruited using local flyers, media, and online advertisements. In an initial telephone interview, families were provided with information about the study protocol and were screened for inclusion/exclusion criteria. Participants were excluded if they could not undergo MRI (e.g., had metal implants, braces), had a history of neurological disorder or major medical illness, had serious cognitive or physical challenges that might interfere with their ability to understand or complete procedures, were not fluent in English, or, for female participants, had experienced the onset of menses. Participants were Tanner stages 1–4 as measured by self-report (Marshall and Tanner 1969). The distributions of Tanner stages at Time 1 and Time 2 are presented in the supplement. Given that girls advance to sexual maturation earlier than boys, girls and boys were matched on self-reported pubertal stage rather than chronological age (Colich et al. 2017; King et al. 2017). Consequently, girls were younger than boys in our sample (mean difference = 0.74 years, t(212) = 5.48, P < 0.001); age was included as a covariate in all analyses. All families signed consent and assent forms to participate in this study, which was approved by the Stanford University Institutional Review Board, and all participants were compensated for their time.
Participants were diverse in terms of race/ethnicity (44.4% White; 20.1% biracial; 10.3% Asian, 8.4% Black; 8.4% Hispanic/Latino; 6.5% other; 1.9% did not report) and family socioeconomic status (mean annual family income = $75 K–$100 K, median annual family income = $100 K–$125 K, range from less than $5 K to greater than $150 K). Of the 214 participants, 62 were missing neuroimaging data due to opting out of the MRI session, to having unusable data (e.g., excessive movement in the scanner), or to technical difficulties (e.g., problems with the task). Two participants were missing saliva samples for assaying telomere length at the baseline assessment of the study (Time 1). A follow-up assessment (Time 2) took place approximately 2 years later (mean = 1.96 years, SD = 0.34, range from 1.28 to 3.37 years) and included 124 adolescents who provided saliva samples (75 girls, 49 boys) and 158 adolescents who provided pubertal stage data (88 girls, 70 boys). The average age at the Time 2 saliva sample was 13.35 years (SD = 1.05, range = 11.15–15.85). Participants who provided saliva samples at both time points did not differ significantly from participants who were missing saliva samples at Time 2 with respect to age at Time 1, pubertal stage at Time 2, sex, race/ethnicity, parental education, family income, or amygdala activation at Time 1 (all Ps > 0.171), but did differ in severity of ELS exposure (P = 0.014) and Time 1 pubertal stage (P = 0.016); participants who were missing saliva samples at Time 2 were exposed to higher levels of ELS and reported being at an earlier pubertal stage at Time 1. Participants who provided pubertal stage data at Time 1 did not differ significantly from participants who were missing pubertal stage data at Time 2 with respect to all other variables in the present study (all Ps > 0.102). Participants who provided useable fMRI data at Time 1 did not differ significantly from participants who were missing fMRI data at Time 2 with respect to age at Time 1, sex, race/ethnicity, parental education, family income, telomere length or pubertal stage at Time 1 and Time 2, and interval between Time 1 and Time 2 (all Ps > 0.050), but did differ in severity of ELS exposure (P = 0.026); participants who were missing fMRI data at Time 2 were exposed to higher levels of ELS.
Early Life Stress Severity
ELS was operationalized in this study as stressful events occurring up to early adolescence. As previously described (King et al. 2017), to assess history of ELS at Time 1, adolescents completed interviews assessing exposure to 30 different types of stressful experiences using a modified version of the Traumatic Events Screening Inventory for Children (TESI-C; Ribbe 1996). For each type of stressful experience that adolescents endorsed, the interviewer asked questions about the details of the event, including age at onset of the event. These interview responses were coded for objective stress severity by a panel of trained raters who were blind to the adolescents’ reactions during the interview and their perceived severity of each event. Responses were coded using a modified version of the UCLA Life Stress Interview coding system (Rudolph and Hammen 1999); the objective severity of each stressful event was rated on a scale ranging from 0 (non-event or no impact) to 4 (extremely severe impact) with half-point increments. Coders discussed cases of disagreement between their ratings to reach consensus. To obtain a cumulative measure of ELS severity for each adolescent, we summed the maximum severity scores from all stressors endorsed across each type of stress. This scoring algorithm is available at: https://github.com/lucysking/els_stress_interview. Descriptions of the types and rates of ELS in our sample, and analyses testing the association between cumulative severity of ELS and age at onset of ELS, are presented in the Supplement.
fMRI Task
At Time 1, we administered a well-validated fMRI task to probe neural activation in response to viewing subliminally and supraliminally presented emotional face stimuli (Korgaonkar et al. 2013; Williams et al. 2015). Because prior neuroimaging research examining the stress acceleration hypothesis in previously institutionalized youth used supraliminally presented emotional faces (Gee et al. 2013b), we present analyses here using data from the supraliminal condition of the task. Analyses using data from the subliminal condition of the task are presented in the Supplement.
Participants passively viewed pictures of faces of men and women expressing six different emotions (anger, disgust, fear, happy, neutral, and sad) (Gur et al. 2002). Faces were presented for 500 ms with an interstimulus interval (ISI) of 750 ms (blank screen). Five 10 s blocks of 8 faces were presented for each emotion (240 stimuli per run). The single run lasted 5 min 8 s. Blocks were presented in a fixed pseudorandom order.
MRI Data Acquisition
Neuroimaging data were acquired using a GE Discovery MR750 3T scanner (GE Medical Systems, Milwaukee, WI, USA) with a 32-channel head coil. For each participant, a high-resolution T1-weighted structural image was obtained using a spoiled gradient echo (SPGR) scan sequence (TR/TE/TI = 6.24/2.34/450 ms; flip angle = 12°; matrix size = 256 × 256, 186 slices; FOV = 23 cm; scan duration = 315 s). Functional data during the task were acquired using a T2*-weighted echo-planar imaging (EPI) pulse sequence (TR/TE = 2500/30 ms; flip angle = 77°; matrix size = 70 × 70; voxel size = 3.2 mm3; 43 slices; FOV = 224 × 224 mm). The first three volumes of each functional run were discarded to allow for magnet stabilization.
fMRI Data Processing and Analysis
The Analysis of Functional and Neural Images (AFNI) software package (Cox, 1996) was used to preprocess and analyze fMRI data. Preprocessing included volume registration to the first volume, slice timing correction, spatial smoothing with a 4.2-mm half-maximum Gaussian kernel, scaling of blood-oxygen-level-dependent (BOLD) intensity to percentage of signal change, and co-registration with the structural images and warping to the Montreal Neurological Institute (MNI) template using a linear transformation. Volumes with head motion greater than 0.5 mm from the previous volume (i.e., relative motion) were censored from the data. Participants for whom more than 20% of volumes were censored were defined as having unusable neuroimaging data. A boxcar plot convolved with the hemodynamic response function was used to model BOLD response for each of the emotion blocks.
