Abstract
Visual processing of other’s actions is supported by sensorimotor brain activations. Access to sensorimotor representations may, in principle, provide the top-down signal required to bias search and selection of critical visual features. For this to happen, it is necessary that a stable one-to-one mapping exists between observed kinematics and underlying motor commands. However, due to the inherent redundancy of the human musculoskeletal system, this is hardly the case for multijoint actions where everyone has his own moving style (individual motor signature—IMS). Here, we investigated the influence of subject’s IMS on subjects’ motor excitability during the observation of an actor achieving the same goal by adopting two different IMSs. Despite a clear dissociation in kinematic and electromyographic patterns between the two actions, we found no group-level modulation of corticospinal excitability (CSE) in observers. Rather, we found a negative relationship between CSE and actor-observer IMS distance, already at the single-subject level. Thus, sensorimotor activity during action observation does not slavishly replicate the motor plan implemented by the actor, but rather reflects the distance between what is canonical according to one’s own motor template and the observed movements performed by other individuals.
Keywords: action observation, individual motor signatures, multijoint actions, transcranial magnetic stimulation, variability
Introduction
The coordination of our own actions with those of others requires the ability to read and anticipate what and how our partner is about to do. Indeed, when observing someone else moving, we can extract useful information such as future bodily displacements (Flanagan and Johansson 2003; Blakemore and Frith 2005; Falck-Ytter et al. 2006) or infer higher order cognitive processes hiding behind those actions (Becchio et al. 2008; Soriano et al. 2018). In principle, knowledge about the invariant properties of movement control (Flash and Hogans 1985; Bennequin et al. 2009) could support inferences about the unfolding of other’s actions (Dayan et al. 2007; Casile et al. 2010). In this regard, it has been proposed that these inferences may be based on a direct match between actor’s sensorimotor activations during action execution (AE) and observer’s sensorimotor activations triggered by action observation (AO; Rizzolatti et al. 2001; Rizzolatti and Craighero 2004; Rizzolatti and Sinigaglia 2016). Indeed, using corticospinal excitability (CSE), motor recruitment during AO was shown to replicate the spatio-temporal sequence of motor commands implemented by the actor (for a review please see: Naish et al. 2014).
This idea is, however, challenged by the redundancy that characterizes the organization of human movement (Kilner 2012; D’Ausilio et al. 2015; Hilt et al. 2017). The abundance of degrees of freedom available during AE suggests that different joint configurations, as well as spatio-temporal patterns of muscle activity, can equally be used to reach the same behavioral goal (Bernstein 1967). In this regard, a strong version of the direct-matching hypothesis (Rizzolatti et al. 2001; Rizzolatti and Craighero 2004; Rizzolatti and Sinigaglia 2016) explains inferences when a direct relationship exists between muscle recruitment, movement kinematics, and behavioral goals (e.g., simple finger movements). However, it is less clear how other’s complex movements (i.e., multijoint movements) are transformed onto the observer’s motor representations. In this case, any sensorimotor-based inference about other’s actions, amount to finding a solution to a many-to-many mapping problem.
Here, we suggest that a simpler mapping exists between behavioral goals and the lower dimensionality space of whole-body configurations (i.e., synergies; Hilt et al. 2017). In fact, although a handful of kinematic solutions is biomechanically valid, everyday actions (i.e., reaching for an object on the floor starting from a standing posture) are usually performed via a limited number of possible kinematic configurations of the biomechanical chain (e.g. “ankle” and “hip” strategies for postural control; Horak and Nashner 1986; Berret et al. 2009). On the top of that, each individual carry his own robust and yet unique way of moving (individual motor signature—IMS; Hilt et al. 2016; Słowiński et al. 2016). For instance, in a whole-body reaching task, Hilt and collaborators (Hilt et al. 2016) showed low intrasubject motor variability, accompanied by a large intersubject variability. The inherent lower dimensionality of whole-body postural control and the presence of robust Individual Motor Signatures (IMS) suggest the existence of a simpler AO-AE mapping that may be the function of everyone’s individual movement style. Backed by this, we hypothesize that while observing others’ multijoint actions, people build sensorimotor-based predictions by referencing what they see to the motor engrams of their own IMS.
To verify our hypothesis, we asked naive participants to first perform and then observe a whole-body reaching action which could be executed with numerous IMSs generally spread within a continuum between two “extreme” patterns (ankle and knee strategies; Hilt et al. 2016). After characterizing subjects’ own IMS during execution, we measured their sensorimotor recruitment (CSE) by administering single-pulse transcranial magnetic stimulation (TMS) on their motor cortex while they observed an actor achieving the same goal by using the two “extreme” patterns of IMSs. CSE was measured from the cortical representation of the tibialis anterior (TA) muscle that shows a clearly dissociable pattern while executing the two IMSs. To exclude potential carry-over effects between action execution (AE) and observation, the same subjects were also tested several months later in the AO task only.
Based on this protocol, we aim at testing the mechanism underlying motor resonance in complex AO. Based on prior data (Naish et al. 2014), the prediction is that all subjects asked to observe the actions should mirror the TA recruitment in the actor. On the contrary, we predict that CSE would reflect, on an individual basis, a measure of the distance between own IMS and observed IMS. Furthermore, if sensorimotor activations are greater for little IMS distance, then it is likely that the motor system is computing the similarity between observed and own IMS. On the contrary, a negative relationship would suggest that sensorimotor inferences about other’s goals might be built by computing the difference or an error measure between one’s own motor template and the observed movement.
Method
Experiment 1
Participants
Twenty right handed volunteers (11 females and 9 males; age: 24 ± 5 years) participated in the study. Data from one subject were removed due to technical problems during the experiment. None of the participants reported neurological, psychiatric, or other contraindications to TMS (Rossi et al. 2009). They had normal or corrected-to-normal visual acuity and were unaware of the purposes of the study. All of them gave informed consent before the experiment, which was approved by the Ferrara University/Hospital unified Ethics Committee and conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki, as revised in 1983.
Procedure and Setups
The experiment was divided into three parts. Participants were first asked to perform the AE task lasting ∼5min. After that the TMS procedure started followed by the AO task (lasting ∼30min). In the last part, participants were asked to repeat the AE task. Notably, between the AE and AO tasks, a pause of about 20–30 min was included to let cortical excitability return to baseline levels (Classen et al. 1998). These two tasks are described below.
