The current wave of COVID‐19 infections, which widely believed as an outbreak that initially sprout within the city of Wuhan in China, is beginning to assume alarming proportions across the globe, as cautioned by reports from the World Health Organization (WHO). Viral incidence rates that began to spike from the early December 2019 were the resultant cause of an infection that had contracted from a novel coronavirus (CoV) (form. 2019‐nCoV). By the mid of April 2020, the global number of positive COVID‐19 cases, as determined by the WHO, stood at 1,699,576 and has been climbing dangerously. Until the same period, the total deaths worldwide due to the disease were 102,734, the worst affected being the United States. It is worthwhile to note that the typically decorated Coronaviridae family, which primarily houses, enveloped single‐stranded RNA viruses, in addition to the 2019‐nCoV, (now classified as SARS‐CoV‐2) also include the Severe Acute Respiratory Syndrome Coronavirus (SARS‐CoV), which primarily interacts with humans and bats, the Middle East Respiratory Syndrome‐related Coronavirus (MERS‐CoV), which causes infection in camels, humans and bats, and several other related forms. There are necessarily four major structural proteins that make up the architecture of the virus; (a) the glycoprotein on the spike surface (S), (b) the protein that envelopes (E), (c) the matrix core protein (M), and (d) the protein in the nucleocapsid (N). It is well known that the virus employs its spike surface glycoprotein to carefully bind with the protein binding sites of the host cells.
Nevertheless, the scenario is slightly different with the spike proteins that belong to SARS‐CoV and the ones that belong to the MERS‐CoV, where they attach themselves to various host protein binding sites through several other receptor‐binding domains (RBDs). The primary RBD for the SARS‐CoV particles is Angiotensin‐converting enzyme 2 (ACE2). On the other hand, the MERS‐CoV employs the dipeptidyl peptidase‐4 (DPP4) or “cluster of differentiation 26” (CD26) as their principal protein‐binding site. Earlier reports claimed that the COVID‐19 virus boasts a close developmental relationship with the SARS virus (Wu et al., 2020; Zhou et al., 2020). However, from a pharmacological viewpoint, we have enough evidence now to believe that the aerosolized droplet transmission of COVID‐19 viral particles to the lungs involves several key pathological molecular mechanisms.
It is well‐observed among most viruses that, the common mechanism of their entry into a host cell is through receptor‐mediated endocytosis. Looking at the bigger picture, we are convinced to think that the receptor employed by the COVID‐19 virus to attack and modify the cells in the lungs could most probably be ACE2, a protein that is found on the surface of the cells in the kidney, heart tissues, artery and veins, and most significantly, on the epithelial cells (AT2) of the alveoli of the lungs. Moreover, AT2 cells are vulnerable to virus borne infections (Richardson et al., 2020; Zhao et al., 2020). Having mentioned that, the COVID‐19 viral particles have the notorious tendency to proteolytically generate mutated, biologically active fragments of RAS, namely, Ang II and Ang 1–7. These biological fragments may subsequently activate the AT2 receptors, leading them to bind with ACE2. AT2 is a type of G‐protein coupled receptor (Clayton et al., 2015; Singh, Singh, & Sharma, 2013). Interestingly, the GRK‐β arrestin system catalyzes the activation of the Gi2/Gi3‐proteins downstream into the cell. This system, also, causes the downregulation of the receptors and pave the way for internalization of the COVID‐19 virus, binding the ligand‐receptor complex into the respiratory alveolar epithelial cell. Earlier reports suggested the possibility that c‐jun NH2‐terminal kinase (JNK) activity can be inhibited through vascular AT2Rs. This might be possible by altering a downstream signal that belonged to Pyk2 is a tyrosine kinase (SHP‐1) dependent manner (Matsubara et al., 2001). The JNK pathway is commonly known as the signaling pathway of “death,” which controls the response of the cell towards harmful extracellular factors, namely, inflammatory cytokines. The JNK interface plays a significant role in cellular apoptosis, tissue level inflammation, tissue cytokine production, and their metabolism. Several extracellular factors activate the JNK module, namely, ionizing radiation, temperature, free radical generation, damage to the DNA, proinflammatory cytokines and growth factors. These stressors signal to the JNK complex and activate targets, namely, transcription factors, in specific, c‐Jun, ATF and also Elk1. There are two major downstream signaling JNK pathways. The first pathway activates c‐Jun and Fos. Besides, this pathway also activates apoptosis signaling. These include upregulating several proteins (BIM, BAD, BAX proteins) that activate apoptosis in the cell. The path also involves active P53 transcription. The second pathway consists of the blocking of cell survival signaling, namely, STATs and CREB. Also, a Nature journal study reported by Kucinski, Dinan, Kolahgar, and Piddini (2017) explored the competitive environment of minute defective cells, which adopt the JNK pathway, while, the neighboring cells extensively activated the JAK–STAT pathway, which enable proliferation at a faster rate in the later stages of apoptosis in defective cell (Figure 1).
