Dear Editor,
We applaud Luo et al 1 for describing their experience with tocilizumab (TCZ) as a treatment option for severe coronavirus disease 2019 (COVID‐19). Patients with COVID‐19 have been reported to have elevated interleukin‐6 (IL‐6) levels. 1 , 2 TCZ, an IL‐6 receptor antibody, has emerged as a viable treatment option in severe COVID‐19 with no acute adverse events described to date. 1 , 2 These safety observations coincide with literature assessing short courses of TCZ in patients receiving chimeric antigen receptor T‐cell therapy that develop cytokine release syndrome. 3 Chronic use of TCZ in rheumatoid arthritis (RA) has been shown to increase lipid parameters, in particular triglycerides. 4 Furthermore, acute pancreatitis (AP) has been associated with chronic TCZ treatment of RA with the development of AP described as early as 2 weeks after initiating therapy. 5 Literature for acute indications of TCZ does not report triglycerides or parameters for AP. 2 , 3 Thus, little is known about the short‐term adverse effects and what parameters should be monitored in those with COVID‐19 receiving TCZ. We have used TCZ in patients with severe COVID‐19 with elevated inflammatory markers (IL‐6, lactate dehydrogenase, d‐dimer, ferritin), and severe acute respiratory distress syndrome (ARDS) with no alternative diagnosis. Here, we outline two cases of acute hypertriglyceridemia in patients with COVID‐19 treated with TCZ (Figure 1), one with elevated inflammatory biomarkers consistent with AP the other without.
Figure 1.
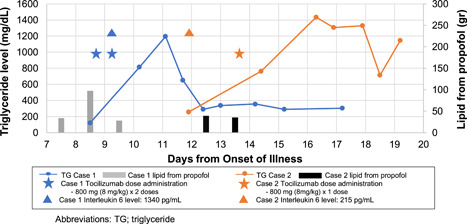
Clinical time course
Case 1 was a 65‐year‐old male admitted to the intensive care unit (ICU) with respiratory failure and ARDS 8 days after symptom onset. At that time, he received lopinavir/ritonavir, ribavirin, and hydroxychloroquine. Sedation was provided with a propofol infusion. TCZ was administered on days 9 and 10 due to persistent fevers, severe ARDS, and elevated inflammatory markers. Propofol was discontinued on day 10 before the second dose of TCZ. On day 11, serum TG levels (1196 mg/dL) and AP biomarkers (amylase: 309 IU/L, lipase: 104 IU/L) were significantly increased. Case 2 was a 43‐year‐old male admitted to the ICU with respiratory failure and ARDS 12 days after symptom onset and was also treated with lopinavir/ritonavir, ribavirin, and hydroxychloroquine. Sedation was provided with propofol. TCZ was initiated on day 13 for persistent fevers, severe ARDS, and elevated inflammatory markers. Propofol was changed to midazolam on day 13, 6 hours before initiating TCZ. After TCZ administration, serum TG levels peaked on day 16 (1436 mg/dL) and AP biomarkers remained normal (amylase: 47 IU/L, lipase: 58 IU/L). In both cases HLH was lower on the differential using the HLH‐2004 diagnostic criteria and HLH‐probability calculator, enteral feeding had not been initiated, and early rapid increases of TG was felt to be uncharacteristic of short courses of propofol (case 1: 54 hours with dose ranging from 5 to 60 µg/kg/min, case 2: 28 hours with dose ranging from 5 to 55 µg/kg/min). The Naranjo Probability Scale assigned a probable (score: 7) relationship between TCZ and hypertriglyceridemia for both cases. Due to safety concerns in the transporting of critically ill patients and infection control implications of patients with COVID‐19, imaging was not conducted in either case to asses for AP.
These cases highlight several important monitoring parameters, pharmacotherapy, and diagnostic considerations regarding the care of critically ill patients with COVID‐19 receiving TCZ. IL‐6 has both immunomodulatory and metabolic actions. Acute IL‐6 elevations mobilize free fatty acid via adipocytes. 6 IL‐6 stimulates skeletal muscle uptake of glucose and free fatty acid from the serum. 7 The exact mechanism by which chronic TCZ use contributes to TG elevations remains to be elucidated. 4 Membrane‐bound and soluble IL‐6 receptor inhibition by acute TCZ administration may result in increased TG levels by interfering with these metabolic pathways. While propofol is known to increase TGs secondary to the lipid emulsion vehicle, this effect is typically seen 2.25 to 7 days after initiating therapy with normalization occurring within 72 hours. 6 , 8 , 9 This population may be more prone to hypertriglyceridemia development with propofol use given the metabolic activities of IL‐6 and may warrant more frequent monitoring.
TCZ is progressing as a viable and promising treatment option in patients with severe COVID‐19. Given the paucity of robust clinical trial data for most COVID‐19 pharmacotherapies at this time, clinicians should continue to remain steadfast in recognition of interventions that improve clinical outcomes and vigilant in monitoring for acute adverse effects that are difficult to detect in clinical trials with small sample sizes. 10 The observations from our two cases highlight the complex, not fully elucidated interrelationship between elevated IL‐6 and pharmacologic interventions impacting this pathway. Clinicians should consider monitoring for hypertriglyceridemia described with chronic TCZ use in patients with COVID‐19 treated with TCZ.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
All authors had a role in writing the letter.
REFERENCES
- 1. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID‐19: a single center experience. J Med Virol. 2020. 10.1002/jmv.25801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCreary EK, Pouge JM. COVID‐19 treatment: a review of early and emerging options. Open Forum Infect Dis. 2020;7(4):ofaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Le RQ, Li L, Yuan W, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell‐induced severe or life‐threatening cytokine release syndrome. Oncologist. 2018;23(8):943‐947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Giles JT, Sattar N, Gabriel S, et al. Cardiovascular safety of tocilizumab versus etanercept in rheumatoid arthritis: a randomized controlled trial. Arthritis Rheumatol. 2020;72(1):31‐40. [DOI] [PubMed] [Google Scholar]
- 5. Flaig T, Douros A, Bronder E, Klimpel A, Kreutz R, Garbe E. Tocilizumab‐induced pancreatitis: case report and review of data from the FDA Adverse Event Reporting System. J Clin Pharm Ther. 2016;41(6):718‐721. [DOI] [PubMed] [Google Scholar]
- 6. Glund S, Krook A, Pannatier A. Role of interleukin‐6 signaling in glucose and lipid metabolism. Acta Physiol. 2008;192(1):37‐48. [DOI] [PubMed] [Google Scholar]
- 7. Devaud JC, Berger MM, Pannatier A, et al. Hypertriglyceridemia: a potential side effect of propofol sedation in critical illness. Intensive Care Med. 2012;38(12):1990‐1998. [DOI] [PubMed] [Google Scholar]
- 8. Devlin JW, Lau AK, Tanios MA. Propofol‐associated hypertriglyceridemia and pancreatitis in the intensive care unit: an analysis of frequency and risk factors. Pharmacotherapy. 2005;25(10):1348‐1352. [DOI] [PubMed] [Google Scholar]
- 9. Barrientos‐Vega R, Mar Sánchez‐Soria M, Morales‐García C, Robas‐Gómez A, Cuena‐Boy R, Ayensa‐Rincon A. Prolonged sedation of critically ill patients with midazolam or propofol: impact on weaning and costs. Crit Care Med. 1997;25(1):33‐40. [DOI] [PubMed] [Google Scholar]
- 10. Singh S, Loke YK. Drug safety assessment in clinical trials: methodological challenges and opportunities. Trials. 2012;13:138. [DOI] [PMC free article] [PubMed] [Google Scholar]