AE Task
The AE task was replicated from a previous study (Hilt et al. 2016) investigating the different motor strategies when pointing towards a homogeneous surface and without a specific target. This protocol was chosen because it keeps free the subjects from external constraints (e.g., a precise point to reach) and evokes natural intersubject variability. Participants were asked to perform a series of whole-body pointing movements towards a uniform opaque curtain fixed to a wooden frame (2.5 tall × 1.5 m large; see Fig. 1) positioned at a 15° angle with respect to the vertical. The surface was a black curtain (tissue) mounted on a wooden frame, soft enough to prevent subjects from using it as a support when finishing the movement but sufficiently elastic to keep its shape and remain flat. Subjects were told that they could point at any position they wanted over the surface. Starting from a standing position and at a distance of 130% of arm’s length from the surface, subjects had to move all body parts with the only constraint to keep the feet fixed and to move both arms simultaneously. The request to move the two arms together ensured that all markers lay approximately along the para-sagittal plane (Berret et al. 2009) to limit the kinematic analysis to this plane (right hemibody in 2D coordinates). All subjects were able to perform the task. Ten trials were run before and after the AO protocol.
Figure 1.

(A) Screenshots of the two AO task video-clips representing ankle and knee strategy. A single-pulse TMS was released at one of two different timings: t1 (start of actor movement) and t2 (end of actor movement) (B) Muscular activity of the actor right TA for each motor strategy. At t1, actor kinematics and TA activation are similar, while at t2, actor kinematics and TA recruitment are different across video-clips. (C) Average and standard error of normalized MEPs amplitude at t1 and t2 when observing the ankle (red) and knee (blue) video-stimuli. No group-level MEPs modulation was present.
More importantly, this protocol by avoiding external constraints (e.g., a precise target to reach) allows subjects to execute the movement they would naturally/spontaneously use (e.g., IMS). A previous study using this task observed a large movement variability across subjects but low intrasubject variability (Hilt et al. 2016). Interestingly, subjects behaviors were a trade-off between the optimization of two distinct cost functions. The first strategy (named Ankle) limits mechanical energy expenditure but uses a kinematic configuration that may be risky for equilibrium maintenance: bending the body forward using mainly ankle and shoulder joints while freezing knee and hip joints (large center of pressure forward displacement). In muscular terms, the ankle strategy is associated with a pre-activation of the TA (anticipatory postural adjustment) followed by an inhibition of this muscle later in the movement (see Fig. 1 in red). The second strategy (named Knee) increases mechanical energy expenditure but uses a kinematic configuration that may be safer for equilibrium maintenance: substantial knee flexion and forward trunk bending associated with a backward hip displacement (limited center of pressure forward displacement). In muscular terms, the knee strategy implied an activation of lower leg muscles (including TA) during the movement (see Fig. 1 in blue).
Kinematic Recordings
Whole-body movements in three axes (mediolateral, X; anteroposterior, Y; and vertical, Z) were recorded using a seven cameras motion capture system (Vicon) sampling at 100 Hz. Eight retro-reflective markers (15 mm in diameter) were recorded. Markers were placed at the following anatomical locations on the right side of the body: the acromial process (named here “shoulder”), the lateral condyle of the humerus (named here “elbow”), the styloid process of the ulnar (named here “wrist”), the last phalanx of the index finger (named here “index”), the greater trochanter (named here “hip”), the knee interstitial joint space (named here “knee”), the ankle external malleolus (named here “ankle”), and the fifth metatarsal head of the foot (named here “toe”).
Electromyographic Recordings
Electromyography (EMG) of left TA muscle (Fig. 1B) was acquired from each subject via a wireless system (Aurion, ZeroWire EMG). The TA muscle was selected because it plays a central role in whole-body forward reaching execution (Stapley et al. 1998; Leonard et al. 2009). Before electrodes placement, the skin was shaved and cleaned with alcohol to obtain low impedance (<5 kΩ). EMG signals were band-pass filtered (50–1000 Hz), digitized (2 kHz), acquired by a CED power1401 board and visualized with Signal 3.09 software (Cambridge Electronic Design).
AO Task
Stimuli
The experimental stimuli consisted in short video clips showing a lateral view of a female actor who executed the action following two different motor strategies, the Ankle strategy (in red, Fig. 1) and Knee strategy (in blue, Fig. 1). Here, the actor was trained to perform the actions and she could reproduce both strategies well. We made the choice of having one unique actor for the two stimuli, to control for body size, segments length, movement speed, etc.
The kinematic data of the actor was measured as previously described for the AE task. Movement onset and offset times were defined as the instant at which the linear tangential velocity of the index fingertip passed, respectively, above or below 5% of the peak value obtained during the reaching movement. Duration of the two movements was around 1.2 s. Video-clips started 400 ms before the beginning of the movement and finished 400 ms after the end of it (Fig. 1B), for a total length of around 2 s. EMG of the actor’s left TA (Fig. 1B) and left soleus was also acquired (for more details, see “AE task”—“EMG recordings”). Activities of the two muscles for each stimulus are presented in Supplementary Material S1.
Procedure
Subjects were seating in a comfortable armchair with their legs resting. A 17″ LCD computer monitor (1024 × 768 pixels; refresh rate 60 Hz) was placed at a distance of 60 cm from their frontal plane. Each trial started with the presentation of a grey central fixation cross displayed on a black screen. After 3 s, a video-clip appeared. During each video-clip, a single-pulse TMS was released at one of two different timings. The first (t1) corresponded to the start, the second (t2) to the end of the movement shown in the video-clips. Defined in this way, the two timings refer to very distinct moments in term of kinematic and muscular activities. At t1, actor body posture is similar across video-clips (Fig. 1A), while TA muscular anticipatory activations are present in the ankle strategy only (Fig. 1B). By contrast, at t2, actor kinematics are different across video-clips (Fig. 1A), and TA is inhibited in the ankle strategy while remains active in the knee strategy (Fig. 1B). At the end of each trial, an attentional question appeared on the screen (for more details, see Supplementary Material S2). In total, 80 trials were randomly presented: 2 video stimuli × 2 timings of stimulation × 20 repetitions. Twenty baseline trials were recorded at rest (eyes closed, subjects imagining a relaxing landscape) half at the beginning and half at the end of the session. The presentation of the stimuli, the timing of the TMS pulses, and response collection were controlled by Psychtoolbox Version 3.0 (PTB-3), implemented in MATLAB (The MathWorks Inc.).
