Abstract
The ongoing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has a drastic impact on national health care systems. Given the overwhelming demand on facility capacity, the impact on all health care sectors has to be addressed. Solid organ transplantation represents a field with a high demand on staff, intensive care units, and follow-up facilities. The great therapeutic value of organ transplantation has to be weighed against mandatory constraints of health care capacities. In addition, the management of immunosuppressed recipients has to be reassessed during the ongoing coronavirus disease 2019 (COVID-19) pandemic. In addressing these crucial questions, transplant physicians are facing a total lack of scientific evidence. Therefore, the aim of this study was to offer an approach of consensus-based guidance, derived from individual information of 22 transplant societies. Key recommendations were extracted and the degree of consensus among different organizations was calculated. A high degree of consensus was found for temporarily suspending nonurgent transplant procedures and living donation programs. Systematic polymerase chain reaction-based testing of donors and recipients was broadly recommended. Additionally, more specific aspects (eg, screening of surgical explant teams and restricted use of marginal donor organs) were included in our analysis. This study offers a novel approach to informed guidance for health care management when a priori no scientific evidence is available.
KEYWORDS: clinical research/practice, guidelines, health services and outcomes research, infection and infectious agents—viral, infectious disease, organ acceptance, organ allocation, organ procurement, organ procurement and allocation, organ transplantation in general
Abbreviations: ABTO, Brazilian Association of Organ Transplantation; AST, American Society of Transplantation; BAL, bronchoalveolar lavage; CMS, Centers for Medicare & Medicaid Services; CNT, Italian National Transplant Center; COVID-19, Coronavirus disease 2019; DTG, German Transplant Society; IPST, Portuguese Institute of Blood and Transplantation; KTS, Korean Transplant Society; NAT, Nucleoid acid testing; NTS, The Dutch Transplant Society; ONT, Organización Nacional de Trasplantes; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SEIMC, Spanish Society of Infectious Diseases and Clinical Microbiology; SET, Spanish Society of Transplantation; SRC, Society recommendation consensus; TTS, The Transplantation Society
1. INTRODUCTION
Currently, the global public health sector is facing an unprecedented challenge in dealing with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. Readjustments in the sector of organ transplantation are critically important for several reasons. All stages of the transplant procedure (pretransplant care, the surgical procedure itself, posttransplant care, and long-term follow-up) require high capacities of trained staff, operating rooms, and—crucially during the pandemic—intensive care unit (ICU) treatment. In apportioning ICU and staff capacities, all involved sectors are forced to triage essential treatments from those that can be delayed or substituted by less complex therapy options. Second, conduction of an organ transplant imposes a state of high-level medication-induced immunosuppression to the recent transplant recipient. Furthermore, the management of long-term immunosuppressed (transplanted) patients has to be reassessed in the given pandemic to account for the risk of infection.1, 2, 3, 4
At the moment, the “Adult Elective Surgery and Procedures Recommendations” by the Centers for Medicare & Medicaid Services (CMS) label transplants as a “tier 3b” (high acuity surgery/unhealthy patients) with the consecutive action directive, “Do not postpone.”5
In practice, there is a rather wide degree of medical urgency among solid organ transplants ranging from elective operations to ultima ratio options. Therefore, the reasonable execution of a transplant program that is affected by capacity shortage during the pandemic demands complex consideration and decision-making.
Given the high-speed dynamic of the SARS-CoV-2 outbreak, no scientific evidence is currently available to guide medical policymakers during this crucial period of readjustment.
Also, comparable viral outbreak in the past, such as the SARS outbreak in 2002-2004, did not impose such a challenge to transplant medicine. Therefore, no official recommendations or guidelines on the subject of transplantation programs during the viral outbreak were published. A single center report from the University of Toronto—an area with relatively high disease burden—is to be found including a case report describing a severe course of suspected SARS in a liver transplant recipient 9.5 years after transplantation.6
As no consensus guidelines or international recommendations have been published on coronavirus disease 2019 (COVID-19) and organ transplant, the aim of this study was to offer a consensus-based approach to manage transplant programs until reliable data on risk and benefits of conducting organ transplants in times of a viral pandemic are available.
2. METHODS
2.1. Study design
An online search was performed and recommendations on the topic of solid organ transplants and COVID-19 published by international and national transplant societies were identified. Two independent researchers (PR and NN) pooled the information, defined thematic areas, and interpreted the statements. Relevant key statements were deduced and the degree of consensus on these statements throughout the recommendations was evaluated. Categories were defined as (1) does support (+), (2) does not support (−), (3) leaves the answer open/case-by-case decision (±), or (4) does not comment (n/a). In case of diverging classification, a third investigator (MS) was consulted. According to counts of allocation to each category, statements were classified in “strong recommendation” (Society Recommendation Consensus [SRC] = A, if more than 9 out of all 19 societies support the statement; >50%), “medium recommendation” (SRC = B, if 5 to 9 societies out of all 19 support the statement, >25%) or “low recommendation” (SRC = C, if 1 to 4 societies support the statement, <25%). The number of negative recommendations was subtracted from the count of positive recommendations. Individual statements were then discussed to available literature.
