Abstract
The outstanding role of spermine in eliciting defense adaptation of soybean to different levels of water deficit (0, -0.1, -0.5 and -1.1 MPa) was investigated by determining the changes in growth, photosynthetic pigments, osmolytes, water relations, and antioxidants. All the studied traits clearly revealed cultivar-dependent variation in response to water deficit where cv. Giza 111 was tolerant and cv. Giza 21 was sensitive. Both cultivars came in agreement that photosynthetic limitation (chlorophylls reduction) was the troubling shot induced by water deficit. Such limitation was reflected on three directions (a) disturbances of water relations (stomatal conductance, transpiration rate, relative water content and water use efficiency), (b) down regulation of metabolites which affect osmotic adjustment and (c) elevated reactive oxygen species (increased hydrogen peroxide) and destruction of membrane stability (increment of electrolyte leakage and lipid peroxidation). The damaging impacts of water deficit on these parameters were obviously coined for sensitive cultivar compared to tolerant one. Although spermine priming did not have apparent stimulatory role on well-watered plants, unequivocal inversion from a state of down regulation to up-regulation was distinct under water stress. In this regard, spermine enhanced pigments, osmolytes accumulation, up-regulated water relations and enhanced membrane stabilization. Furthermore, spermine pre-sowing decreased oxidative stress by lowering hydrogen peroxide via activation of anthocyanins, total antioxidants and phenolic compounds.
Keywords: Agricultural science, Biochemistry, Biotechnology, Ecology, Physiology, Plant biology, Environmental science, Earth sciences, Hydrology, Water deficit, Pigments, Water relation, Spermine, Osmolyte, Antioxidants
Agricultural science, Biochemistry, Biotechnology, Ecology, Physiology, Plant biology, Environmental science, Earth sciences, Hydrology, Water deficit, Pigments, Water relation, Spermine, Osmolyte, Antioxidants
1. Introduction
Soybean is considered as one of the oldest agricultural plants which contain abundant amounts of proteins, carbohydrates, oil, phosphorus, calcium, iron, magnesium, zinc, fibres, and vitamins (Akparobi, 2009). The agricultural productivity of soybean is decreased by a wide array of environmental stresses especially low water availability. Traditional breeding programs have aimed to improve the growth of plants against abiotic stress environments, but with limited success (Richards, 1996) especially under unexpected global climate change, limited water resources and unpredictable rainfall. Such climate changes will increase the impact of water shortage on plants (Sallam et al., 2019b). Furthermore, an increasing frequency of droughts in days ahead will make natural and cultivated vegetation more vulnerable to severe and acute shortage of water (Nawaz et al., 2012). Drought stress imposes drastic effects on plant growth, development, agronomic and yield traits (Sallam et al., 2019a) by altering physio-anatomical mechanisms (Anjum et al., 2017). It disturbs plant-water relations, photosynthetic gas exchange capacities, cell turgor, source-sink relationships and various metabolic processes in plants (Anjum et al., 2011, 2017). This was synchronized with the generation of reactive oxygen species which induces oxidative stress and damages proteins, membrane lipids, and other cellular components and activated other biochemical reactions (Behnamnia, 2015; Sallam et al., 2019a).
Researchers globally seek to apply eco-friendly regulators for crop development against various abiotic and biotic stresses (Dawood et al., 2019). The development of plant growth and preservation condition is a prevalent approach in agriculture, should be parallel to lessening the utility of hazardous chemicals products (Younes et al., 2020). During the last decade, the foliar application of plant growth regulators and biomolecules, such as polyamines (PAs) has become an established procedure in crop production to increase yield and quality of the crop under abiotic stresses as drought (Shallan et al., 2012; Ahmed et al., 2017). During the stress, the signaling properties of polyamines allow adjusting ion homeostasis and ion movement via reacting with ion channel (Podlešáková et al., 2019). So, PAs besides their ability to manage natural plant developmental and physiological pathways, they withstand striking role in abiotic stress tolerance (Hasanuzzaman et al., 2014). Thus, improving the expression of PAs synthetic genes and enhancing PAs production could mitigate stress-induced negative impacts on plant (Liu et al., 2015). Polyamines, mainly diamine putrescine (Put), triamine spermidine (Spd) and tetraamine spermine (Spm), are low molecular weight aliphatic polycations and are ubiquitously distributed in all living organisms (Minocha et al., 2014; Li et al., 2018). Polyamines are involved in many physiological processes, such as cell division, growth and development, and respond to stress tolerance to various environmental factors (Liu et al., 2007; Kusano et al., 2008). Tanou et al. (2014) illustrated that PAs have a vital role in up-regulation of the cellular redox status, the transcript expression and the action of antioxidant enzymes. In addition, PAs may participate in cellular signals, thus manage plant tolerance against the stress (Tanou et al., 2014; Pál et al., 2015). A number of studies have demonstrated that the application of exogenous PAs can improve drought tolerance (Alcázar et al., 2010; Radhakrishnan and Lee, 2013; Yin et al., 2014; Li et al., 2018). Numerous studies are available for outstanding the role of spermine, distinct from the other major polyamines, in eliciting an effective defense response to biotic and abiotic stresses. These studies revealed that spermine functions differently during biotic and abiotic interactions in the regulation of oxidative homeostasis and phytohormone signaling (Seifi and Shelp, 2019). Spermine application may increase the expression levels of the stress-related genes that protect seedlings from stress damage (Pál et al., 2015). Furthermore, considering that Spm contains four nitrogen groups, it could provide greater buffering capacity than Spd and Put (Shi et al., 2010). This is in agreement with previous studies that report exogenous Spm, unlike Spd and Put, has a potent anti-senescence effect on plants (Serafini-Fracassini et al., 2010). So far, it is decisive to further studying the physiological roles of spermine on many plants to address its effects on osmolyte accumulation and water relations. Accordingly, the aim of the present investigation was to examine the fundamental role of spermine in enhancing soybean tolerance against different levels of water deficit.
2. Materials and methods
2.1. Experimental design
The experiment was conducted at Botany and Microbiology Department, Faculty of Science, Assuit University, Egypt during the year 2016. The seeds of two soybean cultivars (namely, Giza 111 and Giza 21), were supplied from Faculty of Agriculture, Assiut University, Assuit, Egypt, were surface sterilized with 0.2 % HgCl2 solution for 5 min and thoroughly rinsed with distilled water. Sterilized seeds were soaked in 0.2 mM spermine for 48 h at 27 ± 2 °C and control plants were soaked in distilled water where the ratio of seed weight to solution volume was 1:5 (w/v). Treated or untreated seeds were sown in pots (20 cm diameter and 25 cm height) containing 1 Kg air-dry soil (sand/clay 1:2 v/v) in rate of 3 seeds/pot. The plants were kept in the greenhouse during experimentation to secure mild climatic conditions for 8 weeks where the soil water content was maintained near field capacity. Meteorological data (average values) recorded at Botany and Microbiology Department, Faculty of Science, Assuit University, Egypt were recorded in Table 1.
Table 1.
