Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) originated in China in late 2019 and has since spread rapidly to every continent in the world. This pandemic continues to cause widespread personal suffering, along with severe pressure on medical and health care providers. The symptoms of SARS‐CoV‐2 and the subsequent prognosis are worsened in individuals who have preexisting comorbidities prior to infection by the virus. Individuals with obesity or overweight, insulin resistance, and diabetes typically have chronic low‐grade inflammation characterized by increased levels of several proinflammatory cytokines and the inflammasome; this state predisposes to greater risk for infection along with more adverse outcomes. Here, we consider whether a high level of cardiorespiratory fitness induced by prior exercise training may confer some innate immune protection against COVID‐19 by attenuating the “cytokine storm syndrome” often experienced by “at risk” individuals.
Background
In December 2019, individuals in Wuhan, the capital city of Hubei province in the People’s Republic of China, presented with pneumonia‐like symptoms of unknown etiology. Several weeks later, the first death attributable to this illness was reported, with the cause confirmed as a novel strain of virus, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). The virus rapidly spread to every continent, causing widespread suffering, panic, social unrest, and economic instability. On April 20, 2020, the Center for Systems Science and Engineering at Johns Hopkins University declared that the deadly pandemic (renamed COVID‐19 by the World Health Organization) had infected 2,475,841 people worldwide, resulting in 170,261 deaths. The rate of diagnosis and mortality continues to rise, with no viable vaccine currently available. Several factors appear to be associated with an increased risk of hospitalization and mortality in patients with COVID‐19, including advanced age (> 60 years), obesity, diabetes, hypertension, cardiovascular disease, a history of smoking, and chronic obstructive pulmonary disease (1, 2). Common to these conditions is a state of chronic low‐grade inflammation. It has recently been suggested that the world is facing two pandemics simultaneously: (1) the COVID‐19 pandemic and (2) a physical inactivity pandemic (3). Both pandemics cause adverse health effects at the population level while inflicting widespread burdens on health care systems.
Exercise elicits potent and wide‐ranging effects on the immune system, principle among these being its anti‐inflammatory effects. Indeed, exercise training offers some protection against the development of several chronic metabolic diseases, including the insulin‐resistant state that typically accompanies obesity and diabetes. As such, individuals with comorbidities who have high levels of cardiorespiratory fitness may mount a stronger host immune defense against SARS‐CoV‐2 and reduce susceptibility to risk of infection in the early stage of the disease. Here, we consider whether high levels of cardiorespiratory fitness induced by prior exercise training may confer some innate immune protection against COVID‐19 by attenuating the “cytokine storm syndrome” often experienced by “at‐risk” individuals.
The Progression of COVID‐19
The progression of COVID‐19 is largely dependent on the initial health status of an individual and the immune response triggered by the infection (4). Though susceptibility to infection is multifactorial, epigenetic and environmental/behavioral factors all impact or contribute to immunity in the early stages of the infection. In this incubation phase, the patient does not present with severe symptoms. In order to eliminate the virus, a specific adaptive immune response is required to halt disease progression, and interventional strategies to boost immune responses (or attenuate proinflammatory responses) should be of prime consideration. At this time, the virus can be asymptomatic, causing no noticeable illness in some individuals, although they remain contagious and can spread infection.
If the protective immune response is impaired or inadequate, the virus will proliferate and destroy affected cells, especially in tissues and organs that have a high expression of angiotensin converting enzyme 2 (ACE2). As a transmembrane protein, ACE2 serves as the main entry point into cells for many coronaviruses, including SARS‐CoV‐2 (5). As ACE2 is attached to the outer surface of cells, especially in the lungs, decreasing the levels of ACE2 in cells might help in fighting infection (6). During this second phase, the damaged cells induce innate inflammation in the lungs, largely mediated by proinflammatory macrophages and granulocytes, which lead to the classic symptoms of fever, coughing, fibrosis, and dramatic rise in proinflammatory cytokine levels. Lung inflammation is the main cause of life‐threatening respiratory disorders at this time.