2.2. Statistics
This study does not compare strategies of different countries nor does it draw causal conclusions. Therefore, no specific tests were applied. No study approval was necessary as all information was already published elsewhere.
3. RESULTS
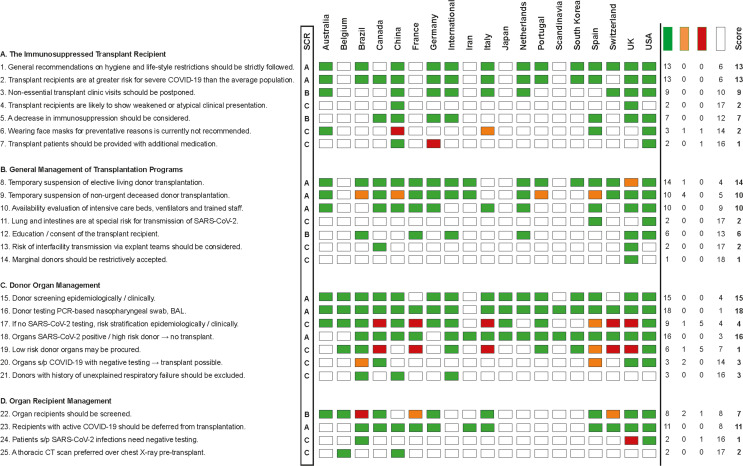
Nineteen society recommendation bulletins on questions concerning organ transplant during the SARS-CoV-2 pandemic could be identified as of March 30, 2020 (countries with society names in brackets): Australia/New Zealand (Transplantation Society of Australia and New Zealand; TSANZ), Belgium (Belgian Transplant Society), Brazil (Brazilian Association of Organ Transplantation; ABTO), Canada (The Canadian Transplant Association/ Canadian Blood Services), China (Branch of Organ Transplantation of Chinese Medical Association), France (Francetransplant, French Biomedical Agency), Germany (German Transplant Society; DTG), International (The Transplantation Society; TTS), Iran (Iranian Society of Organ Donation), Italy (CNT - Italian National Transplant Center), Japan (The Japan Society for Transplantation), Netherlands (Dutch Transplant Society; NTS), Portugal (Portuguese Institute of Blood and Transplantation; IPST), Scandinavia (Scandiatransplant), South Korea (Korean Society for Transplantation; KST), Spain (Spanish Society of Transplantation; SET), Switzerland (Swisstransplant), United Kingdom (British Transplant Society), and USA (American Society of Transplantation; AST). The Austrian Society of Transplantation, Eurotransplant, European Society for Organ Transplantation, Turkish Transplant Foundation, and World Health Organization (WHO) had no published recommendations for transplant professionals or patients on their websites by March 28, 2020. Detailed society recommendations are listed in Figure 1.
FIGURE 1.
Consensus chart of the individual transplant societies. Relevant key statements were deduced and formulated on the basis of information found on the individual transplantation society webpages. Categories were defined as (A) do support (green), (B) do not support (red), (C) leave the answer open/case-by-case decision (orange), or (D) do not comment (white). On the right-hand side, the sum of supporting society recommendations was subtracted by dissenting votes and a final score was calculated. The final score was converted into a society recommendation consensus (SRC; “strong recommendation” = A, if more than 9 societies support the statement, >50%; “medium recommendation” = B, if 5-9 societies support the statement, >25%; “low recommendation” = C, if 1-4 societies support the statement, <25%). BAL, bronchoalveolar lavage; COVID, coronavirus disease 2019; CT, computed tomography; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2. [Color figure can be viewed at wileyonlinelibrary.com]
Conforming with the information given on the individual websites (links see Table 1), 25 recommendation statements were extracted. Statements were pooled according to four subitems of transplant program management:
-
A
Management of long-term immunosuppressed transplant recipients ( Table 2)
-
B
General management of transplantation programs ( Table 3)
-
C
Donor management ( Table 4)
-
D
Recipient evaluation ( Table 5)
TABLE 1.
Sources for recommendations
Date when the recommendation was last updated; authors checked on recommendation updates until March 30, 2020.
TABLE 2.