TMS and EMG Recordings
Motor-evoked potentials (MEPs) were recorded with a wireless EMG system (Aurion, ZeroWire EMG) from the left TA. Since the observed action was bilateral and symmetric, we expected no specific lateralization of the effects, and then decided to record only the left TA to ensure the cleanest signal possible. Before electrodes placement, the skin was shaved and cleaned with alcohol to obtain a low impedance (<5 kΩ). EMG signals were band-pass filtered (50–1000 Hz), digitized (2 kHz), acquired by a CED power1401 board and visualized with Signal 3.09 software (Cambridge Electronic Design). A 70-mm (loop diameter) figure-of-eight shaped conic coil connected to a Magstim stimulator (Magstim Co.) was placed over the right primary motor cortex with antero-posterior directed current orientation. As optimum scalp position was considered, the location on the scalp where maximum amplitude MEPs in the TA were evoked at the lowest possible stimulation intensity (hotspot). Once the optimal site was found, the scalp was marked with a felt pen to ensure consistency between stimulations. The coil was secured by a lockable articulated arm (Fisso, Swiss). The resting Motor Threshold (rMT) was assessed by using standard protocols (5 out of 10 MEPs exceeding 50 μV peak-to-peak amplitude), with an interstimulus interval of about 8 s. During the experiment, single-pulse TMS was applied with an intensity of stimulation corresponding to 120% of the rMT (mean = 57.8%; standard deviation [SD] = ± 4.7% of the maximum stimulator output).
Data Analysis
Kinematic Data
Kinematic trajectories were low-pass filtered using a digital fifth-order Butterworth filter at a cutoff frequency of 10 Hz. We focused the kinematic analysis on the final posture in the sagittal plane (Y, Z) that described the motor strategy used by the subject. Movement onset (tstart) and offset (tend) time were defined as described earlier for the action video-clips. At tend, four intersegmental angles were computed for the four principal joints used: ankle, knee, hip, and shoulder. These intersegmental angles were already used to characterize the motor strategies in previous studies (Hilt et al. 2016; for more details see Supplementary Material S3).
IMS Index
We computed an individual AE index (IMS index) by normalizing (z-score) and averaging the final value of the four intersegmental angles considered. Considering that each joint angle has different maximal amplitude (e.g., ankle vs. hip), the z-score normalization ensures that the final index (average of all angles) is not reflecting the contribution of only the joint having the largest range of motion. This index is a simple way to represent the final kinematic configuration of each subject and may thus be considered as the description of the postural strategy implemented by each participant.
IMS Distances
To complement the IMS index, we evaluated the difference/similarity between the IMS of each subject and the actor’s implementation of the two IMSs. To this aim, we defined a distance by computing the root mean squared error (RMSE) between intersegmental angular trajectories of the actor and each of the subjects. RMSE is commonly used to compute the average magnitude of the errors between experimental values and associated model predictions (Hilt et al. 2016).
 |
All trials were time-normalized (from tstart to tend) to 100 frames. For each subject and each joint (ankle, knee, hip, and shoulder), we computed an averaged angular trajectory that we compared (using RMSE) with the corresponding angular trajectory of the actor in both IMSs. RMSE were then normalized across subjects (z-score) and averaged across joints, to obtain a unique distance value for each pairwise comparison between subject’s and actor’s IMSs. From this point, Dist_ankle and Dist_knee will refer to the distance between the IMSs of subjects and the video-stimuli, respectively, showing the ankle strategy and the knee strategy.
Computing in this way, the distances are taking into account the whole duration of the movement, while the IMS index refers to only the final posture (see Fig. 2). There is obviously some degree of overlapping variance between the two, but they are built using different data, potentially describing very different processes. The first one addressing the dynamic component of reaching the final posture, while the other describing the final posture only.
Figure 2.

Illustration of the different steps to compute the AE index (upper part) and AE distances (lower part) to ankle IMS (red) and knee IMS (blue).
Neurophysiological Data
Trials with EMG activity in the 50-ms period prior to TMS were discarded from the analysis (1% of the trials). Peak-to-peak value (mV) was used to represent MEP amplitude. MEPs exceeding 3 SD from the mean peak-to-peak amplitude, at the single subject level, were excluded from the data set (2% of the trials). The remaining MEPs were then averaged for every experimental condition and each subject. To perform correlation with IMS, we computed and normalized (z-score) the subtraction of the MEPs amplitude recorded when observing the video stimulus 1 (ankle strategy) from the MEPs amplitude recorded when observing the video stimulus 2 (knee strategy), for each subject (i.e., MEPs AO-knee—MEPs AO-ankle). This subtraction will be further called AO index. Computed in this way, a negative value of AO index indicates a greater CSE modulation when observing ankle stimulus compared with knee stimulus; a positive value of AO index indicates a greater CSE modulation when observing knee stimulus compared with ankle stimulus. Thus, an AO index close to null indicates similar CSE modulation when observing the two stimuli.
Statistical Analysis
We used Shapiro–Wilk test to check the normality assumption for parametric tests. MEPs data and kinematic parameters were not normally distributed (P < 0.05), and we then decided to use a two-tail permutation test (5000 permutations; MATLAB function mult_comp_perm_t1). All preprocessing and analyses were performed using custom software written in MATLAB (Mathworks). For each correlation analysis, we estimated the Pearson correlation coefficient (R) and the associated P-value (MATLAB function corcoeff). The data used in the correlation analysis were all normally distributed according to the Shapiro–Wilk test (P > 0.05). All P-values were corrected for multiple comparisons using the Benjamini-Hochberg false discovery rate (MATLAB function fdr_bh).
Experiment 2
Participants
Eleven volunteers (6 females and 5 males; age: 22 ± 3 years) who participated to the first experiment also performed the second experiment. The second experiment took place 6–12 months after the first. Upon recruitment, we did not mention their participation to the previous study nor the fact that this one was a follow-up. All of them gave informed consent before the experiment, which was approved by the Ferrara University/Hospital unified Ethics Committee and conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki, as revised in 1983.