Meteorological data (average values) recorded at Botany and Microbiology Department, Faculty of Science, Assiut University, Egypt during the year of 2016.
| June |
July |
August |
|||||
|---|---|---|---|---|---|---|---|
| Day | Night | Day | Night | day | night | ||
| Temperature °C | Optimum | 35 | 25 | 37 | 27 | 39 | 30 |
| Minimum | 30 | 20 | 32 | 22 | 33 | 25 | |
| Maximum | 40 | 30 | 42 | 32 | 44 | 35 | |
| Relative humidity, % | 48 | 52 | 50 | 55 | 53 | 57 | |
| Rain fall, mm | 0 | 0 | 0 | ||||
| Photoperiod, h | 12 | 12 | 13 | 11 | 14 | 10 | |
| High intensity, ΔT, lux | 2000 | 2300 | 2500 | ||||
Afterwards, each group was divided into four sub-groups corresponding to four different levels of water stress (0, -0.1, -0.5 and -1.1 MPa). Five pots per treatment were conducted. The moisture content of the soil in each pot was adjusted gravimetrically by decreasing the availability of water to the desired levels. Soil water content can be obtained by characteristic curve data of soil moisture desorption curve achieved by the pressure plate technique (Richards, 1947). The plants were kept under the assigned levels for 12 days before starting the measurements.
2.2. Plant analyses
2.2.1. Growth analysis
Fresh weight of the harvested plants was determined immediately then oven dried at 60 °C for 2 days to a constant weight to evaluate dry weight.
2.2.2. Determination of chlorophyll parameters
Chlorophyll a and b were extracted from a definite weight of fresh healthy leaves suspended in 5 ml of 95% ethyl alcohol at 60–70 °C in water bath. Absorbance readings were followed with a spectrophotometer (Unico UV-2100 spectrophotometer). Chlorophyll a and b were calculated as mg/g FW at 663 and 644 nm using equations recommended by Lichtenthaler (1987). Chlorophyll stability index (CSI) calculated according to Murty and Majumder (1962) as the ratio of chlorophyll content in water heated leaf (56 ± 1 °C) to that in fresh leaf, expressed as a percentage.
2.2.3. Preparation of leaf extraction for metabolites and osmotic pressure
The fresh shoots were dried in an oven at 70 °C for 24 h, and then the dry shoots were ground into fine powder and placed in distilled water. The extraction technique was adopted from El-Sharkawi and Michel (1977).
2.2.3.1. Determination of osmolytes
In the plant extract, soluble hydrolysable carbohydrates (in water extract) and total carbohydrates were hydrolyzed into simple sugars by acid hydrolysis (HCl) using procedures described by Dubois et al. (1956). 0.2 ml of extract was completed to 2 ml with distilled water and then add 1 ml 5% phenol followed by 5 ml concentrated H2SO4 rapidly. The tubes were placed in water bath at 25–30 °C for 10–20 min before reading the developed color at 490 nm.
Proteins were determined using alkaline reagent solution according to the method of Lowery et al. (1951). Five ml of the alkaline reagent were added to 0.1 ml of the water extract. The tubes were mixed and allowed to stand at room temperature for 10 min and then add 0.5 ml of diluted Folin Ciocalteau's reagent (1:2 v/v) and were mixed rapidly. After 30 min, the extinction of blue color developed against appropriate blank was read at 750 nm. A calibration curve was constructed using bovine serum albumin and the data were expressed as mg protein/g DW.
Free amino acids were determined according to the method of Moore and Stein (1948) in the previous water extract of soluble sugars and proteins. One ml of Stannus Chloride reagent was mixed with 0.5 ml of the water extract and then the tubes were boiled in water bath for 20 min and then cooling. Four ml of diluent solvent was added and mixed rapidly. The extinction of violet color was recorded at 570 nm against blank containing all the above reagents and distilled water instead of the extract of plant sample. A calibration curve was constructed using glycine and the data were expressed as mg amino acid (glycine)/g D.W.
2.2.3.2. Determination of osmotic pressure
The osmotic pressure of leaf sap was measured by the cryoscopic method (Walter, 1949) and described by El-Sharkawi and Abdel-Rahman (1974). The plant sap was obtained by crushing portions of washed leaves according to Scholander et al. (1966). The sap obtained used for measurement of osmotic potential by the cryoscopic method of Walter (1931a) using a Beckman differential thermometer (calibrated to 0 ± 01 °C). The correction of Walter and Thren (1934) and Walter (1936) for the super-cooling was used to calculate the real freezing point depression. The osmotic potentials (-bar) were then obtained from tables compiled by Walter (1931b).
2.2.4. Water relations
2.2.4.1. Relative water content (RWC)
Relative water content was calculated according to Turner (1981) using the following equation
| RWC = [(FW-DW)/(TW-DW)]×100 | (1) |
Where FW = Fresh weight, TW = Turgid weight measured after 24 h of saturation on deionized water at 4 °C in the dark and DW = dry weight.
2.2.4.2. Water use efficiency (WUE)
Prior to every irrigation, each pot was weighed and the weight differences (kg) were converted to volume (ml). The values obtained for each pot represented the volume of water applied to that particular pot at that period. The average volume of the water used rate was determined for each cultivar. The water use efficiency based on biomass was calculated according to Larcher (2003) as follows:
| WUE (g/kg) = Biomass (g/plant)/Water use rate (kg/plant) | (2) |
2.2.4.3. Transpiration rate
Transpiration rate (TP) was expressed as g water loss/cm2 leaf area/hour according to the equation adopted by Bozcuk (1975). The daily transpiration rate (TP, g day−1) per pot was measured using the gravimetric method. TP is the transpiration rate on day i (g), Wi is the weight of the entire pot after the irrigation on day i (g), and W`i+1 is the weight of the entire pot before irrigation on day i+1 (g). Irrigation was carried out by adding the same amount of water as had been lost through transpiration (i.e., TP). The total TP per pot was divided per number of plants in each pot based on their final plant biomass and the TP and calculated using the following equation.
| Transpiration rate (TP) = Wi-W`i+1 | (3) |
2.2.4.4. Leaf stomatal conductance
Leaf stomatal conductance is the inverse of the stomatal resistance. The stomatal resistance can be calculated from the following equation which has been applied by Slatyer (1967), and as adopted by El-Sharkawi (1981) were
| (4) |
where = r. is the total (stomatal) resistance at the leaf-air interface, then
| (5) |
where: T. = transpiration rate (mg. H2O/cm2/sec), r. = total stomatal resistance (sec/cm), Cleaf = concentration of water vapor in leaf (absolute humidity) (mg/cm3), Cair = concentration of water vapor in air (mg/cm3), eleaf = vapor pressure inside leaf (mm Hg) and eair = vapor pressure of air (mm Hg).
= which is the vapour pressure deficit between the leaf and air bulk outside. The term 0.622 p/p. is a conversion factor to change from c () to . It has a value of approximately 10−6, so 1 mm of vapor pressure is equivalent to approximately 1 mg of water vapor per liter of air. If most of the stomata are on one surface of a leaf, r. will differ significantly for the upper and lower surfaces (Holmgren et al., 1965).