Recommendations for the immunosuppressed transplant recipient
| Statement | The immunosuppressed transplant recipient | Rating | Consensusa |
|---|---|---|---|
| 1 | Preventative policies and general recommendations concerning hygiene and life-style restrictions should be strictly followed by transplant patients and household cohabiters | A | 13/19 |
| 2 | Transplant recipients are at greater risk for severe COVID-19 than the average population | A | 13/19 |
| 3 | Nonessential transplant clinic visits should be postponed | B | 9/19 |
| 4 | Transplant recipients are likely to show weakened or atypical clinical presentation of COVID-19 | C | 2/19 |
| 5 | A decrease in immunosuppression should be considered for SARS-CoV-2 infected transplant recipients | B | 7/19 |
| 6 | Wearing face masks for prophylactic reasons is currently not recommended for transplant recipients | C | 2/19 |
| 7 | Transplant patients should be provided with additional medication In case of unexpected delay or quarantine |
C | 1/19 |
A, high recommendation; B, medium recommendation; C, low recommendation.
Number of societies that support the statement.
TABLE 3.
Recommendations for transplantation programs
| Statement | Transplantation programs | Rating | Consensusa |
|---|---|---|---|
| 8 | Temporary suspension of elective living donor transplantation should be considered during viral pandemic | A | 14/19 |
| 9 | Temporary suspension of nonurgent deceased donor transplantation may be considered during viral pandemic | A | 10/19 |
| 10 | Availability of intensive care beds, ventilators, and trained staff has to be accounted for prior to organ acceptance | A | 10/19 |
| 11 | The lungs and intestines are at special risk for transmission of SARS-CoV-2 and should not be transplanted in case of suspicion | C | 2/19 |
| 12 | Education on the residual risk of SARS-CoV-2 transmission and retrieval of consent of the transplant recipient are recommended | B | 6/19 |
| 13 | The risk of interfacility transmission of SARS-CoV-19 through surgical explant teams should be considered | C | 2/19 |
| 14 | Marginal donors should be restrictively accepted when facility’s capacity is limited | C | 1/19 |
A, high recommendation; B, medium recommendation; C, low recommendation.
Number of societies that support the statement.
TABLE 4.
Recommendations for donor management
| Statement | Donor management | Rating | Consensusa |
|---|---|---|---|
| 15 | Donors should be screened epidemiologically and by clinical history for risk of COVID-19 infection | A | 15/19 |
| 16 | Donor testing should be executed by PCR-based nasopharyngeal swab or bronchoalveolar lavage (BAL) testing | A | 18/19 |
| 17 | If no SARS-CoV-2 testing of the donor is available preprocurement, the risk can be stratified by epidemiological and clinical screening | C | 4/19 |
| 18 | Organs of deceased donors with active SARS-CoV-2 infection should not be considered for transplant, nor those of high-risk donors without available PCR testing | A | 16/19 |
| 19 | Organs of low-risk donors may be procured before test results are available or in the absence of available testing | C | 1/19 |
| 20 | Organs from deceased donors who have recovered from COVID and have resolution of symptoms and negative testing are considered safe for transplants | C | 3/19 |
| 21 | Donors with a history of unexplained respiratory failure should be excluded | C | 3/19 |
A, high recommendation; B, medium recommendation; C, low recommendation; COVID, coronavirus disease 2019; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Number of societies that support the statement.
TABLE 5.
Recommendations for recipient management
| Statement | Recipient management | Rating | Consensusa |
|---|---|---|---|
| 22 | Organ recipients should be screened for SARS-CoV-2 infection | B | 7/19 |
| 23 | Recipients with active COVID-19 should be deferred from transplantation | A | 11/19 |
| 24 | Patients with a history of SARS-CoV-2 infection need a negative COVID-PCR test result and complete symptom resolution before a transplant procedure | C | 1/19 |
| 25 | A thoracic CT scan should be performed in the immediate pretransplant evaluation instead of a chest X-ray | C | 2/19 |
A, high recommendation; B, medium recommendation; C, low recommendation; COVID, coronavirus disease 2019; CT, computed tomography; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Number of societies that support the statement.
The following section offers listing, SRC consensus and further discussion of the retrieved recommendations.