FIGURE 1.
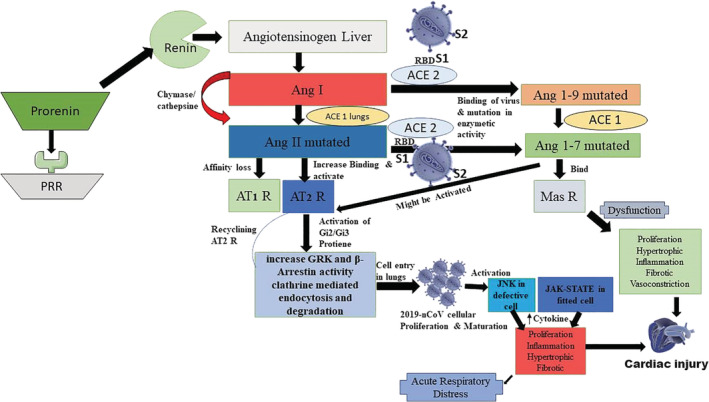
Schematic illustration of the 2019‐novel CoV (2019‐nCoV) transmission through host cell directed network of GPCR
Here, we conclude that the COVID‐19 virus activates the JNK and JAK–STAT mediated biochemical mechanisms in lungs, resulting in the proliferation and transmission of viral cells. Moreover, before recommending ACE2 inhibitors, it is worthwhile to be mindful and aware of the complications that arise during the blockade of the enzyme, which could lead to cardiac hypertrophy and fibrosis. Thus, we summarize that both JNK and JAK–STAT pathways could be targeted pharmacologically for the inhibition of the COVID‐19 infection. Moreover, there must be effective strategies to contain the spread before a vaccine can be developed.
REFERENCES
- Clayton, D. , Hanchapola, I. , Thomas, W. G. , Widdop, R. E. , Smith, A. I. , Perlmutter, P. , & Aguilar, M. I. (2015). Structural determinants for binding to angiotensin converting enzyme 2 (ACE2) and angiotensin receptors 1 and 2. Frontiers in Pharmacology, 6, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucinski, I. , Dinan, M. , Kolahgar, G. , & Piddini, E. (2017). Chronic activation of JNK JAK/STAT and oxidative stress signalling causes the loser cell status. Nature Communications, 8(1), 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara H, Shibasaki Y, Okigaki M, Mori Y, Masaki H, Kosaki A, Tsutsumi Y, Uchiyama Y, Fujiyama S, Nose A and others. 2001. Effect of angiotensin II type 2 receptor on tyrosine kinase Pyk2 and c‐Jun NH2‐terminal kinase via SHP‐1 tyrosine phosphatase activity: Evidence from vascular‐targeted transgenic mice of AT2 receptor. Biochemical and Biophysical Research Communications 282(5):1085–91. [DOI] [PubMed] [Google Scholar]
- Richardson, P. , Griffin, I. , Tucker, C. , Smith, D. , Oechsle, O. , Phelan, A. , & Stebbing, J. (2020). Baricitinib as potential treatment for 2019‐nCoV acute respiratory disease. The Lancet, 395, e30–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, Y. , Singh, K. , & Sharma, P. L. (2013). Effect of combination of renin inhibitor and mas‐receptor agonist in DOCA–salt‐induced hypertension in rats. Molecular and Cellular Biochemistry, 373(1), 189–194. [DOI] [PubMed] [Google Scholar]
- Wu A, Peng Y, Huang B, Ding X, Wang X, Niu P, Meng J, Zhu Z, Zhang Z, Wang J and others. 2020. Genome composition and divergence of the novel coronavirus (2019‐nCoV) originating in China. Cell Host & Microbe, 27, 325, 328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. 2020. Single‐cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019‐nCov. bioRxiv:January 26, 2020.919985.
- Zhou P, Yang X‐L, Wang X‐G, Hu B, Zhang L, Zhang W, … Shi Z‐L. 2020. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. bioRxiv:January 22, 2020.914952.