Procedure and Setups
The second experiment consisted in the AO task (see Experiment 1) without any AE task. TMS and EMG recordings’ procedures were identical to the Experiment 1.
Data Analysis
Neurophysiological data analysis was identical to the first experiments. Notably, no kinematic data were acquired in Experiment 2.
Statistical Analysis
Statistical analysis was identical for the two experiments.
Results
Experiment 1
AE Task
No significant changes in the execution task appeared between the two repetitions of the same AE task, before and after AO. This was verified on the final posture achieved by participants (IMS) and on the measure of IMS distance with respect to actor’s IMSs (Dist_knee and Dist_ankle; for values and statistics refer to Supplementary Material S4 and Supplementary Material S5). Additionally, and in agreement with previous results (Hilt et al. 2016), IMSs showed large between-subjects and small within-subject variability (Supplementary Material S4). Furthermore, as already shown earlier (Hilt et al. 2016), we found a significant negative correlation between the two distances (Distknee vs. Distankle; R = −0.75, P < 0.01; Supplementary Material S6), such that the more a subject had an IMS close to one of the two strategies, the further away will be from the other. This confirms that the two selected IMSs are likely the two ends of a natural behavioral continuum. Also, no correlation was found in our experimental subjects (Supplementary Material S7) between TA activation at t2 and kinematics of the final posture (AE index) suggesting that a many-to-many mapping indeed exists between muscle pattern and movement kinematics.
AO Task
Subjects answered correctly to the attentional question in most of the trials (90 ± 8%). Regarding CSE, a significant decrease was observed in the baseline computed after AO (0.34 ± 0.07 V) compared with before (0.43 ± 0.10; t = 2.88, P < 0.01). A change of baseline before and after observation has already been described and commented in (Hilt et al. 2017). Furthermore, we found a significant increase of MEPs amplitude in the trials recorded during AO (average of the four conditions: 0.53 ± 0.10 V) compared with baseline pre (t = −2.15, P < 0.05) and post (t = −4.25, P < 0.01). These variations are associated to an unspecific AO effect, which may be explained by a generic arousal effect (Hilt et al. 2017; See Supplementary Material S10). Rather, the specificity of the AO task has to be verified across conditions (timing of TMS and properties of the action stimuli). When normalizing on the averaged baseline pre and post, no significant difference was observed between the four experimental conditions: t1knee (1.59 ± 0.18%), t1ankle (1.55 ± 0.16%), t2knee (1.63 ± 0.24%), and t2ankle (1.57 ± 0.19%). In conclusion, no group level significant effects were present between the different conditions (Please see Supplementary materials S11 for additional analyses supporting this claim).
Correlations between IMS Index and CSE Modulation
To further evaluate the link between IMS and CSE modulation, we ran a correlation analysis between the final posture in the AE task of each subject and the AO indexes. A significant correlation was found between IMS and the AO index on timing t2 only (t1: R = −0.12, P = 0.94; t2: R = −0.73, P < 0.01; Fig. 3). Similar modulations were obtained also without z-scoring the two indexes (Supplementary Fig. S9.1). Equivalent results were found when separating for the IMS recorded before (t1: R = −0.01, P = 0.99; t2: R = −0.7, P < 0.01) and after AO (t1: R = −0.22, P = 0.94; t2: R = −0.63, P < 0.01). This result suggests that only in the presence of discriminative kinematic cues (t2), CSE modulation during AO depends on IMS.
Figure 3.

Correlation between the AE index and the AO index at TMS timing t1 (A) and t2 (B). A negative AO index value (lower part—red background) indicates larger CSE when observing ankle IMS compared with knee IMS, and vice versa for positive values (upper part—blue background). Pearson correlation coefficients and P-values are reported above each graph.
Correlations between IMS Distance to Stimuli and CSE Modulation
To complement final posture IMS information, we defined a distance measure (Distankle and Distknee) that evaluates the difference/similarity between the postural trajectory of each subject and the two IMSs implemented by the actor (two video-stimuli). We analyzed these distances in relation to the AO index. The correlation analysis at timing t2 revealed two significant correlations, in opposite directions. The AO index is negatively correlated with Distknee (R = −0.65, P < 0.01; Fig. 4A) and positively correlated with Distankle (R = 0.59, P < 0.05; Fig. 4B). In other terms, subjects exhibited larger MEPs amplitude when observing the action that differed the most from their own IMS (Fig. 5). No significant correlation was present at t1 (Dist knee: R = −0.03, P = 0.90; Dist ankle: R = 0.26, P = 0.37). Similar, but nonsignificant, trends were obtained without z-scoring the two indexes (Supplementary Fig. S9.2).
Figure 4.

Correlation between distances to each stimulus and the AO index at TMS timing t2. The Pearson correlation coefficients and P-values are reported on each graph. Each graph (A, B) can be separated into four regions. The blue region indicates subjects exhibiting a higher CSE when observing knee IMS video-clip compared with ankle IMS video-clip. The red region indicates the position of subjects exhibiting a higher CSE when observing ankle IMS video-clip compared with knee IMS video-clip. Darker areas indicate subjects exhibiting greater CSE when observing their own IMS. On the opposite, lighter areas (and black points) indicate subjects exhibiting greater CSE when observing the IMS opposite to their own behavior in AE.
Figure 5.
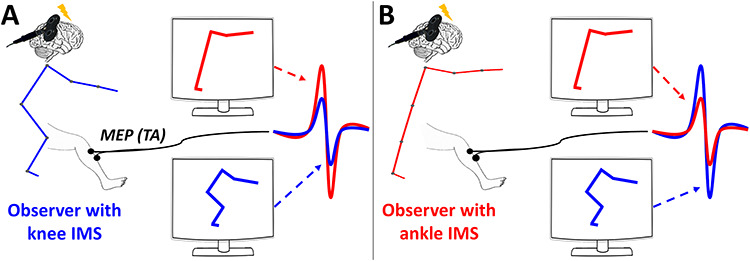
Illustration of the main results for two imaginary subjects having extreme IMSs. MEPs amplitudes are depicted when observing knee (blue stick figure) or ankle (red stick figure) stimulus, for a subject that performed the knee (A) or the ankle (B) IMS in AE. Our results showed that CSE was greater when actor and observer IMSs differ the most. These results agree with the predictive coding hypothesis that hypothesize the existence of a distance computation between observed movement and observer’s IMS.