3.1. The immunosuppressed transplant recipient
3.1.1. Preventative policies and general recommendations concerning hygiene and lifestyle restrictions should be strictly followed by transplant patients and household cohabiters. SRC (A)
Anticipating a higher-than-average susceptibility of transplant patients for COVID-19, a strong consensus was found for the validity of general preventive and hygienic measures for transplant patients. Numerous international sources offer directions, information, and tutorials on preventive policies and lifestyle recommendations such as social distancing, telework, hand hygiene, travel restrictions, and more (Table 2).7 , 8
3.1.2. Transplant recipients are at greater risk for severe COVID-19 than the average population. SRC (A)
Deduced from data on other viruses and SARS, anticipation of courses with high viral load and severe clinical manifestations in immunosuppressed patients are justified. The underlying cause might be via dependence of immunosuppressive drugs itself but as well might be due to comorbidities that accompany solid organ recipients.9
Nevertheless, D’Antiga (Italy) published on March 20, 2020 the first descriptive analysis of clinical observations in SARS-CoV-19 positive transplant patients and suggested that unlike common viral agents (eg, adenovirus, influenza, respiratory syncytial virus), infection with SARS-CoV-19 might not lead to a worse general condition in immunosuppressed patients.10
The TTS discusses paying close attention to medication-induced lymphopenic transplant patients because low lymphocyte count in COVID-19 patients is associated with a severe course of disease.11 The Canadian Society of Transplantation (CST) recommends critical reconsideration of lymphocyte depleting therapies.12
3.1.3. Nonessential transplant clinic visits should be postponed. SRC (B)
Interpersonal and facility contacts should be reduced to a minimum. Possible strategies include telemedical approaches, postponing therapeutic interventions and person-to-person diagnostics as far as medically acceptable, and creation of shortcuts for education. Enhanced communication such as letters delivering general information and required documents (eg, telework requests) should be implemented. Templates are available on various platforms (eg, NATCO “General Letter to Transplant families”13 or WHO7).
3.1.4. Transplant recipients are likely to show weakened or atypical clinical presentation of COVID-19. SRC (C)
Given the high awareness levels of COVID-19 symptoms such as fever, dry coughing, and sneezing, the alertness regarding altered clinical presentations such as gastrointestinal symptoms or fatigue is considered to be low. In a recent case report, an atypical presentation of COVID-19 infection in a kidney transplant recipient has been described.4 Early clinical presentation with gastrointestinal symptoms are attributed to 16% of the SARS-CoV-2 population.14 Consequently, the transplant societies of South Korea and the United Kingdom recommend a low threshold for SARS-CoV-2-testing in transplant patients after contact with a positively tested person or subject to a broader spectrum of COVID-19-associated symptoms.15 This situation may reflect a country’s quantitative resources for SARS-CoV-2 testing. Therefore, the risk of infection due to facility contact while testing has to be carefully weighed against beneficial therapeutic options after early diagnosis.
3.1.5. A decrease in immunosuppression should be considered for SARS-CoV-2-infected transplant recipients. SRC (B)
According to the WHO, there is no specific treatment or vaccination available to treat COVID-19 infection.7 Therefore, the therapy on all patients, including transplant recipients, should be based on the principles of acute respiratory distress syndrome (ARDS) treatment, including fluid restrictions, lung protective ventilation, prophylactic antibiotics, prone position, etc.7 , 16 At the moment, there is insufficient evidence to support any antiviral treatments.16
Nevertheless, in the absence of a recent history of transplant rejection and until further proof, there is a 7/19 society consensus that reduction of immunosuppression may be considered for transplant patients with symptomatic COVID-19. To date, empirical evidence is limited to a single case report of successful recovery from COVID-19 pneumonia of a renal transplant recipient where therapeutic strategies included temporary cessation of immunosuppression.17
A systematic review of observational studies of corticosteroids administered to patients with SARS reported no survival benefit but possible harms (avascular necrosis, psychosis, diabetes, and delayed viral clearance).18 A recently published review recommends a staged approach for immunosuppressed COVID-19 patients with reduction of immunosuppression in the early phase and—among other drugs—corticosteroids in the late phase (inflammatory response in the host).3 The Spanish society advises in case of a confirmed COVID-19 infection in a transplant recipient lopinavir/ritonavir, hydroxychloroquine, interferon-ß, oseltamivir, and antibiotic treatment for superinfections as therapeutic options depending on clinical symptoms.19
3.1.6. Wearing face masks for prophylactic reasons is currently not recommended for transplant recipients. SRC (C)
Society consensus does not recommend general mask use by transplant recipients for infection protection. Limitations in the availability of masks make it necessary to avoid usage with unclear protective value. According to the recommendation of the Centers for Disease Control and Prevention (CDC) and the AST, wearing a face mask is recommended in (unavoidable) public contact for (transplant) patients with clinical symptoms of COVID-19.20 Usage in high contamination risk surroundings (eg, a hospital) has to be weighed against resource limits and substitution measures, such as hand hygiene. N95 masks should be reserved for health care workers according to the AST.20 Only the Chinese Society of Transplantation recommends general prophylactic use of face masks.
3.1.7. Transplant patients should be provided with additional medication in case of unexpected delay or quarantine. SRC (C)
Transplant patients should consider receiving a 90-day medication supply to minimize facility contact and anticipate a quarantine situation. Low-contact supply chains should be enhanced (eg, home mailed, drive-through pharmacies).