2.2.5. Membrane damage traits
2.2.5.1. Electrolyte leakage
Electrolyte leakage (EC %) was estimated as given by Premachuandra et al. (1992). Fresh leaf discs of the test plants washed with deionized water and submerged in 30 ml deionized distilled water for 24 h at 10 °C. The electrical conductivity (conductimeter, YSI Model 35 Yellow Springs, OH, USA) of the bathing solution was measured at 25 °C. Leaf discs were autoclaved for 15 min, cooled to 25 °C, and the electrical conductivity was measured. Electrolyte leakage was evaluated as a percentage injury according to the following equation:
| EC % = (C1/C2) × 100 | (6) |
where C1 and C2 are the electrolyte conductivities measured before and after autoclaving, respectively.
2.2.5.2. Lipid peroxidation
Lipid peroxidation was determined by measuring “malondialdehyde” (MDA) formation using the “thiobarbituric acid (TBA) reaction” as described by Madhava Rao and Sresty (2000). Fresh leaves were homogenized in 0.1 % trichloroacetic acid (TCA) and ten centrifuged for 10 min at 10,000 rpm. 1 ml of the supernatant was mixed with TCA-TBA reagent. The mixture was heated on water bath at 95 °C for 30 min and then cooled rapidly on an ice-bath. Afterwards, the mixture was centrifuged at 10,000 rpm for 15 min and the absorbance of the supernatant was monitored at 532 nm. The concentration of MDA was calculated by using an extinction coefficient (155 mM) and the results expressed as μg/g fresh weight (FW). The concentration of MDA was calculated by using an extinction coefficient (155 mM) and the results expressed as μmol/g FW.
2.2.6. Hydrogen peroxide (H2O2)
Hydrogen peroxide content of leaves samples was measured spectrophotometrically as described by Mukherjee and Choudhuri (1983). The concentration of H2O2 was calculated from a standard curve plotted with known concentration of H2O2 and expressed as μmol/g FW. Fresh leaves were grinded in cold acetone (4 ml). Three ml of the acetone extract was mixed with 1 ml of 0.1 % titanium dioxide in 20 % H2SO4 and the mixture was then centrifuged at 6000 rpm for 15 min. The yellow color developed was measured at 415 nm.
2.2.7. Non-enzymatic antioxidants
2.2.7.1. Phenolic compound content
Phenolic content was determined according to Kofalvi and Nassuth (1995) using the Folin-Ciocalteu's phenol reagent. Hundred μl of the methanol extract were diluted to 1 ml with distilled water and mixed with 0.5 ml of 2 N Folin-Ciocalteu's reagent and 2.5 ml of 20 % Na2CO3. The mixture was left for 20 min at room temperature, and then the absorbance of samples was measured at 725 nm with Unico UV-2100 spectrophotometer. Phenolic concentration in methanol extract was determined from standard curve prepared with gallic acid and the data expressed as mg/gm FW.
2.2.7.2. Total antioxidant
The total antioxidant contents were elicited based on Prieto et al. (1999). Methanolic extract and reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate); was incubated at 95 °C for 90 min, and then cooled to room temperature. The absorbance was measured at 695 nm and the data were expressed in terms of ascorbic acid equivalents as μg g−1 dry matter using molar coefficient of 0.997 μg−1 cm−1 ml−1.
2.2.7.3. Anthocyanin pigments
Anthocyanin pigments were determined according to Krizek et al. (1993) on acidified methanol (1% HCl v/v) extract of leaves. Anthocyanin content was expressed as μmol/g FW using extinction coefficient of 33.000 mol−1 cm−1.
2.2.8. Statistical analysis
The data were subjected to one-way ANOVA using SPSS 18.0 software program. Means were calculated for three replicate values. Means were compared by the Duncan's multiple range tests and statistical significance was determined at 5 % level.
Declaration
I hereby declare that the used chemicals or species in the current study need no approval or permission to be used.
3. Results
3.1. Growth analysis
he data in Table 2 illustrated that water-deficit adversely reduced growth of cultivars Giza 111 and Giza 21 as expressed in reduction of fresh and dry mass as well as inhibition of chl a, chl b and chlorophyll stability index which was prominent for cv. Giza 21 compared to cv. Giza 111. The damaging effect of drought was restrained by spermine priming to be more or less comparable to control especially at moderate water stress levels. Although spermine neither prompted biomass (fresh or dry) nor pigment composition for normally irrigated plants, the stimulatory effect of spermine on biomass and pigments increased as water shortage increased in the soil.
Table 2.