The same significant effect was found when using distances computed from pre-AO data (AOt1—Dist ankle: R = −0.03, P = 0.91; AOt1—Dist knee: R = 0.15, P = 0.54; AOt2—Dist ankle: R = 0.57, P < 0.05; AOt2—Dist knee: R = −0.57, P < 0.05). Differently, using distances computed from post-AO, no significant correlation was observed (AOt1—Dist ankle: R = −0.03, P = 0.89; AOt1—Dist knee: R = 0.31, P = 0.18; AOt2—Dist ankle: R = 0.44, P = 0.11; AOt2- Dist knee: R = −0.42, P = 0.14). This absence of significant correlation (despite a trend similar to pre-AO) revealed a slight change during the AO task (already suggested by the change of CSE between baseline pre- and post-AO).
Experiment 2
AO Task
Subjects answered correctly to the attentional question in most of the trials (92 ± 6%). Regarding CSE, no change was observed in the baseline computed after AO (0.57 ± 0.16 V) compared with before (0.49 ± 0.14; t = 1.13, P = 0.31).
In agreement with experiment 1, we found a significant increase of MEPs amplitude in the trials recorded during AO (average of the four conditions: 0.79 ± 0.19 V) compared with baseline pre (t = −2.34, P < 0.05) and post (t = −3.67, P < 0.01). When normalizing on the averaged baseline pre and post, no significant difference was observed between the four experimental conditions: t1knee (1.59 ± 0.17%), t1ankle (1.54 ± 0.16%), t2knee (1.61 ± 0.17%), and t2ankle (1.41 ± 0.11%). No group-level significant effects were present between the different conditions as in experiment 1.
Correlations between IMS Index (Experiment 1) and CSE Modulation (Experiment 2)
In agreement with experiment 1, a significant correlation was found between IMS and the AO index on timing t2 only (t1: R = −0.27, P = 0.43; t2: R = 0.77, P < 0.05; Supplementary Fig. S8.1). Equivalent results were found when separating for the IMS recorded before (t1: R = −0.21, P = 0.54; t2: R = −0.68, P < 0.05) and after AO (t1: R = −0.28, P = 0.40; t2: R = −0.71, P < 0.05).
Correlations between IMS distance (experiment 1) to stimuli and CSE modulation (experiment 2).
In agreement with experiment 1, the AO index is negatively correlated with Distknee (R = −0.66, P < 0.05). However, the AO index is only marginally (positively) correlated with Distankle (R = 0.55, P = 0.07; Supplementary Fig. S8.2). No significant correlation was present at t1 (Dist knee: R = −0.06, P = 0.85; Dist ankle: R = −0.07, P = 0.83).
Similar but marginally significant effects were found when using distances computed from pre-AO data (AOt1—Dist ankle: R = 0.01, P = 0.97; AOt1—Dist knee: R = 0.03, P = 0.93; AOt2—Dist ankle: R = 0.54, P = 0.08; and AOt2—Dist knee: R = −0.52, P = 0.09). In agreement with experiment 1, using distances computed from post-AO, no significant correlation was observed (AOt1—Dist ankle: R = −0.17, P = 0.62; AOt1—Dist knee: R = 0.21, P = 0.53; AOt2—Dist ankle: R = 0.27, P = 0.42; and AOt2—Dist knee: R = −0.14, P = 0.67).
Discussion
Previous studies on AO mostly investigated mirroring mechanisms evoked by simple goal-directed actions (i.e., involving few degrees of freedom) performed in the canonical way. However, due to motor redundancy, observation of daily life actions is rarely characterized by a univocal relationship between the visual (e.g., observed kinematics) and the motor description (e.g., underlying motor commands) of the action. For the same reason, it is not clear how the predictions about others’ actions (multijoint) would be simplified by a direct access to the motor commands (e.g., muscle-level).
To better understand these mechanisms in the context of multijoint actions, we investigated observers’ motor excitability while seeing two different motoric variants of a whole-body reaching action. To this purpose, we selected the cortical representation of TA muscle, differentially involved in the variants of the IMS used to achieve the goal. During the execution of the first variant (ankle IMS), TA is activated only in the anticipation of the movement onset (at t1, Fig. 1A). In the second variant (knee IMS), TA becomes active only after the initiation of the movement (at t2, Fig. 1A). Group-level analysis did not find any significant difference in CSE modulation, due to intersubject variability.
In agreement with this result, several authors recently reported a quite large intersubject variability in CSE modulations to AO (Palmer et al. 2016; Hilt et al. 2017; Hannah et al. 2018). This variability may have multiple origins and, as we argued earlier, one possibility is that the lack of a clear muscle-to-movement mapping in complex actions leads to mixed results when we observe CSE modulations at the group level. The prediction in this case would be an increase in intersubject variability with task complexity. Indeed, a simple motor task (e.g., finger’s abduction/adduction) is characterized by a simple and unique motor mapping directly translated into coherent group-level AO effects (e.g., Romani et al. 2005). In more complex actions involving a larger number of degrees of freedom (e.g., upper-limb reaching to-grasp movement), the mapping depends upon individual strategies, thus explaining why we did not find robust group-level effect (Palmer et al. 2016; Hilt et al. 2017; Hannah et al. 2018). In other words, our results indicate that CSE-based measures of sensorimotor activations during others’ (complex) AO are subject-dependent and cannot be summarized into a common standard pattern.
CSE was instead modulated at the single-subject level according to the “distance” between actors’ and observer’s IMS: larger CSE modulations are associated with the observation of a more different IMS. This result is schematically illustrated in Figure 5 for two hypothetical subjects having extreme IMSs. Importantly, motor priming effects elicited by the AE task can be excluded considering that the same pattern of results, in same subjects, was shown several months later and in the absence of any AE task.