3.2. General management of transplantation programs
3.2.1. Temporary suspension of elective living donor transplantation should be considered during viral pandemic. SRC (A)
A strong consensus was found regarding reduction of elective transplantations to a minimum, if not complete postponement; in particular living donor kidney programs.5 , 21 , 22 Living donor liver transplantation is being recommended to be carried out preferably in unstable patients with a model end-stage liver disease score > 25 or >30 depending on the ICU capacities, respectively (Table 3).22
3.2.2. Temporary suspension of nonurgent deceased donor transplantation may be considered during viral pandemic. SRC (A)
Restriction of transplant programs is based on the high requirement of both staff and equipment required for a transplantation procedure and follow-up compared to the withholding of health benefits to potential organ recipients. Only a few countries (eg, Iran, Bulgaria) adopted a “top-down” approach of instructed restriction.23 , 24 Most countries allow individual transplant centers to define the threshold for need of pronounced resource restrictions. If transplants are to continue during the pandemic, the AST has introduced a concept of a “COVID-19-free pathway” for recipients.20 This concept includes clear distinction of COVID-19-free zones within a hospital, such as operating room and ICU capacities, and can serve as the hallmark of a center’s capacity to maintain a wider transplant program.
3.2.3. Availability of intensive care beds, ventilators, and trained staff has to be accounted for prior to organ acceptance. SRC (A)
Every consulted society recommendation addresses the crucial need for evaluation of staff and ICU capacity. Kumar et al22 published a staged approach to transplant program reduction according to available capacity.
3.2.4. The lungs and intestines are at special risk for transmission of SARS-CoV-2 and should not be transplanted in case of suspicion. SRC (C)
The AST and Swisstransplant explicitly restrict the use of these organs for transplants due to their key role in the virus’ biology and a potentially high parenchymal viral load in case of undetected infection. In Canada, the lung transplant program was paused due to the pandemic.
3.2.5. Education on the residual risk of SARS-CoV-2 transmission and retrieval of consent of the transplant recipient is recommended. SRC (B)
The first available data show a 2%-22% rate of false negative–polymerase chain reaction (PCR) results for SARS-CoV-2 tests.25 A strong consensus supports educating transplant recipients on a potentially high risk in the case of an undetected SARS-CoV-2 infection. A corresponding statement should be added to the written consent.
3.2.6. The risk of interfacility transmission of SARS-CoV-19 through surgical explant teams should be considered. SRC (C)
Given the work with associated high-contact profile, the mobility of medical staff bears a risk of infection transmission. The Korean Transplant Society and the British National Health Service recommend screening explant teams for SARS-CoV-2 infection.26 The Korean recommendations include clinical and temperature control before entering the organ donor hospital.15 Within the Eurotransplant zone, the Hungarian government is prohibiting foreign explant teams from entering the country as of April 2020.27 The extent of screening (repetitive PCR testing vs screening for symptomatic physicians) should be adapted to local virus spread, availability of testing, and general risk of transmission.
3.2.7. Marginal donors should be restrictively accepted when facility’s capacity is limited. SRC (C)
A unique but equally interesting approach was stated by the British National Health Service. The NHS recommends a qualitative restriction of donor organ retrieval, reducing transplant cases by declining donations from all donors after brain death older than 60 years (reduction by 33%), and all donors after cardiac death older than 50 years (reduction by 33%).26 This concept may be adapted according to available capacity of the individual center.
3.3. Donor management
3.3.1. Donors should be screened epidemiologically and by clinical history for risk of COVID-19 infection. SRC (A)
The AST guideline defines epidemiological screening as the recording of travel history to high-risk areas or contact with suspected or confirmed cases of COVID-19 within 21 days or confirmed diagnosis of the subject within the last 28 days. Clinical screening should contain symptoms like fever (>38°C or 100.3°F), flu-like symptoms, new-onset cough, shortness of breath, and gastrointestinal symptoms. If both screening queries are positive, the donor is considered high risk; if just one query is positive, the donor is considered intermediate risk; if none applies then the donor is assumed low risk. In addition, Kumar et al propose transmission of COVID-19 on the donor ICU as a knockout criterion.22 In countries with high burden of disease, the epidemiological screening can be a priori assumed positive (Table 4).
3.3.2. Donor testing should be executed by PCR-based nasopharyngeal swab or bronchoalveolar lavage (BAL) testing. SRC (A)
The majority of all societies advise testing for SARS-CoV-2. Nucleoid acid testing (NAT) via PCR is currently the state of the art and specimens can be nasopharyngeal or bronchoalveolar.22 Specimen retrieval via BAL on the other side may interfere with availability of bronchoscopes during the COVID-19 pneumonia pandemic should be considered as well as the risk of infection for the examiner.
Although SARS-CoV-2 nasopharyngeal PCR shows reasonable sensitivity, a recently published study demonstrates that of 51 COVID-19 patients only 36 were initially positive in NAT.28 Hence, repetitive testing should be considered in case of doubt.