Proinflammatory cytokines are also elevated in many chronic metabolic diseases, such as insulin resistance, obesity, and type 2 diabetes (7), but with a different order of magnitude. Indeed, chronic inflammation is an underlying pathological condition in which inflammatory cells such as neutrophils and monocyte/macrophages infiltrate into fat and other tissues and accumulate in individuals with chronic metabolic conditions. In this regard, recent evidence has suggested that individuals with obesity and diabetes are at increased risk for complications from SARS‐CoV‐2, including death (8). Indeed, patients with obesity who are infected with SARS‐CoV‐2 have a higher prevalence of invasive mechanical ventilation use, with disease severity strongly associated with a higher BMI (9). The high incidence of diabetes throughout the world and particularly in the elderly make this a major concern as the COVID‐19 pandemic spreads.
The Calm Before the Storm: Cytokine Storm Syndrome, Lung Damage, and Fatal Respiratory Complications
A subgroup of patients with severe COVID‐19 experience “cytokine storm syndrome” (10), referring to the overproduction of immune cells and cytokines that are associated with a surge of activated immune cells into the lungs, usually 7 to 10 days following the onset of symptoms (11). In health, cytokines and chemokines play important roles in immunity and immunopathology, but dysregulated and amplified immune responses in patients infected with SARS‐CoV‐2 are causative of severe lung damage, respiratory complications, and reduced rates of survival. In these patients, interleukin (IL)‐6, IL‐10, and tumor necrosis factor‐alpha (TNFα) surge coincidental with peak adverse clinical symptoms, rapidly declining during recovery. Patients requiring intensive care unit admission have significantly higher levels of IL‐6, IL‐10, and TNFα and fewer T cells (12). This cytokine storm likely dampens innate adaptive immunity against SARS‐CoV‐2 infection.
The precise mechanisms by which the levels of proinflammatory cytokines are increased in patients with COVID‐19 is not known. However, it is believed to be initiated by the binding of SARS‐CoV‐2 to the ACE2 receptor (13) (Figure 1), leading to an increase in the number of inflammasomes and levels of proinflammatory factors. Paradoxically, ACE2 is involved in the conversion of angiotensin II (Ang II) to angiotensin 1‐7 (Ang 1‐7), which plays a protective role against hypertension, cardiovascular diseases, and diabetes (14). Apart from the lungs, ACE2 is also expressed in intestinal epithelial cells, which explains why a certain number of patients infected with SARS‐CoV‐2 develop gastrointestinal disorders. This most likely leads to a permeabilization of the intestine wall, which favors the development of endotoxemia by increasing, among others, the levels of circulating lipopolysaccharide (LPS).
Figure 1.
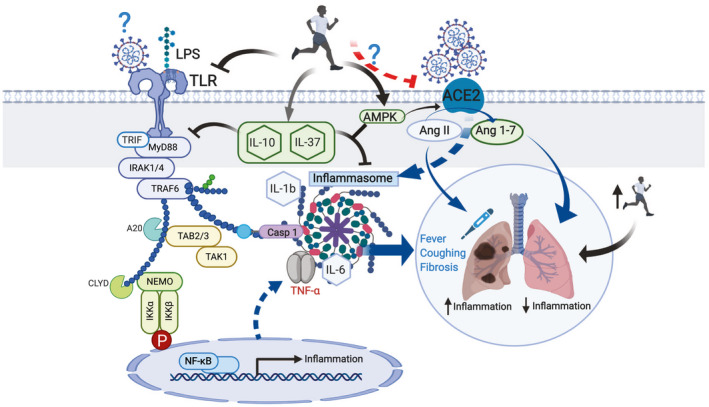
Proposed model for the protective effects of high levels of cardiorespiratory fitness on proinflammatory responses after infection by SARS‐CoV‐2. Binding of the virus SARS‐CoV‐2 to the angiotensin converting enzyme 2 (ACE2) receptor activates the inflammasome, causing the symptomatology in the lungs characteristic of the COVID‐19. Lipopolysaccharide (LPS), and possibly SARS‐CoV‐2, bind to the Toll‐like receptors (TLR) to activate the inflammation pathway. Myeloid differentiation primary response 88 (MyD88) or TIR domain‐containing adapter‐inducing interferon‐β (TRIF) activates tumor necrosis factor receptor associated factor 6 (TRAF6), which in turn stimulates caspase 1, inducing the activation of the inflammasomes as well as tumor growth factor‐β–activated kinase (TAK) and I kappa B kinase (IKK). This cascade ultimately initiates the activation of nuclear factor‐kappa B (NF‐κB) in the nucleus. Subsequently, proinflammatory cytokines, such as tumor necrosis factor‐alpha (TNFα), IL‐6, and IL‐1β, initiate