Fresh weight, dry weight, chlorophyll a (Chl a), chlorophyll b (Chl b) and chlorophyll stability index (CSI) of cv. Giza 111 and cv. Giza 21 under different water levels as affected by 0.2 mM spermine priming. Each value represents a mean value of three replicates ±SE. values with different letters are significantly different at P < 0.05.
| FWT (g/plant) |
DWT (g/plant) |
Chl a (mg/g FW) |
Chl b (mg/g FW) |
CSI (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Giza 111 | Giza 21 | Giza 111 | Giza 21 | Giza 111 | Giza 111 | Giza 21 | Giza 111 | Giza 111 | Giza 21 | ||
| Water priming | 0 | 10.33b ± 1 | 0.86a±0.03 | 0.65d ± 0.014 | 0.29b ± 0.003 | 0.86b ± 0.03 | 0.65d ± 0.014 | 0.29cb ± 0.003 | 3.2a±0.05 | 7c±0.21 | 5d ± 0.3 |
| -0.1 | 9.67b ± 2 | 0.75ab±0.01 | 0.47c±0.011 | 0.25ab±0.001 | 0.75ab±0.01 | 0.47c±0.011 | 0.25b ± 0.001 | 5c±0.2 | 6b ± 0.18 | 4.1c±0.4 | |
| -0.5 | 8.33a±1.2 | 0.64b ± 0.0142 | 0.31b ± 0.004 | 0.20a±0.00142 | 0.64a±0.0142 | 0.31cb ± 0.004 | 0.20a±0.00142 | 5.2c±0.1 | 5a±0.05 | 2.7b ± 0.5 | |
| -1.1 | 7.67a±2 | 0.6b ± 0.004 | 0.28a±0.003 | 0.18a±0.003 | 0.6a±0.004 | 0.28a±0.003 | 0.18a±0.003 | 5.7c±0.1 | 4.7a±0.141 | 1.9a±0.03 | |
| Spermine Priming | 0 | 11b ± 1.9 | 0.83a±0.03 | 0.77e±0.02 | 0.3b ± 0.003 | 0.83b ± 0.03 | 0.77e±0.02 | 0.3c±0.003 | 3a±0.05 | 6.9cb ± 0.207 | 6.4e±0.3 |
| -0.1 | 10.5b ± 2 | 0.83a±0.026 | 0.71d ± 0.017 | 0.27ab±0.0026 | 0.83b ± 0.026 | 0.71e±0.017 | 0.27b ± 0.0026 | 4b ± 0.08 | 7c±0.35 | 5.4d ± 0.2 | |
| -0.5 | 9.83b ± 1.1 | 0.75ab±0.03 | 0.66d ± 0.03 | 0.23ab±0.003 | 0.75ab±0.03 | 0.66ed±0.03 | 0.23b ± 0.003 | 4b ± 0.1 | 7c±0.28 | 3.8c±0.1 | |
| -1.1 | 10b ± 1.5 | 0.70b ± 0.006 | 0.60d ± 0.023 | 0.20a±0.006 | 0.70a±0.006 | 0.60d ± 0.023 | 0.20a±0.006 | 3a±0.08 | 7.9d ± 0.237 | 2.5b ± 0.1 | |
3.2. Osmolytes and total osmotic potential
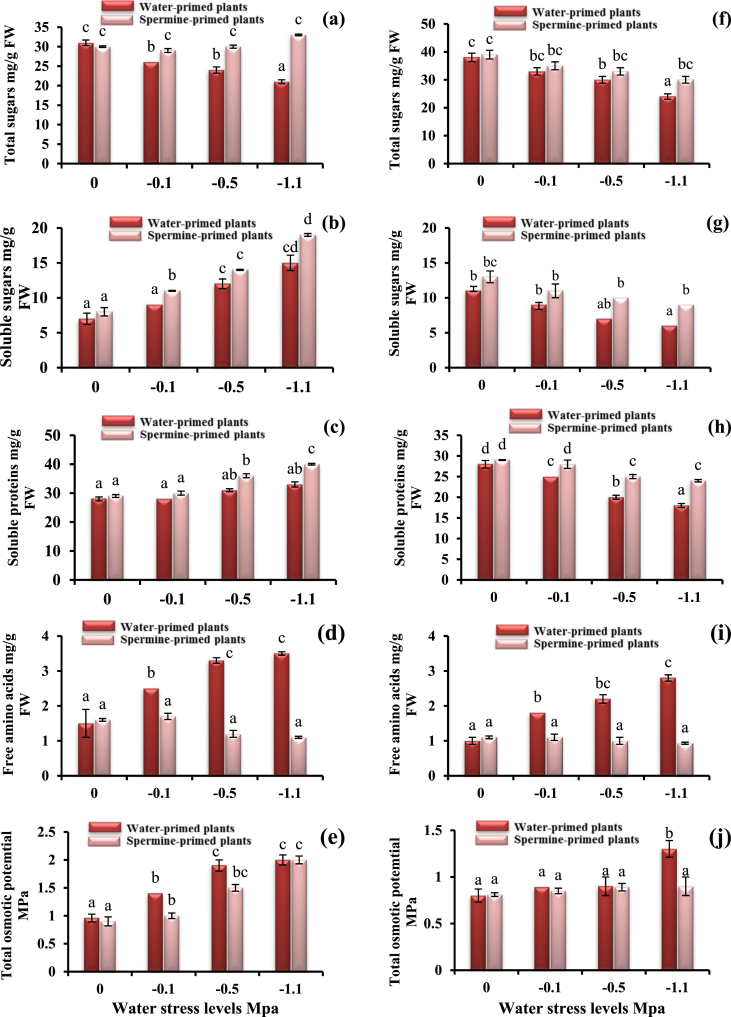
As depicted in Figure 1, water deficit exhibited gradual inhibition of total sugars (Figure 1 a and f) for both cultivars which was more pronounced at the level of -1.1 MPa, whilst soluble sugars and soluble proteins increased for cv. Giza 111 and reduced for cv. Giza 21 (Figure 1 b, c, g and h). On the other hand, free amino acids (Figure 1 d and i) promoted under water stress, but more pronounced for cv. Giza 21 compared to control. For both cultivars, spermine priming significantly enhanced total sugars, soluble sugars and soluble proteins, whilst reduced free amino acids under deficit-water. Total osmotic potential (Figure 1 e and j) vastly enhanced for both cultivars comprehensively for cv. Giza 111 more than cv. Giza 21. Spermine treatment reduced total osmotic potential compared to the drought level to be mainly around the control values.
Figure 1.
Soluble carbohydrates, total carbohydrates, soluble proteins, free amino acids and total osmotic potential of cv. Giza 111 (a, b, c, d, e) and (f, g, h, i, j) for cv. Giza 21 under different matric potential levels as affected by 0.2 mM spermine priming. Each histogram represents a mean value of three replicates, and the vertical bars indicate ±SE. Bars carrying different letters are significantly different at P < 0.05.
3.3. Water relations
The data in Table 3 revealed that RWC and WUE (calculated based on Eqs. (1) and (2), respectively) found to be inhibited slightly for cv. Giza 111 and dramatically for cv. Giza 21 as the water availability reduced in the soil. The transpiration rate and stomatal conductance (calculated based on Eqs. (3), (4), and (5), respectively) reduced for cv. Giza 111 and the reverse cut held was registered for cv. Giza 21. Spermine priming did not affect stomatal conductance either control or different moisture levels, whilst a promotion of WUE and RWC was encountered by the used genotypes especially under water stress. Spermine reduced transpiration rate for both genotypes to be lower than the corresponding level.
Table 3.
Transpiration rate, stomatal conductance, relative water content (RWC) and water use efficiency (WUE) of cv. Giza 111 and cv. Giza 21 under different water levels as affected by 0.2 mM spermine priming. Each value represents a mean value of three replicates ±SE. values with different letters are significantly different at P < 0.05.
| Relative water content (%) |
Transpiration rate (μmol m−2s−1) |
Water use efficiency (mg DW/H2O loss) |
Stomatal conductance (mol m−2s−1) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Giza 111 | Giza 21 | Giza 111 | Giza 21 | Giza 111 | Giza 21 | Giza 111 | Giza 21 | ||
| Water priming | 0 | 89d ± 1.2 | 88e±2 | 5c±0.15 | 3.2a±0.05 | 7d ± 0.21 | 5d ± 0.3 | 0.8c±0.024 | 0.69a±0.15 |