3.3.3. If no SARS-CoV-2 testing of the donor is available preprocurement, the risk can be stratified by epidemiological and clinical screening. SRC (C)
As resources differ worldwide, especially in the current massively challenged health care systems, SARS-CoV-2 NAT might not be available due either to lack of time or resources. In this case, a low consensus was found for proceeding with the explantation after screening through clinical presentation and epidemiology given a highly urgent transplant situation.
3.3.4. Organs of deceased donors with active SARS-CoV-2 infection should not be considered for transplant, nor high-risk donors without available PCR testing. SRC (A)
By publication, no case of the transplant of an organ from a SARS-CoV-2 positive donor has been reported. In a recently published study, RNAemia was shown in 10%-15% of all COVID-19 positive patients. Even though the presence of viral RNA sequences in a blood sample cannot be equated with the presence infectious virions and imminent risk of infection, a high risk of SARS-CoV-2 transmission via solid organ transplantation can be anticipated.29 , 30 A strong consensus (16/19) was found for anticipating a risk of severe infectious complications posttransplant of SARS-CoV-2-positive donor organs also depending on whether a depleting or a nondepleting induction agent is being applied. SARS-CoV-2-positive donor organs should therefore be deferred from transplantation.
3.3.5. Organs of low-risk donors may be procured before test results are available or in the absence of available testing. SRC (C)
Given the lack of evidence-based algorithms, the responsibility for accepting a donor organ with the possible residual risk of SARS-CoV-19 infection lies with the transplant surgeon. There were mixed recommendations for procuring organs of low-risk donors to transplantation without available NAT results. This result may be due to the broad range of disease burden within individual countries. More recently published recommendations have a tendency of demanding mandatory testing; earlier published recommendations (eg, AST, published March 20) considered other screening techniques as sufficient in the absence of available testing. This might reflect the rapid increase of testing availability over just a few days shifting the risk-benefit calculation.
3.3.6. Organs from deceased donors who have recovered from COVID and have resolution of symptoms and negative testing are considered safe for transplants. SRC (C)
Given the vast spread of COVID-19 with more than 100 000 recovered cases at the time of publication, the question will arise as to when these patients can be accepted as organ donors. The CST recommends two negative swabs 24 hours aside, whereas the AST guideline requires an additional 28 days after symptom resolution.12 , 20 Repeated testing is recommended due to false negative results.
3.3.7. Donors with a history of unexplained respiratory failure should be excluded. SRC (C)
Whereas consensus is clear about excluding COVID-19-positive donors, the TTS additionally warns about retrieving organs from donors with unclear lung disease. This is a relevant issue, because pulmonary parenchyma is often strongly affected by the severity of the premortem course of disease. Parenchymal defects may be present without further clarification of diagnosis.
3.4. Recipient management
3.4.1. Organ recipients should be screened for SARS-CoV-2 infection. SRC (B)
Until now, no solid organ transplant procedure has reportedly been performed on a SARS-CoV-2-infected patient. Therefore, the expected negative impact on outcome remains speculative. It is widely recommended that prior to transplant, a possible infection should be ruled out by NAT.31 The pretransplant screening routine should include SARS-CoV-2 PCR testing. If possible, organ procurement or at least the transplant should be delayed until PCR results are available. The German society of transplantation recommends systematic PCR-based testing for epidemiological and scientific gain but states that for asymptomatic recipients the transplant should not be delayed until test results are available (Table 5).
3.4.2. Recipients with active COVID-19 should be deferred from transplantation. SRC (A)
There is unanimous assent in transplants societies, that patients testing positive should not undergo a transplant procedure. Not a single society recommended differently, although the evidence level is zero to date.
3.4.3. Patients with a history of SARS-CoV-2 infection need a negative COVID-PCR test result and complete symptom resolution before a transplant procedure. SRC (C)
The AST recommends 28 days of symptom resolution prior to procurement of a former COVID-19-positive donor; however, they do not comment on the duration in the recipient. Together with the CST, they recommend two negative NATs before the transplant.12 According to CST, tests should be performed with a 24 hours gap. In contrast, the British recommendation states that patients “recovering from an acute COVID-19 infection should not undergo transplantation.”26 Zhou et al could demonstrate that the duration of viral shedding in survivors was 20 days (ranging from 8 to 37 days), which may indicate benefit of an even longer symptom-free period before transplantation.2 The final responsibility lies once again with the physicians in charge of the procedure. The infectious risk of pursuing transplantation must be weighed against the risk of depriving a patient of organ replacement therapy.