Defining Amygdala Subdivision and vmPFC Regions of Interest (ROIs)
All of our analyses focused on the two major subdivisions of the amygdala nuclei: the basolateral amygdala (BLA) and centromedial amygdala (CMA). The BLA serves as the sensory information input site of the amygdala and is involved in associative learning and evaluation of emotional significance of stimuli (Davis and Whalen 2001). The CMA serves as the output of the amygdala and is involved in allocating attention and enhancing arousal by way of organizing autonomic, endocrine, and behavioral responses to emotionally evocative stimuli (LeDoux 2007). Given both that ELS may differentially affect the functioning of specific amygdala subdivisions (Suzuki et al. 2014; Rau et al. 2015), and that distinct psychological mechanisms may be related to cellular aging (O’Donovan et al. 2012), we explored whether the two subdivisions of the amygdala are differentially associated with ELS, telomere shortening, and pubertal tempo.
Probabilistic maps of cytoarchitectonic boundaries from the Juelich anatomic atlas from FSL (Jenkinson et al. 2012) were used to define BLA and CMA subdivisions as regions of interest (ROIs; see Fig. 1a) (Amunts et al. 2005). Given previous work suggesting a potential lateralization of amygdala activation for socioemotional processing (Cahill 2003; Baas et al. 2004), separate masks for right and left BLA and CMA were created. A bilateral anterior vmPFC ROI was defined using the Mackey atlas in AFNI (see Fig. 1b) (Mackey and Petrides 2014). Previous studies have used this voxel resolution to study functional connectivity of the BLA and CMA subdivisions (Roy et al. 2009; Qin et al. 2014; Brown et al. 2014; Jalbrzikowski et al. 2017).
Figure 1.

Regions of interest (ROIs) including (a) basolateral amygdala (BLA; yellow) and centromedial amygdala (CMA; red) and (b) ventromedial prefrontal cortex (vmPFC; red), used in the examination of ROI-to-ROI connectivity.
Frontoamygdala Functional Connectivity
We used psychophysiological interaction (PPI) analysis to examine individual differences in BLA–mPFC and CMA–vmPFC connectivity during the presentation of emotion faces relative to implicit baseline. For each participant, general linear models included regressors for emotion face type, left and right BLA and CMA seed time series (for the respective analysis), the interactions of each emotion face type and each time series, and six motion regressors. Correlations for each BLA and CMA PPI interaction term were extracted from the bilateral vmPFC ROI and converted using Fisher’s r-to-z transformation. PPI values were used in subsequent analyses focusing on the association between ELS and connectivity, connectivity and telomere shortening, and connectivity and pubertal tempo. PPI values were highly correlated across different emotion face types for amygdala–vmPFC circuits (rs ranging from 0.61 to 0.92, all P < 0.001). Thus, to examine overall associations with the processing of emotional faces (i.e., processing not specific to emotion face type), we averaged PPI values across emotions to form indices of BLA–vmPFC and CMA–vmPFC connectivity. It is important to note that although most task-based studies of frontoamygdala connectivity have used fearful faces as stimuli (Di et al. 2017), the amygdala is sensitive to other types of emotional faces, including neutral faces (Marusak et al. 2013). In addition, findings that neutral faces can be perceived as negative (Blasi et al. 2009), particularly in adolescence (Marusak et al. 2017), call into question the use of adult neutral faces as baseline conditions in developmental neuroimaging studies. Thus, our analyses focused on frontoamygdala connectivity in response to faces in general (including fear, happy, sad, anger, disgust, and neutral faces) relative to implicit baseline rather than to neutral faces.
Change in Telomere Length
Telomere assessment in this sample was previously described in Humphreys et al. (2019b). Briefly, genomic DNA was purified from 500 μL of saliva collected in the Oragene DNA Kit (DNA Genotek, Kanata, ON, Canada) with the DNA Agencourt DNAdvance Kit (cat. No. A48705; Beckman Coulter Genomics, Brea, CA, USA). DNA was quantified by Quant-iT PicoGreen dsDNA Assay Kit (cat. No. P7589; Life Technologies, Grand Island, NY, USA) and run on 0.8% agarose gels to check the integrity. DNA samples were stored at −80°C. Parameters for assaying telomere length are presented in the Supplement. As expected, telomere length was significantly shorter at Time 2 than at Time 1 (mean difference = 0.11, t(119) = 6.14, P < 0.001), and telomere length was correlated across time points (r = 0.68, P < 0.001). Telomere shortening was defined as the rate of change in telomere length from Time 1 to Time 2 (i.e., the difference in telomere length from Time 1 to Time 2 over the interval between Time 1 and Time 2). Longer telomere length at Time 1 was associated with faster telomere shortening (r = −0.34, P < 0.001).
Pubertal Tempo
Pubertal stage was assessed using the Tanner staging self-report questionnaire (Morris and Udry 1980). Participants reported on their own pubic hair growth and breast/genitalia growth, and these two Tanner scores were averaged to generate a measure of pubertal stage. As expected, pubertal stage was significantly higher at Time 2 than at Time 1 (mean difference = 1.42, t(157) = 20.86, P < 0.001), and pubertal stage was correlated across time points (r = 0.46, P < 0.001). Pubertal tempo was defined as the rate of change in pubertal stage from Time 1 to Time 2 (i.e., the difference in pubertal stage from Time 1 to Time 2 over the interval between Time 1 and Time 2). Pubertal tempo was negatively correlated with pubertal stage at Time 1 (r = −0.44, P < 0.001).
Data Analyses
We first conducted correlation analyses to examine unadjusted associations among ELS severity, amygdala activation and connectivity, telomere shortening, and pubertal tempo. All analyses of telomere shortening and pubertal tempo are controlled for telomere length and pubertal stage at baseline (i.e., Time 1), respectively. Next, we conducted regression analyses to examine whether significant associations among these variables remained after adjusting for age and sex. Extreme outlier values for all variables were identified using boxplots (values greater or less than the mean by three times the interquartile range); these values were replaced with the next most extreme value in the dataset prior to analyses. All analyses were conducted using the lavaan package (Rosseel 2012) in R software (R Core Team 2017). Model parameters were estimated using full information maximum likelihood estimation to account for missing data.
Results
Participant Characteristics and Associations between ELS and Amygdala Activation and Connectivity
Descriptive statistics and unadjusted correlations are presented in Table 1.
Table 1.