the inflammatory processes in the lung, giving rise to the classic COVID‐19 symptoms. A high level of cardiorespiratory fitness at the onset of symptoms may reduce the susceptibility to infection and be beneficial for host immune defense against a proinflammatory state. Exercise training may act to reduce inflammatory pathways via the following three putative mechanisms: (1) by reducing the expression of the TLRs; (2) by increasing the levels of anti‐inflammatory cytokines such as IL‐10 and IL‐37, which in turn will inhibit the TLR‐inflammation pathway and counteract the inflammatory response induced by the inflammasomes; and (3) by activating the AMPK‐activated protein kinase (AMPK) in the lung, reducing the inflammatory processes by allowing for the transformation of Ang II to Ang 1‐7. [Color figure can be viewed at wileyonlinelibrary.com]
Like many other viruses, SARS‐CoV‐2 is likely to be recognized by Toll‐like receptors 2, 3, and 4 (TLR 2, 3, and 4), a family of transmembrane proteins with direct roles in the innate immune system, found in many tissues, including skeletal muscle, liver, and the lungs. First, proteins such as myeloid differentiation primary response 88, or TIR domain‐containing adapter‐inducing interferon‐β are recruited to an intracellular TLR domain to initiate two distinct inflammatory pathways (15). One pathway increases IL1‐β and inflammasome levels via caspase 1 activation, whereas the other initiates a phosphorylation cascade that activates key proteins, including tumor growth factor‐β activated kinase and I kappa B kinase. These in turn increase proinflammatory cytokine levels such as IL‐6 and TNFα via the nuclear factor‐kappa B, the so‐called “master regulator” of inflammation. TLR4 has been implicated in LPS‐induced acute lung disease (16) and is likely to mediate some of the harmful effects of COVID‐19 in this organ.
Does High Cardiorespiratory Fitness Boost Immunity and Attenuate the Proinflammatory State Induced by COVID‐19?
Exercise training elicits potent and wide‐ranging effects on the immune system; high cardiorespiratory fitness and moderate‐intensity aerobic exercise training improve immune responses to vaccination, lower chronic low‐grade inflammation, and improve various immune markers in several disease states including cancer, human immunodeficiency virus, cardiovascular disease, diabetes, cognitive impairment, and obesity (17, 18). Furthermore, exercise training may reduce the risk, duration, and severity of viral infections (19). The cytokine profile induced by exercise is classically anti‐inflammatory, comprising marked increases in the levels of several potent anti‐inflammatory cytokines such as IL‐10, IL‐1 receptor antagonist, and IL‐6 (20). The “myokine” IL‐6 appears to be the major contributor to the anti‐inflammatory effects of exercise (21), with contracting human skeletal muscles producing and releasing significant amounts of IL‐6 into circulation to mobilize energy substrates in a similar manner to stress hormones (21).
Another mechanism whereby exercise induces an anti‐inflammatory response is a downregulation of the expression/activation of proinflammatory TLRs (22). Both an acute bout of aerobic exercise (23) as well as resistance‐based training (24) decrease the expression of TLR4 on the surface of monocytes. As circulating monocytes are the precursors to tissue macrophages, the exercise‐induced decreases in monocyte TLR4 expression may be an important mechanism by which the anti‐inflammatory effects of exercise are mediated (25, 26), specifically in patients with chronic low‐grade inflammation, such as patients with obesity and/or diabetes (27). In addition to attenuating the production of proinflammatory cytokines induced by TLR signaling, exercise stimulates the release of anti‐inflammatory cytokines. For instance, the IL‐1 family cytokine IL‐37 is a natural suppressor of innate inflammation and acquired immunity (28).