3.4.4. A thoracic CT scan should be performed in the immediate pretransplant evaluation instead of a chest X-ray. SRC (C)
The Chinese and the Belgian Transplantation Societies strictly recommend a CT scan of the chest during evaluation immediate before the transplant. In a retrospective cohort study, 51 SARS-CoV-2-positive patients underwent both a CT scan and a reverse transcription (RT)-PCR testing for COVID-19 at time of admittance to the hospital. Of these, 15/51 had a negative PCR test, but just 1/51 showed no pathological findings in the CT. In this cohort, a CT scan without contrast medium showed a higher sensitivity than the RT-PCR testing (98% vs 71%).28
4. DISCUSSION
These days, medical professionals are facing unprecedented challenges in handling a pandemic-induced health care crisis. Scientific evidence is scarce and thus strategies can be based only on expert opinion rather than evidence. Especially in the field of transplantation, many questions remain with regard to COVID-19 that need to be addressed as quickly as possible. As long as scientific evidence is not available, expert consensus can serve as a basis for decision-making. Our study offers a structured approach to consensus-based immediate decision-making bridging the time span until evidence becomes available.
Generating a “conventional” consensus usually requires months or years. As special situations require special solutions, an “unconventional” way of consensus-building was attempted: Websites of renowned transplant organizations and societies were screened for recommendations and consents on organ transplantation and COVID-19 (Table 1).
Concrete action plans should be evaluated by each transplant center in view of the individual situation at a site (evaluation of operating room, staff, and ICU capacity and viral burden on campus). Therefore, a temporarily accorded phased reduction of transplant programs is highly recommended.22 The first tapering steps with strong SRC include:
-
•
Reduction of nonurgent deceased donor programs—especially kidney transplants—due to the “elective” character and existing organ replacement options (SRC A)
-
•
Reduction of elective living donor programs (SRC A)
All retrieved recommendations consider SARS-CoV-2 screening as essential. PCR-based methods are preferred. A positive screening results of SARS-CoV-2 testing is seen as a strict contraindication for both organ donation and transplantation to a recipient.
Susceptibility of immunosuppressed patients to SARS-CoV-2 infections and a higher risk to severe courses are anticipated (SRC A). Therefore, the importance of general preventive and hygienic measures is important (SRC A). The extension of telemedical approaches are broadly recommended (SRC B). There is a B-level consensus for considering reduction of immunosuppression as a therapeutic approach in transplanted COVID-19 patients. Without signs of infection, standard immunosuppression should not be adapted. No consensus recommendation can be made in the use of discussed antiviral medication. There is anecdotal evidence of six cases (Iacovoni, ASST Papa Giovanni XXIII, Bergamo, Italy; AST Transplant Town Hall 23.03.2020), in which immunosuppression reduction was conducted for stable outpatient cases and the application of center-specific (experimental) medication protocol was applied for patients with severe symptoms.
Standardized expert consensus is not available for managing transplant programs during the SARS-CoV-2 pandemic due to the lack of scientific evidence and time constraints. This work offers a substitute approach by summarizing and aligning existing society recommendations.
An inevitable limitation of this work is the interpretation and translational bias because some recommendations were available only in national languages. This issue was addressed as well as possible through consultation of medically trained staff speaking that native language. Second, due to the lack of scientific literature, the aforementioned consensus statements are themselves based on expert opinion. In addition, the retrieved information was not intended to serve as a basis for consensus recommendation. Hence, reinterpretation by the authors was sometimes necessary to confirm or dismiss the aforementioned statements. Consequently, all recommendations, even if supported by a high SRC, have to be classified as lowest (scientific) evidence level and should be interpreted as such.
In striving toward a rapid transition back to the domain of evidence-based medicine, accurate documentation and publication of prospectively collected clinical data on COVID-19 in transplanted patients are needed. For European centers, the Lean European Open Survey on SARS-CoV-2 Infected Patients (LEOSS) was created and a transplant-specific subunit was incorporated. We strongly encourage participation and promotion of a unified and structured collection of data.
Acknowledgments
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
AUTHOR CONTRIBUTIONS
PVR: research design, paper writing, performance of the research, data analysis. NN: research design, paper writing, performance of the research, data analysis. LW: paper writing, performance of the research. HW: paper writing, performance of the research. PM: paper writing, performance of the research. AB: paper writing, performance of the research. KH: paper writing, performance of the research. FT: paper writing, data analysis. FF: paper writing, data analysis. TS: paper writing, data analysis. WS: paper writing, data analysis. RO: paper writing, data analysis. MS: research design, paper writing, data analysis. JP: research design, paper writing, data analysis.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Footnotes
Moritz Schmelzle and Johann Pratschke contributed equally as senior authors.
Paul Viktor Ritschl and Nora Nevermann contributed equally as first authors.