Visuomotor contingencies have often been proposed to drive the emergence of mirror-like responses (van Elk et al. 2008; Calvo-Merino et al. 2010; Stapel et al. 2010; Hunnius and Bekkering 2014). Notably, our data cannot confirm nor exclude the contribution of previous visual experience of the observed action: participants never experienced such a lateral and full view of their movements, but they can get visual access to their ankle, knee, and hip bending—which are, here, the fundamental discriminative properties of the two strategies. However, it is also true that own hand visual experience (for which eye-hand coordination is a central example) is not paired to the same amount of visual exposure to own shoulder movements. In this regard, we can extend the same observation to lower limbs and say that feet visual experience is fundamental (while learning to walk or while walking on thin line) and far larger than the one we have for the hips. At the same time, this does not mean that the observer cannot reconstruct proximal leg motion from vision (Betti et al. 2015; Sartori et al. 2015). Indeed, a refined biomechanical model of the body paired with the accurate sampling of end-point positions (hands or feet) is sufficient to reconstruct the whole kinematic chain down to the shoulder or hips. However, the key issue, here, is that each observer has access only to his/her own biomechanical model. As a consequence, individual differences in biomechanical models, added with the obvious noise in end point (visual) sampling, might explain our results.
In a somewhat similar fashion, neurophysiological studies conducted on experts have also shown a relationship between sensorimotor recruitment and motor familiarity or similarity with other’s action (i.e., Calvo-Merino et al. 2005, 2006; musicians: D’Ausilio et al. 2006; sport players: Aglioti et al. 2008; Jola et al. 2012; and dancers: Candidi et al. 2014). Greater activation was also found while observing impossible movements (Romani et al. 2005) or difficult dance movements in dancers (Cross et al. 2011). These results suggest a positive correlation between the amount of sensorimotor activity while observing skilled actions and the individual expertise in that skill. These findings seem to contradict what we found in the present experiment. However, it is important to bear in mind the fundamental difference existing between common everyday actions (as in our study) and over-trained ones (as in studies with experts). In fact, extensive and highly specific training isolate one skillset also by reducing generalization to adjacent ones (negative transfer: Schmidt and Young 1986; Schmidt and Lee 1999; Ajemian et al. 2010). In this regard, expertise could amount to a greater ability to compute very precise distances in one specific skill only (Aglioti et al. 2008). Instead, here, we show evidence that the sensorimotor system, while observing complex but perfectly common whole-body actions, computes differences rather than similarities.
At this point, it is important to discuss how CSE modulations translate into sensorimotor activities capable of supporting inferences about others’ action. Our results are at odds with a strictly simulative account of other’s action. Instead, the fact that sensorimotor activities during AO are shaped around a measure of distance between observed and own IMSs agrees with the predictive coding framework. In this model, prior motor knowledge provides critical top-down signals that are integrated with bottom-up sensory-based processing (Friston 2010; Friston et al. 2011). To do so, a comparison between predicted (own IMS) and observed kinematic information (others’ IMS) generates a prediction error signal that is used to update the representation of other’s action. Neurophysiological studies on simple goal-directed actions indicated that sensorimotor recruitment during AO reflect a prediction error signal (Aglioti et al. 2008; Candidi et al. 2014; Cardellicchio et al. 2018). Interestingly, previous behavioral studies found an increase in perceptual discrimination performance of other’s actions, when actor-observer motor distance was small (Macerollo et al. 2015; Koul et al. 2016). From these data, we speculate that actor-observer similarity may induce smaller prediction errors, and consequently more accurate perceptual performances. On the opposite side, large actor-observer IMS distance is associated to a decline in perceptual performance (Macerollo et al. 2015; Koul et al. 2016) while sensorimotor activations increased, possibly playing a compensatory role (D’Ausilio et al. 2014; Bartoli et al. 2015; Schmitz et al. 2019). A compensatory role of motor activation during AO, in the perception and anticipatory simulation of actions, has already been suggested by a previous study interfering with the activity of regions within and upstream the AON (Avenanti et al. 2013).
Overall, our data suggest that a greater uncertainty about other’s action will call for a greater need of trustful predictions and consequently greater sensorimotor recruitment. In this context, the present study adds direct neurophysiological evidence that prediction errors are estimated by accessing IMS-related information. In fact, the many-to-many mapping problem in other’s (multijoint) action discrimination might be solved by accessing knowledge about IMSs. Indeed, the stability of IMSs (Słowiński et al. 2016; Coste et al. 2017) may reflect the implicit control and prioritization of a limited number of internal parameters during action planning and execution, partly solving the motor redundancy problem.
In this context, IMSs are defined by their large interindividual variability combined with low intra-individual variability across repetitions and time. Here, we capitalized on a motor behavior which has previously been shown to comply with the properties of IMSs (Hilt et al. 2016). In this task, individual anatomical differences contribute but do not fully explain the properties of the two IMSs (Hilt et al. 2016). Instead, IMSs could derive from long-term processes of learning and adaptation to slow but constant changes of our body and neural circuits involved in the control of movements and sensations (Thoroughman and Shadmehr 1999, 2000). Indeed, these neurobehavioral factors could be intertwined with other similarly important psychosocial aspects. For instance, the relatively small intrasubject variability observed in IMS (Hilt et al. 2016) could reflect variation in the emotional states of participants which are discriminable by an attentive observer (Montepare et al. 1987). On the other hand, the relative stability of IMS may be associated to personality traits (e.g., knee IMS was associated to increased anxiety [Carpenter et al. 2006]) or even psychiatric condition (e.g., in schizophrenia [Slowiński et al. 2017]). These data are promising in the framework of developing experimental procedures to investigate individual behavior and complement group-level averaged results with potentially important idiosyncratic differences.
In conclusion, we demonstrated that individual differences in the execution of a multijoint action shape the sensorimotor activities during the observation of the same action. This shaping is made visible by our experimental design but should in principle be an ingredient of any multijoint action. Beside the general suggestion that intersubject variability should be considered as a tool rather than a problem, our results force us to redefine the core properties of the motor simulative account. At the same time, we acknowledge that showing generalization to different motor domains or action types is of critical importance. In this regard, a strong parallel can already be drawn with findings in the speech domain. Here, the degree of motor recruitment during listening to syllables scales for the perceived distance between listener and speaker (Bartoli et al. 2015). Moreover, lip corticobulbar excitability during speech listening is greater for speech sounds that are far from the listener’s motor repertoire (Schmitz et al. 2018). Supported by converging results from two relatively far domains of action-perception integration, we propose that the AO effects reflect sensitivity to differences rather than similarities with respect to other’s behavior.