Regular bouts of moderate‐ to vigorous‐intensity exercise have direct and positive effects on lung function (18) and help lower the risk of respiratory infections/illness (18). In an experimental asthma model in mice, aerobic exercise increased the production of the anti‐inflammatory IL‐10 in response to lung inflammation (29). A 3‐week swim training protocol in mice had protective effects against LPS‐induced systemic inflammation (as measured by reduced circulating concentrations of TNFα, IL‐6, and IL‐1β) and lung inflammation (30). Similar results were reported after a 4‐week treadmill running program (31). Exposure of rat epithelial alveolar cells to cyclic mechanical stretching increased acetyl‐CoA carboxylase phosphorylation (a surrogate marker for AMP‐activated protein kinase [AMPK] activity) by 50%, suggesting that aerobic exercise has beneficial effects on airway epithelial cells (32). AMPK inhibits nuclear factor‐kappa B, thereby reducing proinflammatory cytokines in airway epithelial cells (33). While AMPK activation increases ACE2 phosphorylation and elevates Ang 1‐7 in pulmonary endothelium cells (34), regular moderate‐intensity exercise favors the conversion of Ang II to Ang 1‐7 via ACE2. As such, mice treated with an ACE2 activator and submitted to a 4‐week endurance training were less susceptible to pulmonary fibrosis induced by bleomycin (35). Thus, exercise may counteract, at least partially, the detrimental effects of the binding of SARS‐CoV‐2 to ACE2 receptor, reducing the inflammatory response in the lungs.
Summary
Preliminary evidence has suggested that the severity of symptoms associated with COVID‐19 and the eventual outcome of being infected with this virus are associated with the health status of individuals prior to infection. Among the factors linked to an increased risk of hospitalization and mortality are overweight/obesity, insulin resistance, and diabetes; these lifestyle‐related diseases, underpinned by patterns of sedentary behavior, poor dietary habits, and a lack of physical exercise, are characterized by chronic low‐grade inflammation. Whether a high level of cardiorespiratory fitness can reduce the early amplified proinflammatory responses in patients infected with SARS‐CoV‐2 and confer some protective effect against the development and severity of the disease remains to be established from retrospective epidemiological data. Given the positive effects of moderate doses of exercise on select immune markers associated with many disease states, we suggest that prior exercise training and high levels of cardiorespiratory fitness are likely to be immunoprotective in patients who contract SARS‐CoV‐2.
Disclosure
The authors declared no conflict of interest
References
- 1. Muniyappa R, Gubbi S. COVID‐19 pandemic, corona viruses, and diabetes mellitus. Am J Physiol Endocrinol Metab 2020;318:E736‐E741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID‐ 19: evidence from meta‐analysis. Aging (Albany NY) 2020;12:6049‐6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hall G, Laddu DR, Phillips SA, Lavie CJ, Arena R. A tale of two pandemics: how will COVID‐19 and global trends in physical inactivity and sedentary behavior affect one another? (Published online April 8, 2020) Prog Cardiovasc Dis 2020. doi: 10.1016/j.pcad.2020.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shi Y, Wang Y, Shao C, et al. COVID‐19 infection: the perspectives on immune responses. Cell Death Differ 2020;27:1451‐1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS‐CoV‐2 by full‐length human ACE2. Science 2020;367:1444‐1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sun P, Lu X, Xu C, Sun W, Pan B. Understanding of COVID‐19 based on current evidence. J Med Virol 2020;92:548‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860‐867. [DOI] [PubMed] [Google Scholar]
- 8. Hill MA, Mantzoros C, Sowers JR. Commentary: COVID‐19 in patients with diabetes. Metabolism 2020;107:154217. doi: 10.1016/j.metabol.2020.154217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28:1195‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020;130:2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moon C. Fighting COVID‐19 exhausts T cells. Nat Rev Immunol 2020;20:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Santos SH, Andrade JM. Angiotensin 1‐7: a peptide for preventing and treating metabolic syndrome. Peptides 2014;59:34‐41. [DOI] [PubMed] [Google Scholar]