REFERENCES
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the chinese center for disease control and prevention. JAMA. 2020;323(13):1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal [published online ahead of print 2020]. J Heart Lung Transplant. 10.1016/j.healun.2020.03.012 [DOI] [PMC free article] [PubMed]
- 4.Guillen E, Pineiro GJ, Revuelta I, et al. Case report of COVID-19 in a kidney transplant recipient: does immunosuppression alter the clinical presentation [published online ahead of print 2020]? Am J Transplant. 10.1111/ajt.15874 [DOI] [PMC free article] [PubMed]
- 5.Services CfMM. Adult elective surgery and procedures recommendations. https://www.cms.gov/files/document/31820-cms-adult-elective-surgery-and-procedures-recommendations.pdf. Accessed March 28, 2020.
- 6.Kumar D, Tellier R, Draker R, Levy G, Humar A. Severe Acute Respiratory Syndrome (SARS) in a liver transplant recipient and guidelines for donor SARS screening. Am J Transplant. 2003;3(8):977–981. doi: 10.1034/j.1600-6143.2003.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Organization WH. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Accessed March 28, 2020.
- 8.Prevention CoDCa. https://www.cdc.gov/coronavirus/2019-ncov/prepare/prevention.html. Accessed March 28, 2020.
- 9.Park JY, Kim MH, Bae EJ, et al. Comorbidities can predict mortality of kidney transplant recipients: comparison with the Charlson Comorbidity Index. Transplant Proc. 2018;50(4):1068–1073. doi: 10.1016/j.transproceed.2018.01.044. [DOI] [PubMed] [Google Scholar]
- 10.D’Antiga L. Coronaviruses and immunosuppressed patients. The facts during the third epidemic [published online ahead of print 2020]. Liver Transpl. 10.1002/lt.25756 [DOI] [PubMed]
- 11.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Transplantation CSo. https://profedu.blood.ca/sites/msi/files/20200327_covid-19_consensus_guidance_final.pdf. Accessed March 27,2020.
- 13.Professionals N-TOfT. http://www.natco1.org/Industry-News/updates.asp. Accessed March 28, 2020.
- 14.Luo S, Zhang X, Xu H. Don’t overlook digestive symptoms in patients with 2019 novel coronavirus disease (COVID-19) [published online ahead of print 2020]. Clin Gastroenterol Hepatol. 10.1016/j.cgh.2020.03.043 [DOI] [PMC free article] [PubMed]
- 15.Transplantation KSo. http://www.mykst.org/. Accessed March 28, 2020.
- 16.Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) [published online ahead of print 2020]. Crit Care Med. 10.1007/s00134-020-06022-5 [DOI] [PMC free article] [PubMed]
- 17.Zhu L, Xu X, Ma KE, et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.15869 [DOI] [PMC free article] [PubMed]
- 18.Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Medicine. 2006;3(9):e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.(SEIMC) SSoT. https://seimc.org/contenidos/gruposdeestudio/gesitra/documentos/GESITRA-IC_Recomendaciones_COVID-19_en_TOS.pdf. Accessed March 28, 2020.
- 20.Transplantation ASo. https://www.myast.org/sites/default/files/COVID19%20FAQ%20Tx%20Centers%2003.20.2020-FINAL.pdf. Accessed March 28, 2020.
- 21.Ranney ML, Griffeth V, Jha AK. Critical supply shortages - the need for ventilators and personal protective equipment during the Covid-19 pandemic [published online ahead of print 2020]. N Engl J Med. 10.1056/NEJMp2006141 [DOI] [PubMed]
- 22.Kumar D, Manuel O, Natori Y, et al. COVID-19: a global transplant perspective on successfully navigating a pandemic [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.15876 [DOI] [PMC free article] [PubMed]
- 23.Transplantation ISo. https://ehda.center/fa/page/NPEBP/. Accessed March 28, 2020.
- 24.Sofia GEi. https://sofia.diplo.de/bg-de/aktuelles/-/2318146?fbclid=IwAR1gUwDxaBty8NXvYaRZmdaI0SnJ_9hAv4Ove1-wyDVISuNgg29iyfJjTQ0. Accessed March 28, 2020.
- 25.Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens [published online ahead of print 2020]. JAMA. 10.1001/jama.2020.3786 [DOI] [PMC free article] [PubMed]
- 26.Service BNH. https://www.odt.nhs.uk/deceased-donation/covid-19-advice-for-clinicians/. Accessed March 28, 2020.
- 27.Transplantation GSo. 2020. http://www.d-t-g-online.de/index.php/covid-19. Accessed June 4, 2020.
- 28.Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020;200432. 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed]
- 29.Chen W, Lan Y, Yuan X, et al. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg Microbes Infect. 2020;9(1):469–473. doi: 10.1080/22221751.2020.1732837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malinis M, Boucher HW, AST Infectious Diseases Community of Practice Screening of donor and candidate prior to solid organ transplantation-Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9):e13548. doi: 10.1111/ctr.13548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.