Descriptive statistics and correlations
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Sex (female = 1) | 1 | |||||||||||||
| 2. Age at T1 sample | −0.35*** | 1 | ||||||||||||
| 3. T1–T2 interval | 0.17 | −0.10 | 1 | |||||||||||
| 4. ELS | 0.02 | −0.06 | −0.00 | 1 | ||||||||||
| 5. L CMA | −0.16* | 0.12 | −0.05 | 0.09 | 1 | |||||||||
| 6. R CMA | −0.03 | 0.08 | −0.05 | 0.19* | .49*** | 1 | ||||||||
| 7. L BLA | 0.02 | 0.06 | −0.11 | 0.12 | 0.28*** | −0.06 | 1 | |||||||
| 8. R BLA | 0.00 | 0.10 | −0.04 | 0.13 | 0.41*** | 0.37*** | 0.43*** | 1 | ||||||
| 9. L CMA–vmPFC | 0.04 | 0.00 | −0.10 | −0.20* | 0.01 | −0.12 | −0.05 | −0.12 | 1 | |||||
| 10. R CMA–vmPFC | 0.01 | 0.12 | −0.26* | −0.32*** | 0.03 | −0.14 | 0.16 | 0.04 | 0.49*** | 1 | ||||
| 11. L BLA–vmPFC | −0.09 | 0.03 | 0.11 | −0.08 | 0.14 | 0.05 | 0.05 | −0.03 | 0.17* | 0.21** | 1 | |||
| 12. R BLA–vmPFC | −0.06 | −0.03 | 0.19 | 0.06 | 0.09 | 0.03 | −0.07 | 0.03 | 0.36*** | 0.19* | 0.41*** | 1 | ||
| 13. TL change | 0.01 | −0.04 | 0.11 | −0.12 | −0.10 | −0.19* | −0.10 | −0.09 | −0.26** | −0.17 | −0.10 | 1 | ||
| 14. Pubertal tempo | −0.06 | 0.28*** | 0.13 | −0.07 | 0.09 | 0.03 | 0.12 | 0.06 | −0.03 | 0.01 | 0.23* | −0.11 | 1 | |
| Mean or % | 57% | 11.38 | 1.97 | 6.96 | 0.07 | 0.19 | −0.02 | 0.02 | 0.02 | 0.00 | 0.00 | 0.00 | −0.05 | 0.70 |
| SD | 1.04 | 0.34 | 5.44 | 0.75 | 0.67 | 0.87 | 0.95 | 0.10 | 0.09 | 0.09 | 0.09 | 0.10 | 0.43 |
Note: ***P < 0.001, **P < 0.01, *P < 0.05. ELS = early life stress; CMA = centromedial amygdala; BLA = basolateral amygdala; T1 = Time 1; T2 = Time 2; L = left; R = right; TL change = (T2 telomere length minus T1 telomere length)/T1–T2 interval; Pubertal tempo = T2 Tanner stage minus T1 Tanner stage)/T1–T2 interval. All correlations with TL change and pubertal tempo controlled for T1 telomere length and T1 Tanner stage, respectively.
Participants who experienced higher levels of ELS had greater activation in the right CMA (r = 0.19, P = 0.022) and more negative right and left CMA–vmPFC connectivity (r = −0.32 and r = −0.20, P < 0.001 and P = 0.020, respectively). Figure 2 presents the associations between ELS and CMA–vmPFC connectivity. Associations between ELS and right CMA activation (β = 0.20, P = 0.019), right CMA–vmPFC connectivity (β = −0.31, P < 0.001), and left CMA–vmPFC connectivity (β = −0.20, P = 0.020) remained statistically significant after adjusting for sex and age at T1. These covariates were not associated with amygdala activation and connectivity in these models (all P > 0.073).
Figure 2.
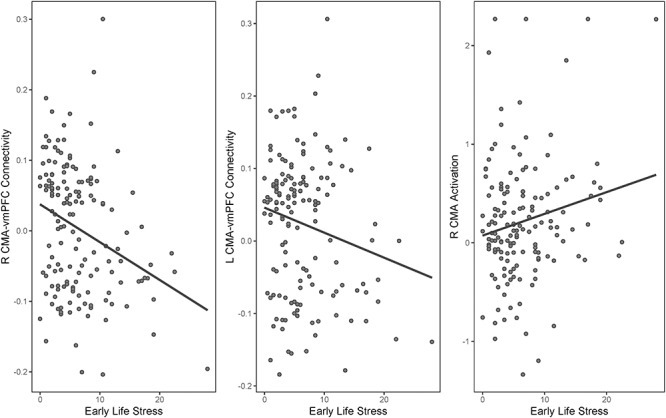
Centromedial amygdala–vmPFC connectivity and right centromedial amygdala activation associated with early life stress.
Associations among Frontoamygdala Connectivity, Telomere Shortening, and Pubertal Tempo
Participants who demonstrated more positive right CMA–vmPFC connectivity showed accelerated telomere shortening over the course of approximately 2 years (β = −0.26, P = 0.005), controlling for telomere length at Time 1. Participants who demonstrated more positive right BLA–vmPFC connectivity showed accelerated pubertal tempo over the course of approximately 2 years (β = 0.23, P = 0.015), controlling for pubertal stage at Time 1. These associations remained statistically significant (β = −0.25, P = 0.007 and β = 0.22, P = 0.015 for telomere shortening and pubertal tempo, respectively) after adjusting for sex and age at T1. Other frontoamygdala connectivity variables and ELS were not significantly associated with telomere shortening (all P > 0.092) or pubertal tempo (all P > 0.192), and telomere shortening and pubertal tempo were not significantly associated with each other. Participants who demonstrated greater left BLA activation showed accelerated telomere shortening (β = 0.19, P = 0.041). This association remained statistically significant after adjusting for sex and age at Time 1 (β = −0.19, P = 0.043). Activation in other amygdala subdivisions was not significantly associated with telomere shortening or pubertal tempo (all P > 0.228).
Interactive Effects of ELS and Frontoamygdala Connectivity on Telomere Shortening and Pubertal Tempo
These associations, combined with previous research examining frontoamygdala connectivity as an adaptation to ELS (Gee et al. 2013b; Keding and Herringa, 2016; Colich et al. 2017), raise the possibility that connectivity is associated with telomere shortening and pubertal tempo differently at low and high levels of ELS severity. Thus, we conducted additional analyses to explore whether ELS moderates the associations between frontoamygdala connectivity and telomere shortening and pubertal tempo. All models included covariates to adjust for sex, age at Time 1, telomere length or pubertal stage at Time 1, as well as the main effects of ELS and frontoamygdala connectivity. We centered all variables prior to forming interaction terms and probed significant interactions using simple slope tests at 1 SD above and below the mean of ELS severity. This revealed significant interactions between ELS and right CMA–vmPFC (β = −0.37, P = 0.004) and between ELS and right BLA–vmPFC connectivity (β = −0.36, P = 0.003) on change in telomere length (Fig. 3). The associations of right CMA–vmPFC and right BLA–vmPFC connectivity with telomere shortening were significantly stronger for adolescents who reported higher levels of ELS (β = −0.71, P < 0.001 and β = −0.52, P = 0.001 for right CMA–vmPFC and right BLA–vmPFC, respectively) than for adolescents who reported exposure to levels of ELS at the sample mean (β = −0.35, P < 0.001 and β = −0.19, P = 0.028 for right CMA–vmPFC and right BLA–vmPFC, respectively) or lower levels of ELS (β = −0.00, P = 0.979 and β = −0.14, P = 0.255 for right CMA–vmPFC and right BLA–vmPFC, respectively). ELS did not interact significantly with other connectivity variables to predict telomere shortening (both Ps > 0.151).