| -0.1 | 80c±2.4 | 60b ± 0.77 | 4.3c±0.129 | 5c±0.2 | 6c±0.18 | 4.1c±0.4 | 0.5b ± 0.015 | 0.67a±0.129 | |
| -0.5 | 78b ± 0.87 | 58b ± 0.7 | 2.4b ± 0.029 | 5.2c±0.1 | 5b ± 0.05 | 2.7b ± 0.5 | 0.3a±0.003 | 0.9b ± 0.024 | |
| -1.1 | 75a±2.25 | 48a±0.9 | 1a±0.03 | 5.7c±0.1 | 4.7a±0.141 | 1.9a±0.03 | 0.34a±0.012 | 0.87b ± 0.03 | |
| Spermine Priming | 0 | 90d ± 2.7 | 88e±0.9 | 2ab±0.06 | 3a±0.05 | 6.9d ± 0.207 | 6.4e±0.3 | 0.8c±0.015 | 0.77a±0.06 |
| -0.1 | 88d ± 0.88 | 77d ± 1.7 | 2.4b ± 0.024 | 4b ± 0.08 | 7d ± 0.35 | 5.4d ± 0.2 | 0.46b ± 0.0046 | 0.8ab±0.024 | |
| -0.5 | 80c±3.2 | 66c±2 | 1.1a±0.04 | 4b ± 0.1 | 7d ± 0.28 | 3.8c±0.1 | 0.44b ± 0.017 | 0.93c±0.04 | |
| -1.1 | 78b ± 2.34 | 59b ± 1.4 | 1.1a±0.033 | 3a±0.08 | 7.9e±0.237 | 2.5b ± 0.1 | 0.39ab±0.0117 | 0.9c±0.033 | |
3.4. Membrane damage criteria and hydrogen peroxide
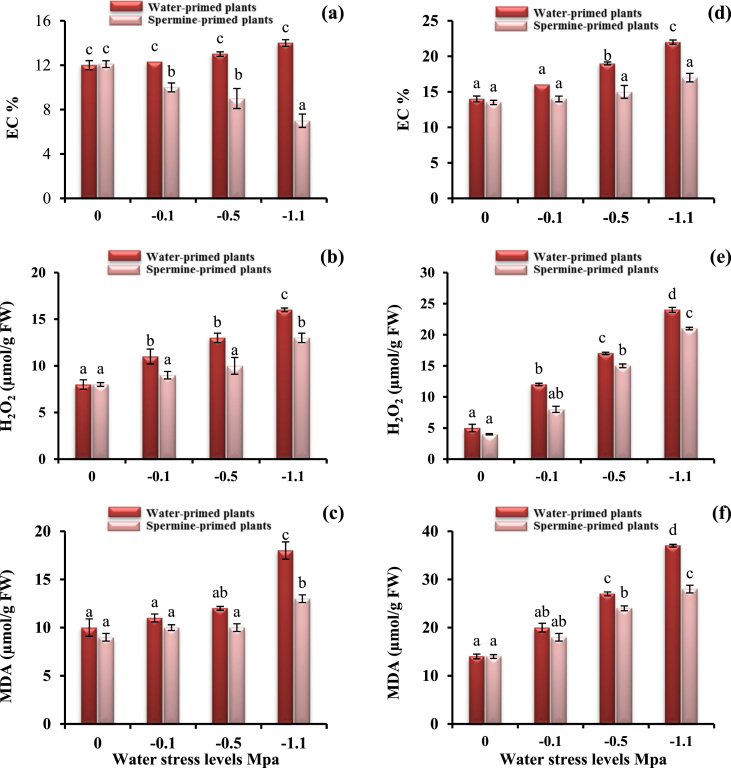
The data in Figure 2 a-f revealed drought tolerance variation of the two studied cultivars in terms of hydrogen peroxide, membrane leakage in terms of EC% (calculated based on Eq.(6)) and lipid peroxidation of the leaves. In this regard, the magnitude of these traits increment positively correlated to cultivars tolerance to water stress which displayed significant increase even at mild drought level. The increase in H2O2 was 200 and 480%; that of electrolyte leakage was 16% and 57% as well as 110% and 164% for lipid peroxidation compared to control for cv. Giza 111 and Giza 21, respectively at the highest level of water stress. Unequivocally, spermine increased membrane rigidity by reduction electrolyte leakage, lipid peroxidation and the causal agent of the oxidative stress (H2O2), especially for the stressed plants to be lower than the corresponding stress level for both cultivars.
Figure 2.
Electrolyte leakage (EC %), hydrogen peroxide (H2O2) and lipid peroxidation (MDA), of cv. Giza 111 (a, b, c) and (d, e, f) for cv. Giza 21 under different matric potential levels as affected by 0.2 mM spermine priming. Each histogram represents a mean value of three replicates, and the vertical bars indicate ±SE. Bars carrying different letters are significantly different at P < 0.05.
3.5. Non-enzymatic antioxidants
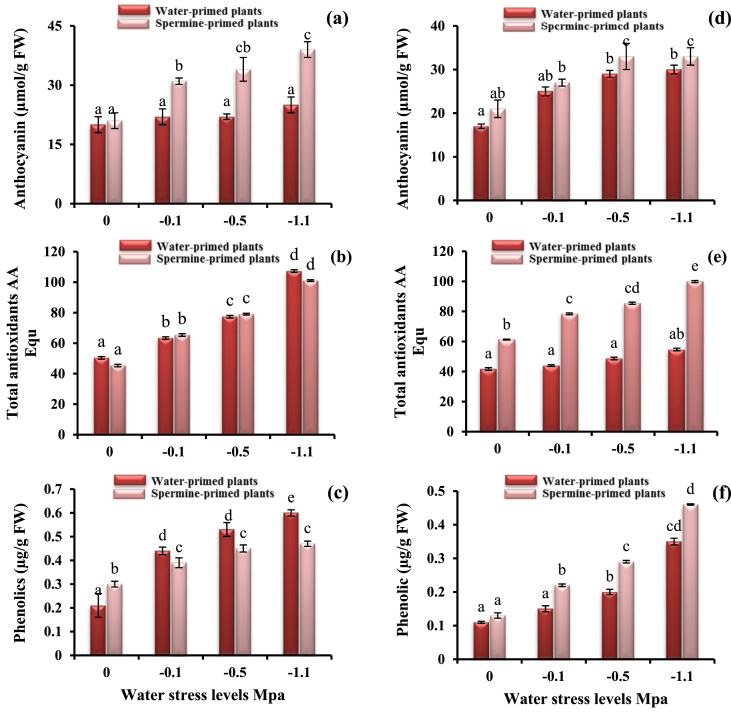
As registered in Figure 3 a-f, anthocyanins, total antioxidants and phenolic compounds stimulated by drought stress for both cultivars, but prominently for cv. Giza 111. Exacerbation of anthocyanin and phenolic compounds was registered for both cultivars irrespective to the water stress level used under spermine priming. Phenolic compounds found to be enhanced by spermine application, but this increment was lower than the corresponding stress level for cv. Giza 21. The total antioxidants enhanced progressively by spermine priming which was higher than the corresponding level for cv. Giza 21 and comparable to the corresponding water stress level for cv. Giza 111.
Figure 3.
Anthocyanin pigments, total antioxidants and phenolic compounds of cv. Giza 111 (a, b, c) and (d, e, f) for cv. Giza 21 under different matric potential levels as affected by 0.2 mM spermine priming. Each histogram represents a mean value of three replicates, and the vertical bars indicate ±SE. Bars carrying different letters are significantly different at P < 0.05.
4. Discussion
Polyamines are considered a plant growth regulators and a secondary messenger in signaling pathways which involved in abiotic stress tolerance (Hasanuzzaman et al., 2019). In the present study, change in growth due to water deficit was appraised in terms of reduction of fresh and dry matter where cv. Giza 21 suffered the highest growth reduction, suggesting that it was the most water deficit-sensitive and Giza 111 was the most water deficit–tolerant one. Such cultivar dependent effect in response to drought stress was similar to the finding (Abeed and Dawood, 2020). As has been described, spermine priming was effective in enhancing fresh and dry mater accumulation of soybean cultivars, which was more efficient under deficit irrigation and for the tolerant cultivar Giza 111 compared to cv. Giza 21. It is worth mentioning that the selected dose of spermine for both cultivars was not efficient enough to improve growth parameters under controlled conditions which indicated that spermine was promoter factor to dry matter acquisition especially under stress conditions. Ameliorative effect of spermine on different species has been reported by (Alcázar et al., 2010; Shi et al., 2010; Amooaghaie and Moghym, 2011; Xu et al., 2011; Minocha et al., 2014; Romero et al., 2018).