Funding
Ministero Salute e Ricerca Finalizzata 2016 (GR-2016-02361008) and 2018 (GR-2018-12366027) - Giovani Rivercatori to QD; PRIN 2015 and EnTimeMent (H2020-FETPROACT-824160) to LF 2016 & 2018—Giovani Ricercatori to AD; PRIN 2015 and EnTimeMent (H2020-FETPROACT-824160 to LF).
Notes
Conflict of Interest: None declared.
Supplementary Material
References
- Aglioti SM, Cesari P, Romani M, Urgesi C. 2008. Action anticipation and motor resonance in elite basketball players. Nat Neurosci. 11:1109–1116. [DOI] [PubMed] [Google Scholar]
- Ajemian R, Ausilio AD, Moorman H, Bizzi E, Ajemian R, Ausilio AD, Moorman H, Bizzi E, Ajemian R, Ausilio AD et al. 2010. Why professional athletes need a prolonged period of warm-up and other peculiarities of human motor learning why professional athletes need a prolonged period of warm-up and other peculiarities of human motor. Learning. 2895:37–41. [Google Scholar]
- Avenanti A, Annella L, Candidi M, Urgesi C, Aglioti SM. 2013. Compensatory plasticity in the action observation network: virtual lesions of STS enhance anticipatory simulation of seen actions. Cereb Cortex. 23:570–580. [DOI] [PubMed] [Google Scholar]
- Bartoli E, D’Ausilio A, Berry J, Badino L, Bever T, Fadiga L. 2015. Listener-speaker perceived distance predicts the degree of motor contribution to speech perception. Cereb Cortex. 25:281–288. [DOI] [PubMed] [Google Scholar]
- Becchio C, Sartori L, Bulgheroni M, Castiello U. 2008. The case of Dr. Jekyll and Mr. Hyde: a kinematic study on social intention. Conscious Cogn. 17:557–564. [DOI] [PubMed] [Google Scholar]
- Bennequin D, Fuchs R, Berthoz A, Flash T. 2009. Movement timing and invariance arise from several geometries. PLoS Comput Biol. 5:e1000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein NA. 1967. In: Pergamon P, editor. The Coordination and Regulation of Movements. Oxford: Pergamon Press. [Google Scholar]
- Berret B, Bonnetblanc F, Papaxanthis C, Pozzo T. 2009. Modular control of pointing beyond arm’s length. J Neurosci. 29:191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betti S, Castiello U, Sartori L. 2015. Kick with the finger: symbolic actions shape motor cortex excitability. Eur J Neurosci. 42:2860–2866. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Frith C. 2005. The role of motor contagion in the prediction of action. Neuropsychologia. 43:260–267. [DOI] [PubMed] [Google Scholar]
- Calvo-Merino B, Ehrenberg S, Leung D, Haggard P. 2010. Experts see it all: configural effects in action observation. Psychol Res. 74:400–406. [DOI] [PubMed] [Google Scholar]
- Calvo-Merino B, Glaser DE, Grèzes J, Passingham RE, Haggard P. 2005. Action observation and acquired motor skills: an fMRI study with expert dancers. Cereb Cortex. 15:1243–1249. [DOI] [PubMed] [Google Scholar]
- Calvo-Merino B, Grèzes J, Glaser DE, Passingham RE, Haggard P. 2006. Seeing or doing? Influence of visual and motor familiarity in action observation. Curr Biol. 16:1905–1910. [DOI] [PubMed] [Google Scholar]
- Candidi M, Sacheli LM, Mega I, Aglioti SM. 2014. Somatotopic mapping of piano fingering errors in sensorimotor experts: TMS studies in pianists and visually trained musically naïves. Cereb Cortex. 24:435–443. [DOI] [PubMed] [Google Scholar]
- Cardellicchio P, Hilt PM, Olivier E, Fadiga L, D’Ausilio A. 2018. Early modulation of intra-cortical inhibition during the observation of action mistakes. Sci Rep. 8:1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MG, Adkin AL, Brawley LR, Frank JS. 2006. Postural, physiological and psychological reactions to challenging balance: does age make a difference? Age Ageing. 35: 298–303. [DOI] [PubMed] [Google Scholar]
- Casile A, Dayan E, Caggiano V, Hendler T, Flash T, Giese MA. 2010. Neuronal encoding of human kinematic invariants during action observation. Cereb Cortex. 20:1647–1655. [DOI] [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. 1998. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol. 79:1117–1123. [DOI] [PubMed] [Google Scholar]
- Coste A, Slowinski P, Tsaneva-Atanasova K, Bardy BG, Marin L. 2017. Mapping individual postural signatures In: Weast-Knapp JA, Pepping GJ, editors. Studies in Perception & Action XIV. London: Taylor & Francis. [Google Scholar]
- Cross ES, Kirsch L, Ticini LF, Schütz-Bosbach S. 2011. The impact of aesthetic evaluation and physical ability on dance perception. Front Hum Neurosci. 5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ausilio A, Altenmüller E, Belardinelli OM, Lotze M. 2006. Cross-modal plasticity of the motor cortex while listening to a rehearsed musical piece. Eur J Neurosci. 24: 955–958. [DOI] [PubMed] [Google Scholar]
- D’Ausilio A, Bartoli E, Maffongelli L. 2015. Grasping synergies: a motor-control approach to the mirror neuron mechanism. Phys Life Rev. 12:91–103. [DOI] [PubMed] [Google Scholar]
- D’Ausilio A, Maffongelli L, Bartoli E, Campanella M, Ferrari E, Berry J, Fadiga L. 2014. Listening to speech recruits specific tongue motor synergies as revealed by transcranial magnetic stimulation and tissue-Doppler ultrasound imaging. Philos Trans R Soc B Biol Sci. 369:20130418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan E, Casile A, Levit-Binnun N, Giese MA, Hendler T, Flash T. 2007. Neural representations of kinematic laws of motion: evidence for action-perception coupling. Proc Natl Acad Sci. 104:20582–20587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falck-Ytter T, Gredebäck G, Von Hofsten C. 2006. Infants predict other people’s action goals. Nat Neurosci. 9:878–879. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Johansson RS. 2003. Action plans used in action observation. Lett to Nat. 424:769–771. [DOI] [PubMed] [Google Scholar]