Figure 3.
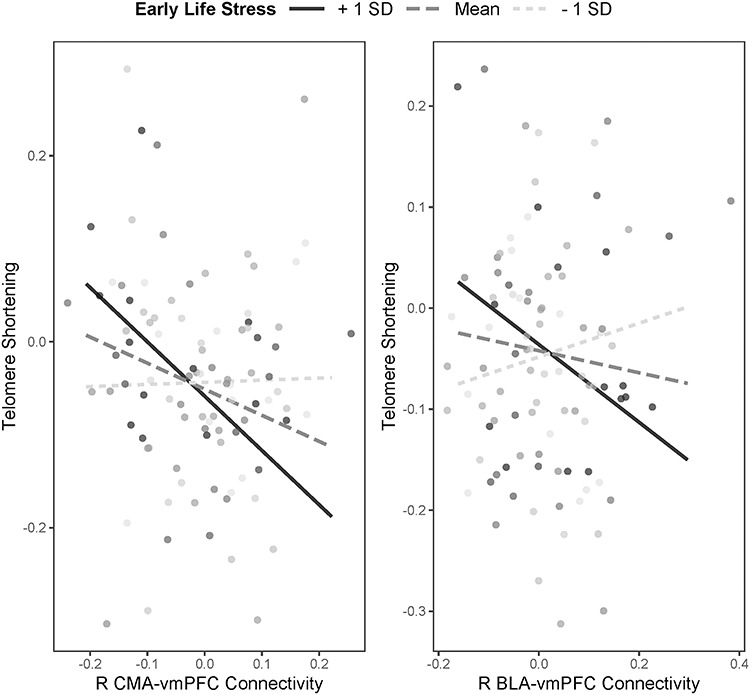
Interactions between severity of early life stress and magnitude of right frontoamygdala connectivity in predicting telomere shortening.
For models predicting pubertal tempo, the interactions between ELS and right CMA–vmPFC connectivity (β = 0.34, P = 0.015), ELS and right BLA–vmPFC connectivity (β = 0.33, P = 0.007), and ELS and left BLA–vmPFC connectivity (β = 0.23, P = 0.044) were also statistically significant (Fig. 3). The positive associations of right CMA–vmPFC and left BLA–vmPFC connectivity with pubertal tempo were significant only for those adolescents who reported higher levels of ELS (β = 0.43, P = 0.030 and β = 0.38, P = 0.034 for right CMA–vmPFC and left BLA–vmPFC, respectively), but not for adolescents who reported exposure to levels of ELS at the sample mean (β = 0.11, P = 0.294 and β = 0.13, P = 0.165 for right CMA–vmPFC and left BLA–vmPFC, respectively) or lower levels of ELS (β = −0.22, P = 0.084 and β = −0.12, P = 0.361 for right CMA–vmPFC and left BLA–vmPFC, respectively). The association of right BLA–vmPFC connectivity with pubertal tempo was significantly stronger for adolescents who reported higher levels of of ELS (β = 0.58, P < 0.001) than for adolescents who reported exposure to levels of ELS at the sample mean (β = 0.38, P < 0.001) or lower levels of ELS (β = −0.01, P = 0.912). The main effects of ELS on pubertal tempo were significant across regression models (βs ranging from 0.19 to 0.29, all P < 0.038), with the exception of the model considering right BLA–vmPFC connectivity (ELS main effect β = 0.15, P = 0.082). Age at Time 1 was significantly associated with pubertal tempo across all regression models (βs ranging from 0.31 to 0.35, all P < 0.001) (Fig. 4).
Figure 4.
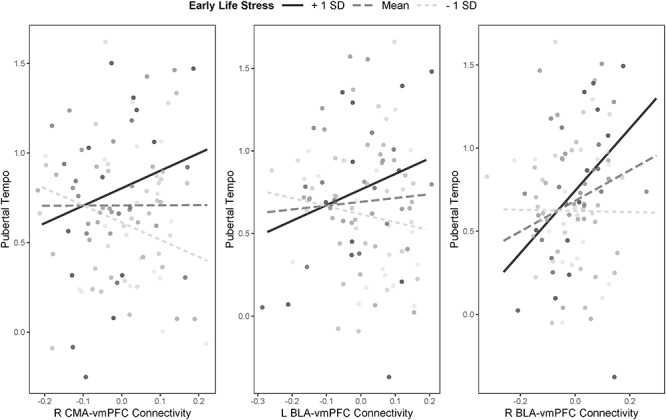
Interactions between severity of early life stress and magnitude of frontoamygdala connectivity in predicting pubertal tempo.
Discussion
In a community sample of adolescents (9–13 years of age at Time 1), we examined (1) whether ELS is associated with amygdala activation and connectivity in response to viewing emotional faces; (2) whether frontoamygdala connectivity is related to cellular aging measured by the rate of change in telomere length and pubertal tempo measured by the rate of change in self-reported Tanner stages, in adolescence; and (3) whether the association between frontoamygdala connectivity and putative measures of biological aging is stronger following exposure to higher levels of ELS. We found that higher ELS scores were associated with higher right CMA reactivity, as well as with stronger negative bilateral CMA–vmPFC connectivity, in response to emotional faces. We also found that more negative right CMA–vmPFC connectivity was associated with slower shortening of telomeres, whereas more negative right BLA–vmPFC was associated with slower pubertal development. In secondary analyses, we examined whether frontoamygdala connectivity was differentially associated with changes in telomere length and changes in pubertal stage as a function of ELS severity. We found that the association between more negative right frontoamygdala connectivity (for both amygdala subdivisions) and reduced telomere shortening was significantly stronger following exposure to higher ELS. We also found a similar pattern for predicting pubertal development; more negative right CMA–vmPFC connectivity was associated with slower pubertal tempo following greater exposure to ELS. Our findings suggest that exposure to ELS is associated with a specific neurophenotype potentially reflecting greater maturation of socioemotional processing and, further, that this neurophenotype may confer protection against accelerated biological aging, particularly in adolescents who report experiencing higher ELS. Our findings are consistent with (1) experimental nonhuman animal research suggesting that ELS accelerates maturation of the amygdala and its connections with the PFC (Ono et al. 2008; Muhammed et al. 2012); (2) human studies linking early psychosocial deprivation and threatening experiences with amygdala hyperactivity and with negative frontoamygdala connectivity (Tottenham et al. 2011; Gee et al. 2013b; Colich et al. 2017); and (3) studies suggesting that stronger negative frontoamygdala connectivity is an adaptation to ELS (Gee et al. 2013b). The current study builds on this literature by providing novel evidence that frontoamygdala connectivity during socioemotional processing appears to have implications for cellular aging and pubertal development in adolescence and that ELS potentiates the association between frontoamygdala connectivity and these measures of biological aging.