The main repairing mechanism of spermine priming under drought stress was the chlorophyll restoration chiefly at severe drought level used, in a way to redress food factory of stressed plants to adapt harsh conditions and save energy to a large extent for normal growth. This ameliorative effect of spermine may be attributed to increasing the stability of thylakoids membranes and plastids biogenesis (Pandey et al., 2000; Chattopadhayay et al., 2002). Polyamines exert positive effects on photosynthetic efficiency under stress conditions due to their acid–neutralizing and antioxidant properties, as well as their membrane stabilizing activity (Mapelli et al., 2008). PAs with a high net positive charge can stabilize photosystem II proteins such as D1 and D2 under photo–inhibition conditions. So, PAs binding to membrane proteins may stabilize the protein structure during stress and consequently preserve photosynthetic activity (Hamdani et al., 2011).
Photosynthesis limitation under drought mainly occurs through stomatal closure or metabolic impairment (Anjum et al., 2003; Abeed and Dawood, 2020). In the present study, the tolerant cultivar Giza 111 exhibited low stomatal conductance under drought stress which in turn decreased the transpiration rate. This might be reflected on conserving water status of cv. Giza 111 that RWC and WUE reduced only by 15 and 33%, respectively at -1.1 MPa compared to control. Thus, cv. Giza 111 suffered from relative reduction of stomatal conductance. On the other hand, the sensitive cultivar (Giza 21) exhibited increment of the stomatal conductance and transpiration rate under drought stress. So, water status disturbed of cv. Giza 21 as registered from dramatic reduction of RWC even at the lower drought level (-0.1 MPa) by 32% and up to 46% at -1.1 MPa compared to control as well as minimization of WUE to be 38% of control at -1.1 MPa. These results notably indicating that the growth retardation of cv. Giza 21 could be ascribed to troubling of plant metabolism, thereby metabolic impairment rather than stomatal conductance limitation (non-stomatal limitation). Our findings declared with the interpretation of Reddy et al. (2004) who suggested that the limitation of photosynthesis under drought through metabolic impairment is more complex phenomenon than stomatal limitation. Furthermore, when stomatal and non-stomatal limitations to photosynthesis are compared, the former can be quite small. This recommended that the main sensitivity criteria of soybean cultivars were photosynthetic depletion joined with the non-stomatal limitation and metabolic impairment.
The tolerance mechanism mediated by Spm priming on stressed plants efficiently restored water status by increasing both RWC and WUE through reduction of transpiration rate. Similarly, Farooq et al. (2009) reported that spermine capable of enhancing the water relations of the plants thereby allowing rice to grow under drought stress. The enhancement of RWC and the reduction of water loss rate was corroborated with the reduction of transpiration rate was at the same par of the findings (Fu et al., 2014; Nahar et al., 2017). Thus, better plant water status by spermine pre-treatment may be ascribed to conserving stomatal conductance restraining water use efficiency to be higher than control. It has been found that the exogenous application of spermine to pine trees under drought conditions caused a decline in transpiration rates, enhanced photosynthesis, and promoted osmotic adjustment (Anisul et al., 2003).
Spermine priming up-regulated cultivars metabolism by upregulation metabolic pathway especially the sensitive cultivar via exacerbation of soluble proteins and carbohydrates in a reflection of its role of enhancing plant body architecture to decrease dry matter loss during stress period. This is compatible with the general reputation that spermine is anti-senescence agents able to retard protein and chlorophyll loss in detached leaves (Pandey et al., 2000). Previous reports have shown that PAs was concerned with carbohydrate metabolism under multiple abiotic stresses. Spd effectively alleviated chill-induced metabolic disturbance of carbohydrate in spinach leaves (He et al., 2002). Exogenous Put improved drought tolerance of wheat by increasing accumulation of soluble sugar in leaves (Zhao et al., 2009). Li et al. (2015) reported that Spm application led to accumulation of high levels of water soluble carbohydrates, sucrose and fructose in white clover cultivars under drought stress. It suggests that there is powerful role related to Spm regulation of active osmolytes in drought-susceptible than -resistant soybean genotype as a way to retain water status under harsh conditions. Also, Spm priming reversed N-metabolism impairment for susceptible genotype Giza 21 under drought stress by converting free amino acids in favor of soluble protein accumulation. Under severe stress conditions the synthesis of osmolytes can ameliorate the detrimental effects of stress, by contributing to osmotic adjustment and/or acting as osmoprotectants (Gil et al., 2013).
Differential adaptation of both cultivars to water deficit stress appeared through increasing of total osmotic potential for cv. Giza 111 rather than cv. Giza 21 which logically explained by higher osmolytes produced for the former under drought stress compared to the latter cultivar. In this regard, cv. Giza 111 which exhibited stimulation of soluble carbohydrates, soluble proteins and free amino acids under reduced water availability. Conversely, cv. Giza 21 greatly hampered C-metabolism with reduction of soluble and total sugars which may be due to hampering of photosynthetically active chlorophylls which resulted from low expression of enzymes involved in photosynthesis under drought conditions Bayramov et al. (2010). The same cultivar reduced soluble proteins under withholding water regime in a relation of down regulation metabolic processes. Such reduction of soluble proteins may be in favor of free amino acids increment as recommended by Karimi et al. (2012) who pointed out that the progressive reduction of total soluble proteins during water deficiency in the plants was induced by proteolysis, with the liberated amino acids used during the plant osmotic adjustment. Conversely, cv. Giza 111 which exhibited stimulation of soluble carbohydrates, soluble proteins and free amino acids under reduced irrigation water. Due to aggregation of solutes in the cell under water stress, the osmotic potential of the cell becomes highly negative, which causes endosmosis of water into the cell and maintains the turgor of the cell (Sharma et al., 2019). This case shed light on the importance of free amino acids contribution in osmotic adjustment because it's the only metabolite enhanced osmotic potential of cv. Giza 21. Although the regulatory metabolic role of spermine priming on the studied metabolites for both genotypes, surprisingly it kept the total osmotic potential at the control of cv. Giza 21 and lower than the corresponding level for cv. Giza 111. This situation may be indicated that spermine primed cultivars less suffering from water stress to direct whole cell energy towards plant production or these metabolites may be directed to be osmoprotectant and/or radical scavenging. Moreover, Spm priming had a positive effect on modeling free amino acids in both cultivars under water stress by inducing incorporation of free amino acids into soluble protein synthesis as cell matric water binder ultimately imparting the ability of stabilizing protoplasmic colloids.
The effectiveness of seed priming with spermine in improving the emergence and seedling growth could be also related to scavenging of ROS, thus spermine limited drought-induced oxidative stress. This could be ascribed to PAs compete with metal ions necessary for ROS formation, such as Fe2+ and Cu2+, which are considered as indirect roles of PAs in reducing ROS production (Farooq et al., 2009). Sequera-Mutiozabal et al. (2016) suggested that Spm is a metabolic defense mechanism against senescence-induced oxidative stress and cell death. The production of ROS, especially H2O2, which is relatively long-lived ROS, is a major cause of oxidative burst (Farooq et al., 2009). For water-deficit treated plants, inhibition of CO2 assimilation, coupled with the changes in photosystem activities and photosynthetic electron transport capacity, results in accelerating the production of active oxygen in the chloroplast (Asada, 2000). Such postulation correlated differential elevated values of hydrogen peroxide of both cultivars with variance reduction percentages of chlorophyll content under drought stress. For instance, water-deficit stress increased hydrogen peroxide of cv. Giza 21 by 380% at -1.1 MPa corresponding to reduction of chlorophyll content more than 50% of control. At the same level, limited reduction of chlorophyll content of cv. Giza 111 by 30% opposite to increment of hydrogen peroxide by about 100%, which again confirmed that pigment reduction was the main problem of the studied cultivars under drought stress. Moreover, this also confirmed that H2O2 production went linear with the severity of water stress on the studied cultivars.