- Flash T, Hogans N. 1985. The coordination of arm movements: an experimentally confirmed mathematical model. J Neurosci. 5:1688–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. 2010. The free-energy principle: a unified brain theory? Nat Rev Neurosci. 11:127–138. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Mattout J, Kilner JM. 2011. Action understanding and active inference. Biol Cybern. 104:137–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah R, Rocchi L, Rothwell J. 2018. Observing without acting: a balance of excitation and suppression in the human corticospinal pathway? Frontiers in neuroscience. 12:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilt PM, Bartoli E, Ferrari E, Jacono M, Fadiga L, D’Ausilio A. 2017. Action observation effects reflect the modular organization of the human motor system. Cortex. 95:104–118. [DOI] [PubMed] [Google Scholar]
- Hilt PM, Berret B, Papaxanthis C, Stapley P, Pozzo T. 2016. Evidence for subjective values guiding posture and movement coordination in a free-endpoint whole-body reaching task. Sci Rep. 6:23868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak FB, Nashner LM. 1986. Central programming of postural movements: adaptation to altered support-surface configurations. J Neurophysiol. 55:1369–1381. [DOI] [PubMed] [Google Scholar]
- Hunnius S, Bekkering H. 2014. What are you doing? How active and observational experience shape infants’ action understanding. Philosophical Transactions of the Royal Society B: Biological Sciences. 369:20130490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jola C, Abedian-Amiri A, Kuppuswamy A, Pollick FE, Grosbras MH. 2012. Motor simulation without motor expertise: enhanced corticospinal excitability in visually experienced dance spectators. PLoS One. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM. 2012. More than one pathway to action understanding. Trends Cogn Sci. 15:352–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koul A, Cavallo A, Ansuini C, Becchio C. 2016. Doing it your way: how individual movement styles affect action prediction. PLoS One. 11:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard JA, Brown RH, Stapley PJ. 2009. Reaching to multiple targets when standing: the spatial organization of feedforward postural adjustments. J Neurophysiol. 101:2120–2133. [DOI] [PubMed] [Google Scholar]
- Macerollo A, Bose S, Ricciardi L, Edwards MJ, Kilner JM. 2015. Linking differences in action perception with differences in action execution. Soc Cogn Affect Neurosci. 10:1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montepare JM, Goldstein SB, Clausen A. 1987. The identification of emotions from gait. J Nonverbal Behav. 11:33. [Google Scholar]
- Naish KR, Houston-Price C, Bremner AJ, Holmes NP. 2014. Effects of action observation on corticospinal excitability: muscle specificity, direction, and timing of the mirror response. Neuropsychologia. 64:331–348. [DOI] [PubMed] [Google Scholar]
- Palmer CE, Bunday KL, Davare M, Kilner JM. 2016. A causal role for primary motor cortex in perception of observed actions. J Cogn Neurosci. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. 2004. The mirror-neuron system. Annu Rev Neurosci. 27:169–192. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. 2001. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci. 2:1–10. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Sinigaglia C. 2016. The mirror mechanism: a basic principle of brain function. Nat Rev Neurosci. 17:757–765. [DOI] [PubMed] [Google Scholar]
- Romani M, Cesari P, Urgesi C, Facchini S, Aglioti SM. 2005. Motor facilitation of the human cortico-spinal system during observation of bio-mechanically impossible movements. NeuroImage. 26:755–763. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. 2009. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 120:2008–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartori L, Betti S, Chinellato E, Castiello U. 2015. The multiform motor cortical output: kinematic, predictive and response coding. Cortex. 70:169–178. [DOI] [PubMed] [Google Scholar]
- Schmidt RA, Lee TD. 1999. Motor Control and Learning: A Behavioral Approach. Champaign, IL: Human Kine. [Google Scholar]
- Schmidt RA, Young DE. 1986. Transfer of movement control in motor skill training In: Cormier SM, Hagman JD, editors. Transfer of Learning. Orlando: Academic Press. [Google Scholar]
- Schmitz J, Bartoli E, Maffongelli L, Fadiga L, Sebastian-Galles N, D’Ausilio A. 2019. Motor cortex compensates for lack of sensory and motor experience during auditory speech perception. Neuropsychologia. 128:290–296. [DOI] [PubMed] [Google Scholar]
- Slowiński P, Alderisio F, Zhai C, Shen Y, Tino P, Bortolon C, Capdevielle D, Khoramshahi M, Billard A et al. 2017. Unravelling socio-motor biomarkers in schizophrenia. npj Schizophrenia. 3:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Słowiński P, Zhai C, Alderisio F, Salesse R, Gueugnon M, Marin L, Bardy BG, di Bernardo M, Tsaneva-Atanasova K, Slowinski P et al. 2016. Dynamic similarity promotes interpersonal coordination in joint action. J R Soc Interface. 13:20151093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano M, Cavallo A, D’Ausilio A, Becchio C, Fadiga L. 2018. Movement kinematics drive chain selection toward intention detection. Proceedings of the National Academy of Sciences. 115:10452–10457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapel JC, Hunnius S, van Elk M, Bekkering H. 2010. Motor activation during observation of unusual versus ordinary actions in infancy. Soc Neurosci. 5:451–460. [DOI] [PubMed] [Google Scholar]
- Stapley P, Pozzo T, Grishin A. 1998. The role of anticipatory postural adjustments during whole body forward reaching movements. Neuroreport. 9:395–401. [DOI] [PubMed] [Google Scholar]
- Thoroughman KA, Shadmehr R. 1999. Electromyographic correlates of learning an internal model of reaching movements. J Neurosci. 19:8573–8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoroughman KA, Shadmehr R. 2000. Learning of action through adaptative combination of motor primitives. Nature. 407:742–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elk M, van Schie HT, Hunnius S, Vesper C, Bekkering H. 2008. You’ll never crawl alone: neurophysiological evidence for experience-dependent motor resonance in infancy. NeuroImage. 43:808–814. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