More negative CMA–vmPFC connectivity in early adolescence following higher ELS appears to reflect altered development of this circuitry. Specifically, negative connectivity between the mPFC and amygdala has been posited to indicate top-down regulation of the mPFC on amygdalar signals and, thus, more mature neural circuitry and effective emotion regulation. In contrast, positive connectivity between these regions is more typical in childhood and reflects less mature circuitry (Gee et al. 2013a, 2013b). Negative frontoamygdala connectivity related to ELS may serve an adaptive, compensatory role in emotion regulation, whereas other ELS-related neural abnormalities could contribute to socioemotional difficulties.
Our findings suggest that connectivity with the centromedial subdivision of the amygdala is particularly affected by ELS. The CMA generates autonomic, endocrine, and behavioral responses to facilitate attention to salient stimuli (LeDoux 2007), a feature of socioemotional processing that may be sensitive to ELS and adaptive in stressful environments (Frankenhuis and de Weerth 2013). Gee et al. (2013b) proposed that amygdala hyperactivity in previously institutionalized youth may facilitate the development of connections between amygdala and prefrontal regions. Here, we also observed heightened amygdala activation in the right CMA in adolescents who were exposed to higher ELS, which is consistent with some previous human neuroimaging research (Suzuki et al. 2014). Despite the promise of our findings, more research is necessary to determine whether the CMA is more sensitive to ELS than are other amygdala subdivisions. In addition, longitudinal research spanning a wider time span is necessary to determine whether the early emergence of accelerated amygdala functioning precedes the early maturation of frontoamygdala connectivity.
Evidence for the formulation that stress accelerates frontoamygdala maturation (i.e., results in the early emergence of negative functional connectivity) has come primarily from studies of previously institutionalized youth (Gee et al. 2013b) and clinical samples (Wolf and Herringa 2016). Our findings provide further support for this formulation in a relatively low-risk, community sample. Importantly, even in this sample nearly all of the participants reported having experienced at least one adverse event in their lifetime; in fact, the majority of children in the United States of America have been exposed to adverse experiences (Cohen et al. 2006; McLaughlin et al. 2012). Bartlett and colleagues (2019) recently found that common stressful life events in a community sample of adolescent girls were associated with accelerated maturation of parietal/cortical morphology. Taken together, it appears that exposure to more intense forms of stressful, albeit prevalent, life events could accelerate neurobiological development (Ho 2019), including in frontoamygdala functional connectivity. The stress acceleration hypothesis (Callaghan and Tottenham 2016) may help us gain a better understanding of adolescent development related to stress in general rather than development that is specific to stress in at-risk or clinical populations.
In addition to supporting the hypothesized links between ELS and amygdala functioning and connectivity, our study provides novel evidence that frontoamygdala connectivity during socioemotional processing is related to measures of biological aging in adolescence. Previous research with adolescents and adults suggests that socioemotional factors are associated with telomere length and/or change (Epel and Prather 2018; Humphreys et al. 2019b) and with measures of pubertal development (Marceau et al. 2011; Mendle 2014). Far fewer studies have considered the associations between socioemotional processing at the neural level and cellular aging or pubertal tempo. In fact, only one previous study has linked telomere length with amygdala reactivity to emotional faces, and that study was cross-sectional, conducted with adults (Powell et al. 2019). The present study is the first to focus on rates of change in telomere length and self-reported pubertal stage over time in relation to frontoamygdala connectivity in adolescence. Stronger negative frontoamygdala connectivity may reflect processes underlying regulation and decreased negative affect (Urry et al. 2006; Gee et al. 2013b; Fowler et al. 2017). Importantly, the centromedial subdivision of the amygdala and negative affect have been related to biological processes that may be involved in telomere shortening and puberty, such as cortisol (Burke et al. 2005; Belsky et al. 2015; Saxbe et al. 2015). One interpretation of our findings is that negative right frontoamygdala connectivity in early adolescence represents a neural capacity for socioemotional and biological processes that buffer against accelerated biological aging in early adolescence. Conversely, positive right frontoamygdala connectivity in early adolescence appears to be a neurophenotype related to increased risk for faster telomere shortening and pubertal tempo. This finding suggests that frontoamygdala connectivity during socioemotional processing has implications for biological aging at the cellular and reproductive system level. Further research is necessary to replicate these findings, to elucidate the specific psychological and biological paths that link frontoamygdala connectivity with biological aging, and to determine the potential long-term outcomes of faster telomere shortening and pubertal tempo related to adolescent frontoamygdala connectivity.
Our findings do not provide support for the perspective that negative frontoamygdala connectivity, cellular aging, and pubertal tempo in adolescence are similar measures of developmental acceleration. It is possible that frontoamygdala connectivity is not a reliable marker of neural development (Colich et al. 2019) or that the processes of biological aging and neural development in adolescence are not strongly overlapping constructs. Indeed, although recent theory suggests that accelerated development across different constructs (e.g., pubertal maturation, telomere shortening, brain connectivity) reflect a single process (Belsky 2019), research indicates that different measures of biological aging are not necessarily related to one another (Belsky et al. 2018). Our findings build on this literature by further indicating that proposed indicators of biological aging may not measure the same developmental process.
Our results also suggest that negative frontoamygdala connectivity during socioemotional processing is an adaptation to ELS that confers some protection against accelerated cellular aging and pubertal development. Interestingly, Gee et al. (2013b) found that previously institutionalized youth tended to demonstrate negative frontoamygdala connectivity that appeared to mitigate anxiety; however, previously institutionalized youth who exhibited positive frontoamygdala connectivity had the most severe difficulties with anxiety. Similarly, in our community sample, positive right BLA–vmPFC and right CMA–vmPFC connectivity were related to faster telomere shortening, and right CMA–vmPFC was related to faster pubertal tempo, in adolescents who experienced greater cumulative severity of ELS. Thus, moderate to severe ELS may potentiate the link between frontoamygdala connectivity and biological aging in adolescence. Frontoamygdala connectivity appears to be unrelated to biological aging in adolescents exposed to less severe ELS. Other circuitry may be more important for biological aging in these adolescents, whereas the socioemotional processes related to negative frontoamygdala connectivity (e.g., emotion regulation) may be particularly important for effectively meeting the demands of more stressful environments (Callaghan and Tottenham 2016). Positive frontoamygdala connectivity, potentially indicating less mature socioemotional functioning, may be particularly disadvantageous in the context of higher ELS. Although negative frontoamygdala connectivity may indicate neural adaptation to ELS, given that it has been linked to resilience against early anxiety (Gee et al. 2013b) and, in the present study, to accelerated biological aging, the long-term outcomes associated with this pattern of connectivity are not clear. Maintaining cortical states of immaturity may help to extend periods of neuronal plasticity that facilitate learning in humans (Somel et al. 2009). Typical trajectories of prefrontal development are characterized by initial synaptogenesis, followed by synaptic pruning starting in late childhood (Petanjek et al. 2011). ELS may alter these trajectories, however, in such a way that terminates or limits neuronal plasticity. Indeed, stress has been shown to downregulate the expression of genes in the vmPFC that are involved in synaptic plasticity (Karssen et al. 2007). In the context of our findings, a potential consequence of accelerated maturation of amygdala–vmPFC circuitry related to ELS is reduced sensitivity to certain kinds of environmental input (Callaghan and Tottenham 2016), such as positive experiences that are important for healthy development (Ho 2019). In addition, alterations in cortical synaptogenesis and pruning may serve as developmental antecedents of the later onset of mental health difficulties (Rakic et al. 1994). Taken together, it is possible that there are costs associated with having both exposure to higher ELS and stronger negative frontoamygdala connectivity that emerge over time.