The difference in the permeability of cell membrane may arise from changes in the extent of ROS to attack the cell membrane phospholipids (Nabi et al., 2019). So, the content of MDA increases in both cultivars proportional to their tolerance which adversely affected membrane integrity and increased cell leakiness in terms of electrolyte leakage. The sensitive cultivars displayed exacerbation of MDA and electrolyte leakage at an earlier water stress level, proving it to be more sensitive to low water availability. On the other hand, the elevated tolerance of cv. Giza 111 was furthermore ascertained under mild and moderate drought levels used where electrolyte leakage and MDA content was not significantly affected. Spm effectively enhanced membrane dysfunction by reducing electrolyte leakage and lipid peroxidation as a result to suppression of H2O2. Studies have shown that PAs act directly as a scavenger of free radicals against the oxidative injury in the plants or bind to antioxidant enzymes to break up the free radicals (Roychoudhury et al., 2011). The spatial separation of positive charges in PAs at physiological pH could enable PAs to bind negatively-charged molecules such as nucleic acids, phospholipids and proteins, thereby protecting the structure and function of these macromolecules from degradation and modification (D'Agostino et al., 2005). This property would also enable the scavenging of free radicals and stabilization of intracellular membranes under stress conditions (Alcázar et al., 2010; Radhakrishnan and Lee, 2013).
Such preservation of the cell membrane and reduction of hydrogen peroxide help delay the senescence and keep leaves long lived. Notably, elevated levels of Spm in an Arabidopsis mutant that lacks the PAs back-conversion pathway are associated with delayed dark-induced senescence, suggesting that Spm is a metabolic defense mechanism against senescence-induced oxidative stress and cell death (Sequera-Mutiozabal et al., 2016).
In addition to sugars, proteins and amino acids, plants are rich in several compounds considered as ‘health-promoting’, such as anthocyanins, flavonoids and other phenolics which may play roles in scavenging of ROS of plants induced under different stress conditions and causing oxidative stress (Al Hassan et al., 2015). Anthocyanins and phenolics enhanced under drought for both cultivars opposite to increment of hydrogen peroxide which might be a way to minimize extra production of reactive oxygen species. In this respect, the increase in antioxidant phenolic compounds levels in leaves can be considered as part of the response induced to cope with oxidative stress (Al Hassan et al., 2015; Bashandy et al., 2020). This recommended by spermine priming which greatly accumulated anthocyanins and phenolics in cultivars leaves where overall correcting steps aided in ameliorating damaging effects of deficit irrigation. Parallel to the presented results, Farooq et al. (2009) found that PAs application led to accumulation of high levels of free proline, soluble phenolics and anthocyanins, whilst curtailing the production of H2O2 and MDA and reducing the relative membrane permeability. Also, PAs have been reported to facilitate the accumulation of phenolic compounds and free proline to protect against oxidative damage (Kumar et al., 2003). Thus, it had been stated that pretreatment with Spm conferred drought tolerance of in vitro citrus plants via modulation of antioxidative capacity and stomatal response (Shi et al., 2010).
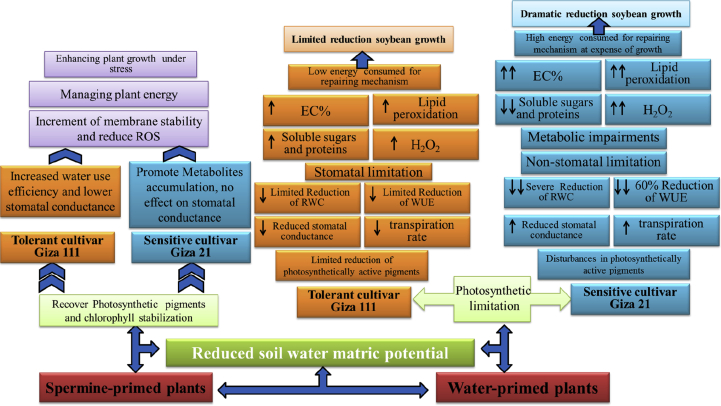
PAs play effective role in regulation of the antioxidative mechanisms to mitigate the overproduction of ROS (Shi et al., 2010; Tian et al., 2012; Fu et al., 2014). Plant synthesizes PAs endogenously, which further enhanced antioxidant defense mechanisms, including energizing antioxidants, ROS scavenging, metal chelation, and membrane stability (Nahar et al., 2017; Chen et al., 2013). In the present study, spermine was effective in enhancing the total antioxidants estimated for both cultivars under drought stress. The tolerant cultivar (Giza111) kept the total antioxidant at the same level of droughted plants only which generally higher than control plants. Otherwise, the sensitive cultivar (Giza 21) further accumulated the total antioxidants over that of the corresponding droughted plants as well as control. Therefore, it is apparent that Spm pretreatment of cv. Giza 111 prevented oxidative stress, thus sufficed with total antioxidants and phenolics to lower than produced under stress, so Spm could be act as H2O2 scavenger. On the other hand, the sensitive cultivar mitigated the H2O2 production via exacerbation of total antioxidants, flavonoids and anthocyanins, so Spm could act as an elicitor of the antioxidant production. These results revealed the variance responses of the tested cultivars to spermine pre-sowing. A summarization of differential effect of water deficit stress on both cultivars and the role of spermine in augmentation of water stress tolerance of the studied cultivars was registered in Figure 4.
Figure 4.
A summarization of differential susceptibilities cv. Giza 111 and cv. Giza 21 to water stress and the role of spermine in augmentation of water stress tolerance of the studied cultivars.
5. Conclusion
Overall, the spermine priming increased tolerance of soybean cultivars irrespective of water availability regimes applied, so it may be a direct stress-protecting compound and a stress signaling regulator. This mechanism professionally recovers pigmentation which left cells less energized by lessen H2O2 production and to some extent protect cell membrane from oxidative damage. Moreover, the adjustments of osmotic potential by enhancing cell fundamental components and efficiently maintain water status of leaves. Spermine effectively enhanced antioxidant system of the tested cultivars which left the membrane system of the cells less affected by drought stress. Thus, spermine priming could efficiently produce vigor soybean plants able to face arid, semi-arid and less irrigated agricultural systems.