- 15. Dinarello CA. Overview of the IL‐1 family in innate inflammation and acquired immunity. Immunol Rev 2018;281:8‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang G, Cao K, Liu K, et al. Kynurenic acid, an IDO metabolite, controls TSG‐6‐mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ 2018;25:1209‐1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pedersen BK, Saltin B. Exercise as medicine ‐ evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports 2015;25:1‐72. [DOI] [PubMed] [Google Scholar]
- 18. Simpson RJ, Campbell JP, Gleeson M, et al. Can exercise affect immune function to increase susceptibility to infection? Exerc Immunol Rev 2020;26:8‐22. [PubMed] [Google Scholar]
- 19. Laddu DR, Lavie CJ, Phillips SA, Arena R. Physical activity for immunity protection: inoculating populations with healthy living medicine in preparation for the next pandemic [published online April 9, 2020]. Prog Cardiovasc Dis 2020. doi: 10.1016/j.pcad.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lancaster GI, Febbraio MA. The immunomodulating role of exercise in metabolic disease. Trends Immunol 2014;35:262‐269. [DOI] [PubMed] [Google Scholar]
- 21. Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle‐derived interleukin‐6. Physiol Rev 2008;88:1379‐1406. [DOI] [PubMed] [Google Scholar]
- 22. Abbasi A, Hauth M, Walter M, et al. Exhaustive exercise modifies different gene expression profiles and pathways in LPS‐stimulated and un‐stimulated whole blood cultures. Brain Behav Immun 2014;39:130‐141. [DOI] [PubMed] [Google Scholar]
- 23. Lancaster GI, Khan Q, Drysdale P, et al. The physiological regulation of toll‐like receptor expression and function in humans. J Physiol 2005;563:945‐955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Flynn MG, McFarlin BK, Phillips MD, Stewart LK, Timmerman KL. Toll‐like receptor 4 and CD14 mRNA expression are lower in resistive exercise‐trained elderly women. J Appl Physiol 2003;95:1833‐1842. [DOI] [PubMed] [Google Scholar]
- 25. Collao N, Rada I, Francaux M, Deldicque L, Zbinden‐Foncea H. Anti‐inflammatory effect of exercise mediated by toll‐like receptor regulation in innate immune cells ‐ a review. Int Rev Immunol 2020;39:39‐52. [DOI] [PubMed] [Google Scholar]
- 26. Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti‐inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 2011;11:607‐615. [DOI] [PubMed] [Google Scholar]
- 27. Rada I, Deldicque L, Francaux M, Zbinden‐Foncea H. Toll like receptor expression induced by exercise in obesity and metabolic syndrome: a systematic review. Exerc Immunol Rev 2018;24:60‐71. [PubMed] [Google Scholar]
- 28. Nold MF, Nold‐Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA. IL‐37 is a fundamental inhibitor of innate immunity. Nat Immunol 2010;11:1014‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fernandes P, de Mendonca Oliveira L, Bruggemann TR, Sato MN, Olivo CR, Arantes‐Costa FM. Physical exercise induces immunoregulation of TREG, M2, and pDCs in a lung allergic inflammation model. Front Immunol 2019;10:854. doi: 10.3389/fimmu.2019.00854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cardoso GH, Petry DM, Probst JJ, et al. High‐intensity exercise prevents disturbances in lung inflammatory cytokines and antioxidant defenses induced by lipopolysaccharide. Inflammation 2018;41:2060‐2067. [DOI] [PubMed] [Google Scholar]
- 31. Rigonato‐Oliveira NC, Mackenzie B, Bachi ALL, et al. Aerobic exercise inhibits acute lung injury: from mouse to human evidence Exercise reduced lung injury markers in mouse and in cells. Exerc Immunol Rev 2018;24:36‐44. [PubMed] [Google Scholar]
- 32. Budinger GR, Urich D, DeBiase PJ, et al. Stretch‐induced activation of AMP kinase in the lung requires dystroglycan. Am J Respir Cell Mol Biol 2008;39:666‐672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cholewa JM, Paolone VJ. Influence of exercise on airway epithelia in cystic fibrosis: a review. Med Sci Sports Exerc 2012;44:1219‐1226. [DOI] [PubMed] [Google Scholar]
- 34. Zhang J, Dong J, Martin M, et al. AMP‐activated protein kinase phosphorylation of angiotensin‐converting enzyme 2 in endothelium mitigates pulmonary hypertension. Am J Respir Crit Care Med 2018;198:509‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Prata LO, Rodrigues CR, Martins JM, et al. Original research: ACE2 activator associated with physical exercise potentiates the reduction of pulmonary fibrosis. Exp Biol Med (Maywood) 2017;242:8‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]