Although stress has been linked to telomere length and pubertal tempo (Mendle, 2014; Ridout et al. 2018), it is worth noting that we did not find these direct associations in the current study for either baseline or change; moreover, some prior studies have also found no association between these variables or have reported mixed results (Mendle et al. 2011; Negriff et al. 2015; Bürgin et al. 2019). These inconsistent findings may be due, in part, to differences across studies in samples and measures of adversity (Bürgin et al. 2019). In the present study, we used a measure of objective severity of ELS; it is possible, however, that subjective stress is implicated more directly in biological aging processes. Indeed, we have previously documented in this sample that adolescents who self-report more severe depressive symptoms have accelerated telomere shortening over time (Humphreys et al. 2019b), and other studies have also found associations between subjective stress and telomere length (Epel et al. 2004; Simon et al. 2006). Moderators of the association between ELS and biological aging, such as timing of ELS exposure (Ridout et al. 2018) and individual differences in reactivity to social conditions (Ellis et al. 2011), could also contribute to inter-study heterogeneity.
We should note three limitations of this study. First, our measure of ELS was based on retrospective self-report of the cumulative severity of previously experienced stressful events. There have been calls for studies that consider potential sensitive periods during which stress may have particularly strong and enduring effects on the brain (Humphreys et al. 2019a; Zhu et al. 2019). Longitudinal studies with repeated assessments of ELS will be critical for determining whether adolescent frontoamygdala circuitry is sensitive to ELS experienced during specific developmental windows. Second, in addition to the timing of ELS, a growing body of research suggests that specific forms of ELS have distinct effects on adolescent development (Sheridan and McLaughlin 2016; King et al. 2019; Sumner et al. 2019). Future studies should examine whether studying different forms of ELS, compared with cumulative stress, yields greater insight concerning frontoamygdala connectivity and its association with proposed measures of biological aging. Finally, some research suggests that ELS affects functional connectivity originating from BLA and CMA subdivisions differently (Grant et al. 2015). In this study we did not determine the direction of influence between the amygdala and vmPFC; PPI analyses utilize correlations that do not provide temporal information about neural activation or about whether activity is inhibitory or excitatory.
In conclusion, we found evidence that ELS is associated with stronger reactivity to socioemotional stimuli in the right CMA, an amygdala subdivision that mediates arousal and orienting. We also found that ELS was associated with more mature frontoamygdala circuitry as indexed by stronger negative CMA–vmPFC coupling. Negative frontoamygdala coupling was associated with slower telomere shortening and slower pubertal tempo 2 years later, and this effect was significantly stronger in adolescents who had experienced higher ELS. ELS may accelerate frontoamygdala development, and our findings offer novel evidence that this circuitry may have important implications for cellular aging and pubertal tempo, particularly for adolescents who have been exposed to higher ELS. Taken together, our findings are consistent with the formulation that negative frontoamygdala connectivity is an adaptation to ELS, but also suggest that frontoamygdala connectivity, cellular aging, and pubertal tempo are not measures of the same developmental or aging process.
Funding
This work was supported by the National Institute of Mental Health (R37MH101495 to IHG, T32MH019908 to JGM, K01MH117442 to TCH, F32MH107129 to KLH, K01MH106805 to SJO, F32MH114317 to NLC), the Brain and Behavior Research Foundation (Young Investigator Award 23819 to KLH), the Klingenstein Third Generation Foundation (Fellowship Awards in Child and Adolescent Depression to TCH and KLH), the Jacobs Foundation (Early Career Research Fellowship 2017–1261-05 to KLH), the National Science Foundation (Graduate Fellowship Awards to LSK and NLC), and Precision Health and Integrated Diagnostics Center at Stanford (PHIND to IHG and TCH).
Conflict of Interest: The authors declare no conflict of interest.
Supplementary Material
References
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. 2005. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol. 210:343–352. [DOI] [PubMed] [Google Scholar]
- Baas D, Aleman A, Kahn RS. 2004. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Res Rev. 45:96–103. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. 2007. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett EA, Klein DN, Li K, DeLorenzo C, Kotov R, Perlman G. 2019. Depression severity over 27 months in adolescent girls is predicted by stress-linked cortical morphology. Biol Psychiatry. 86:769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J. 2019. Early-life adversity accelerates child and adolescent development. Curr Dir Psychol Sci. 28:241–246. [Google Scholar]
- Belsky J, Ruttle PL, Boyce WT, Armstrong JM, Essex MJ. 2015. Early adversity, elevated stress physiology, accelerated sexual maturation, and poor health in females. Dev Psychol. 51:816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Moffitt TE, Cohen AA, Corcoran DL, Levine ME, Prinz JA, Schaefer J, Sugden K, Williams B, Poulton R, et al.. 2018. Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: do they measure the same thing? Am J Epidemiol. 187:1220–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn EH. 2000. Telomere states and cell fates. Nature. 408:53–56. [DOI] [PubMed] [Google Scholar]
- Blasi G, Hariri AR, Alce G, Taurisano P, Sambataro F, Das S, Bertolino A, Weinberger DR, Mattay VS. 2009. Preferential amygdala reactivity to the negative assessment of neutral faces. Biol Psychiatry. 66:847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VM, LaBar KS, Haswell CC, Gold AL, Mid-Atlantic MIRECC Workgroup, McCarthy G, Morey RA. 2014. Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology. 39:351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürgin D, O’Donovan A, d’Huart D, Gallo A, Eckert A, Fegert J, Schmeck K, Schmod M, Boonmann C. 2019. Adverse childhood experiences and telomere length a look into the heterogeneity of findings – a narrative review. Front Neurosci. 13:490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. 2005. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 30:846–856. [DOI] [PubMed] [Google Scholar]
- Cahill L. 2003. Sex- and hemisphere-related influences on the neurobiology of emotionally influenced memory. Prog Neuropsychopharmacol Biol Psychiatry. 27:1235–1241. [DOI] [PubMed] [Google Scholar]
- Callaghan BL, Tottenham N. 2016. The stress acceleration hypothesis: effects of early-life adversity on emotion circuits and behavior. Curr Opin Behav Sci. 7:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Hitsman BL, Paul RH, McCaffery J, Stroud L, Sweet L, Gunstad J, Niaura R, MacFarlane A, Bryant RA, et al.. 2006. Early life stress and adult emotional experience: an international perspective. Int J Psychiatry Med. 36:35–52. [DOI] [PubMed] [Google Scholar]
- Colich NL, Rosen ML, Williams ES, McLaughlin KA. 2019. Biological aging in childhood and adolescence following experiences of threat and deprivation: a systematic review and meta-analysis. BioRxiv. 1-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colich NL, Williams ES, Ho TC, King LS, Humphreys KL, Price AN, Ordaz SJ, Gotlib IH. 2017. The association between early life stress and prefrontal cortex activation during implicit emotion regulation is moderated by sex in early adolescence. Dev Psychopathol. 29:1851–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW.. 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 29:162–173. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. 2001. The amygdala: vigilance and emotion. Mol Psychiatry. 6:13–34. [DOI] [PubMed] [Google Scholar]
- Di X, Huang J, Biswal BB. 2017. Task modulated brain connectivity of the amygdala: a meta-analysis of psychophysiological interactions. Brain Struct Funct. 222:619–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Shirtcliff EA, Boyce WT, Deardorff J, Essex MJ. 2011. Quality of early family relationships and the timing and tempo of puberty: effects depend on biological sensitivity to context. Dev Psychopathol. 23:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthorn RM. 2004. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 101:17312–17315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Prather AA. 2018. Stress, telomeres, and psychopathology: toward a deeper understanding of a triad of early aging. Annu Rev Clin Psychol. 14:371–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CH, Miernicki ME, Rudolph KD, Telzer EH. 2017. Disrupted amygdala-prefrontal connectivity during emotion regulation links reactive rumination and adolescent depressive symptoms. Dev Cogn Neurosci. 27:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenhuis WE, Weerth C. 2013. Does early-life exposure to stress shape or impair cognition? Curr Dir Psychol Sci. 23:466–470. [Google Scholar]
- Ganella DE, Barendse MEA, Kim JH, Whittle S. 2017. Prefrontal-amygdala connectivity and state anxiety during fear extinction recall in adolescents. Front Hum Neurosci. 11:587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Hare TA, Bookheimer SY, Tottenham N. 2013a. A developmental shift from positive to negative connectivity in human-amygdala prefrontal circuitry. J Neurosci. 33:4584–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY, Tottenham N. 2013b. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A. 110:15638–15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant MM, Wood K, Sreenivasan K, Wheelock M, White D, Thomas J, Knight DC, Deshpande G. 2015. Influence of early life stress on intra- and extra-amygdaloid causal connectivity. Neuropsychopharmacology. 40:1782–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Schroeder L, Turner T, McGrath C, Chan RM, Turetsky BI, Alsop D, Maldjian J, Gur RE. 2002. Brain activation during facial emotion processing. Neuroimage. 16:651–662. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. 2008. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 63:927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henje Blom E, Han LK, Connolly CG, Ho TC, Lin J, LeWinn KZ, Simmons AN, Sacchet MD, Mobayed N, et al.. 2015. Peripheral telomere length and hippocampal volume in adolescents with major depressive disorder. Transl Psychiatry. 5:e676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T. 2019. Stress and neurodevelopment in adolescent depression. Biol Psychiatry. 86:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys KL, King LS, Sacchet MD, Camacho MC, Colich NL, Ordaz SJ, Ho TC, Gotlib IH. 2019a. Evidence for a sensitive period in the effects of early life stress on hippocampal volume. Dev Sci. 22:e12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys KL, Sisk LM, Manczak EM, Lin J, Gotlib IH. 2019b. Depressive symptoms predict change in telomere length and mitochondrial DNA copy number across adolescence. J Am Acad Child Adolesc Psychiatry. pii:S0890-8567(19)32100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalbrzikowski M, Larsen B, Hallquist MN, Foran W, Calabro F, Luna B. 2017. Development of white matter microstructure and intrinsic functional connectivity between the amygdala and ventromedial prefrontal cortex: associations with anxiety and depression. Biol Psychiatry. 82:511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. 2012. FSL Neuroimage. 62:782–790. [DOI] [PubMed] [Google Scholar]
- Karssen AM, Her S, Li JZ, Patel PD, Meng F, Bunney WE Jr, Jones EG, Watson SJ, Akil H, Myers RM, et al.. 2007. Stress-induced changes in primate prefrontal profiles of gene expression. Mol Psychiatry. 12:1089–1102. [DOI] [PubMed] [Google Scholar]
- Keding TJ, Herringa RJ. 2016. Paradoxical prefrontal-amygdala recruitment to angry and happy expressions in pediatric posttraumatic stress disorder. Neuropsychopharmacology. 41:2903–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. 2003. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 14:2317–2322. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ. 2011. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res. 223:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LS, Colich NL, LeMoult J, Humphreys KL, Ordaz SJ, Price AN, Gotlib IH. 2017. The impact of severity of early life stress on diurnal cortisol: the role of puberty. Psychoneuroendocrinology. 77:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LS, Humphreys KL, Camacho MC, Gotlib IH. 2019. A person-centered approach to the assessment of early life stress: associations with the volume of stress-sensitive brain regions in early adolescence. Dev Psychopathol. 31:643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korgaonkar MS, Grieve SM, Etkin A, Koslow SH, Williams LM. 2013. Using standardized fMRI protocols to identify patterns of prefrontal circuit dysregulation that are common and specific to cognitive and emotional tasks in major depressive disorder: first wave results from the iSPOT-D study. Neuropsychopharmacology. 38:863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. 2007. The amygdala Curr Biol. 17:R868–R874. [DOI] [PubMed] [Google Scholar]
- Mackey S, Petrides M. 2014. Architecture and morphology of the human ventromedial prefrontal cortex. Eur J Neurosci. 40:2777–2796. [DOI] [PubMed] [Google Scholar]
- Marceau K, Ram N, Houts RM, Grimm KJ, Susman EJ. 2011. Individual differences in boys’ and girls’ timing and tempo of puberty: modeling development with nonlinear growth models. Dev Psychol. 47:1389–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JA. 1969. Variations in pattern of pubertal changes in girls. Arch Dis Child. 44:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Carré JM, Thomason ME. 2013. The stimuli drive the response: an fMRI study of youth processing adult or child emotional face stimuli. Neuroimage. 83:679–689. [DOI] [PubMed] [Google Scholar]
- Marusak HA, Martin KR, Etkin A, Thomason ME. 2015. Childhood trauma exposure disrupts the automatic regulation of emotional processing. Neuropsychopharmacology. 40:1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Zundel CG, Brown S, Rabinak CA, Thomason ME. 2017. Convergent behavioral and corticolimbic connectivity evidence of a negativity bias in child and adolescents. Soc Cogn Affect Neurosci. 12:517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Greif Green J, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. 2012. Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Arch Gen Psychiatry. 69:1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Weissman D, Bitrán D. 2019. 2019. Childhood adversity and neural development: a systematic review. Annu Rev Dev Psychol. 1:277–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendle J, Leve LD, Van Ryzin M, Natsuaki MN, Ge X. 2011. Associations between early life stress, child maltreatment, and pubertal development among girls in foster care. J Res Adolesc. 21:871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendle J. 2014. Why puberty matters for psychopathology. Child Dev Perspect. 8:218–222. [Google Scholar]
- Morris NM, Udry JR. 1980. Validation of a self-administered instrument to assess stages of adolescent development. J Youth Adolesc. 9:271–280. [DOI] [PubMed] [Google Scholar]
- Muhammad A, Carroll C, Kolb B. 2012. Stress during development alters dendritic morphology in the nucleus accumbens and prefrontal cortex. Neuroscience. 216:103–109. [DOI] [PubMed] [Google Scholar]
- Negriff S, Blankson AN, Trickett PK. 2015. Pubertal timing and tempo: associations with childhood maltreatment. J Res Adolesc. 25:201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Tomiyama AJ, Lin J, Puterman E, Adler NE, Kemeny M, Wolkowitz OM, Blackburn EH, Epel ES. 2012. Stress appraisals and cellular aging: a key role for anticipatory threat in the relationship between psychological stress and telomere length. Brain Behav Immun. 26:573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. 2012. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 1251:E1–E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Kikusui T, Sasaki N, Ichikawa M, Mori Y, Murakami-Murofushi K. 2008. Early weaning induces anxiety and precocious myelination in the anterior part of the basolateral amygdala of male Balb/c mice. Neuroscience. 156:1103–1110. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judaš M, Šimić G, Rašin MR, Uylings HBM, Rakic P, Kostović I. 2011. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci U S A. 108:13281–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Siegle GJ, Lee KH, Nelson EE, Stroud LR, Dahl RE.. 2014. Increased neural response to peer rejection associated with adolescent depression and pubertal development. Soc Cog Affect Neurosci. 9:1798–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell TR, De Jong S, Breen G, Lewis CM, Dima D. 2019. Telomere length as a predictor of emotional processing in the brain. Hum Brain Mapp. 40:17550–11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Young CB, Duan X, Chen T, Supekar K, Menon V. 2014. Amygdala subregional structure and intrinsic functional connectivity predicts individual differences in anxiety during early childhood. Biol Psychiatry. 75:892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, Bourgeois J-P, Goldman-Rakic PS. 1994. Synaptic development of the cerebral cortex: implications for learning, memory, and mental illness. Prog Brain Res. 102:227–243. [DOI] [PubMed] [Google Scholar]
- Rau AR, Chappell AM, Butler TR, Ariwodola OJ, Weiner JL. 2015. Increased basolateral amygdala pyramidal cell excitability may contribute to the anxiogenic phenotype induced by chronic early-life stress. J Neurosci. 35:9730–9740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbe D. 1996. Psychometric review of traumatic event screening instrument for children (TESI-C) In: Stamm BH, editor. Measurement of Stress, Trauma, and Adaptation. Lutherville, MD: Sidran Press. [Google Scholar]
- Ridout KK, Levandowski M, Ridout SJ, Gantz L, Goonan K, Palermo D, Price LH, Tyrka AR. 2018. Early life adversity and telomere length: a meta-analysis. Mol Psychiatry. 23:858–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosseel Y. 2012. Lavaan: an R package for structural equation modeling. J Stat Softw. 48:1–36. [Google Scholar]
- Roy AK, Shehzad Z, Marguilies DS, Kelly AM, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP. 2009. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 45:614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Hammen C. 1999. Age and gender as determinants of stress exposure, generation, and reactions in youngsters: a transactional perspective. Child Dev. 70:660–677. [DOI] [PubMed] [Google Scholar]
- Saxbe DE, Negriff S, Susman EJ, Trickett PK. 2015. Attenuated hypothalamic-pituitary-adrenal axis functioning predicts accelerated pubertal development in girls 1 year later. Dev Psychopathol. 27:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, McLaughlin KA. 2016. Neurobiological models of the impact of adversity on education. Curr Opin Behav Sci. 10:108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, Nierenberg AA, Fava M, Wong KK. 2006. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 60:432–435. [DOI] [PubMed] [Google Scholar]
- Somel M, Franz H, Yan Z, Lorenc A, Guoa S, Giger T, Kelso J, Nickel B, Dannemann M, Bahn S, et al.. 2009. Transcriptional neoteny in the human brain. Proc Natl Acad Sci U S A. 106:5743–5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffaroni AM, Tosun D, Lin J, Elahi FM, Casaletto KB, Wynn MJ, Patel N, Neuhaus J, Walters SM, Epel ES, et al.. 2018. Telomere attrition is associated with declines in medial temporal lobe volume and white matter microstructure in functionally independent older adults. Neurobiol Aging. 69:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, Colich NL, Uddin M, Armstrong D, McLaughlin KA. 2019. Early experiences of threat, but not deprivation, are associated with accelerated biological aging in children and adolescents. Biol Psychiatry. 85:268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Luby JL, Botteron KN, Dietrich R, McAvoy MP, Barch DM. 2014. Early life stress and trauma and enhanced limbic activation to emotionally valenced faces in depressed and healthy children. J Am Acad Child Adolesc Psychiatry. 53:800–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RC 2017. R: A Language and Environment for Statistical Computing. https://www.R-project.org/
- Teicher MH, Samson JA, Anderson CM, Ohashi K. 2016. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci. 17:652–666. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gihooly T, Zevin JD, Casey BJ. 2011. Elevated amygdala response to faces following early deprivation. Dev Sci. 14:190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, et al.. 2006. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 26:4415–44125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanTieghem MR, Tottenham N. 2018. Neurobiological programming of early life stress: functional development of amygdala-prefrontal circuitry and vulnerability to stress-related psychopathology. Curr Top Behav Neurosci. 38:117–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Korgaonkar MS, Song YC, Paton R, Eagles S, Goldstein-Piekarski A, Grieve SM, Harris AW, Usherwood T, Etkin A. 2015. Amygdala reactivity to emotional faces in the prediction of general and medication-specific responses to antidepressant treatment in the randomized iSPOT-D trial. Neuropsychopharmacology. 40:2398–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf RC, Herringa RJ. 2016. Prefrontal-amygdala dysregulation to threat in pediatric posttraumatic stress disorder. Neuropsychopharmacology. 41:822–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkowitz OM, Mellon SH, Lindqvist D, Epel ES, Blackburn EH, Lin J, Reus VI, Burke H, Rosser R, Mahan L, et al.. 2015. PBMC telomerase activity, but not leukocyte telomere length, correlates with hippocampal volume in major depression. Psychiatry Res. 232:58-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Lowen SB, Anderson CM, Ohashi K, Khan A, Teicher MH. 2019. Association of prepubertal and postpubertal exposure to childhood maltreatment with adult amygdala function. JAMA Psychiat. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