Declarations
Author contribution statement
Mona F.A. Dawood: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Amany H.A. Abeed: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abeed A.H., Dawood M.F. Comparative impact of different iso-osmotic solutions on osmotic adjustment in Gossypium barbadense. Global Nest J. 2020;22(1):75–84. [Google Scholar]
- Ahmed H.H.A., Darwish E., Alobaidy M.G. Impact of putrescine and 24-epibrassinolide on growth, yield and chemical constituents of cotton (Gossypium barbadense L.) plant grown under drought stress conditions. Asian J. Plant Sci. 2017;16:9–23. [Google Scholar]
- Akparobi S.O. Effect of farmyard manures on the growth and yield of Amaranthus cruentus. Agric. Tropica Subtropica. 2009;42(1):1–4. [Google Scholar]
- Al Hassan M., Martínez F.M., Ramos S.F.J., Vicente O., Boscaiu O.M. Effects of salt and water stress on plant growth and on accumulation of osmolytes and antioxidant compounds in cherry tomato. Not. Bot. Horti. Agrobo. 2015;43(1):1–11. [Google Scholar]
- Alcázar R., Altabella T., Marco F., Bortolotti C., Reymond M., Koncz C. Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta. 2010;231:1237–1249. doi: 10.1007/s00425-010-1130-0. [DOI] [PubMed] [Google Scholar]
- Amooaghaie R., Moghym S. Effect of polyamines on thermotolerance and membrane stability of soybean seedling. Afr. J. Biotechnol. 2011;47:9673–9679. [Google Scholar]
- Anisul I., Blake T., Kocacinar F., Lada R. Ambiol, spermine, and amino ethoxyvinyl glycine prevent water stress and protect membranes in Pinus strobus L. under drought. Trees. 2003;17:278–284. [Google Scholar]
- Anjum F., Yaseen M., Rasul E., Wahid A., Anjum S. Water stress in barley (Hordeum vulgare L.). II. Effect on chemical composition and chlorophyll contents. Pakistan J. Agric. Sci. 2003;40:45–49. [Google Scholar]
- Anjum S.A., Ashraf U., Tanveer M., Khan I., Hussain S., Shahzad B., Zohaib A., Abbas F., Saleem M.F., Ali I., Wang L.C. Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front. Plant Sci. 2017;8:69. doi: 10.3389/fpls.2017.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjum S.A., Wang L.C., Farooq M., Hussain M., Xue L.L., Zou C.M. Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J. Agron. Crop Sci. 2011;197:177–185. [Google Scholar]
- Asada K. The water-water cycle as alternative photon and electron sinks. Phil. Trans. R. Soc. Lond. B Biol. Sci. 2000;355:1419–1431. doi: 10.1098/rstb.2000.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashandy S.R., Abd-Alla M.H., Dawood MFA. Alleviation of the toxicity of oily wastewater to canola plants by the N2- fixing, aromatic hydrocarbon biodegrading bacterium Stenotrophomonas maltophilia-SR1. Appl. Soil Ecol. 2020;154:103654. [Google Scholar]
- Bayramov M.S., Babayen G.H., Khaligzade N.M., Guliyev M.N., Raines A.C. Effect of water stress on protein content of some Calvin cycle enzymes in different wheat genotypes. Pro. ANAS Bio. Sci. 2010;65(5-6):106–111. [Google Scholar]
- Behnamnia M. Protective roles of brassinolide on tomato seedlings under drought stress. Intl. J. Agric. Crop Sci. 2015;8(3):455–462. [Google Scholar]
- Bozcuk S. Effect of sodium chloride upon growth and transpiration in statice sp. and Pisum sativum. L. Proc. Third MPP Meet. Izmir. 1975:37–42. [Google Scholar]
- Chattopadhayay M.K., Tiwari B.S., Chattopadhyay G., Bose A., Sengupta N.D., Ghosh B. Protective role of exogenous polyamines on salinity-stressed rice (Oryza sativa) plants. Physiol. Plant. 2002;116:192–1999. doi: 10.1034/j.1399-3054.2002.1160208.x. [DOI] [PubMed] [Google Scholar]
- Chen L., Wang L., Chen F., Korpelainen H., Li C. The effects of exogenous putrescine on sex-specific responses of Populus cathayana to copper stress. Ecotoxicol. Environ. Saf. 2013;97:94–102. doi: 10.1016/j.ecoenv.2013.07.009. [DOI] [PubMed] [Google Scholar]
- D’Agostino L., Di Pietro M., Di Luccia A. Nuclear aggregates of polyamines are supramolecular structures that play a crucial role in genomic DNA protection and conformation. FEBS J. 2005;272:3777–3787. doi: 10.1111/j.1742-4658.2005.04782.x. [DOI] [PubMed] [Google Scholar]
- Dawood M.F., Abeed A.H., Aldaby E.E. Titanium dioxide nanoparticles model growth kinetic traits of some wheat cultivars under different water regimes. Plant Physiol. Rep. 2019;24(1):129–140. [Google Scholar]
- Dubois M., Gilles K.A., Hamilton K., Rabers P., Smith A. Colorimetric method for the determination of sugars and related substances. Anal. Chem. 1956;28:350–356. [Google Scholar]
- El-Sharkawi H.M. Transpiration resistance in olive and almond trees under semi-arid conditions. Egypt J. Physiol. Sci. 1981;6(l):31–42. [Google Scholar]
- El-Sharkawi H.M., Abdel-Rahman A.A. Response of olive and almond orchards to partial irrigation under dry farming practices in semi-arid regions. ΙΙ- Plant soil water relations in olive during the growing season. Plant Soil (Netherland) 1974;41:13–32. [Google Scholar]
- El-Sharkawi H.M., Michel B.E. Effects of soil water matric potential and air humidity on CO2 and water vapour exchange in two grasses. Photosynthetica. 1977;11:176–182. [Google Scholar]
- Farooq M., Wahid A., Lee D.J. Exogenously applied poly-amines increase drought tolerance of rice by improving leaf water status, photosynthesis and membrane properties. Acta Physiol. Plant. 2009;31:937–945. [Google Scholar]
- Fu X., Xing F., Wang N., Peng L., Chun C., Cao L., Ling L., Jiang C. Exogenous spermine pretreatment confers tolerance to combined high-temperature and drought stress in vitro in trifoliate orange seedlings via modulation of antioxidative capacity and expression of stress-related genes. Biotechnol. Biotechnol. Equip. 2014;28(2):192–198. doi: 10.1080/13102818.2014.909152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil R., Boscaiu M., Lull C., Bautista I., Lidón A., Vicente O. Are soluble carbohydrates ecologically relevant for salt tolerance in halophytes? Funct. Plant Biol. 2013;40:805–818. doi: 10.1071/FP12359. [DOI] [PubMed] [Google Scholar]
- Hamdani S., Gauthier A., Msilini N., Carpentier R. Positive charges of polyamines protect PSII in isolated thylakoid membranes during photoinhibitory conditions. Plant Cell Physiol. 2011;52(5):866–873. doi: 10.1093/pcp/pcr040. [DOI] [PubMed] [Google Scholar]
- Hasanuzzaman M., Alhaithloul H.A.S., Parvin K., Bhuyan M.H.M., Tanveer M., Mohsin S.M., Nahar K., Soliman M.H., Al Mahmud J., Fujita M. Polyamine action under metal/Metalloid stress: regulation of biosynthesis, metabolism, and molecular interactions. Int. J. Mol. Sci. 2019;20(13):3215. doi: 10.3390/ijms20133215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanuzzaman M., Nahar K., Fujita M. Regulatory role of polyamines in growth, development and abiotic stress tolerance in plants. In: Anjum N.A., Gill S.S., Gill R., editors. Plant Adaptation to Environmental Change: Significance of Amino Acids and Their Derivatives. CABI; Wallingford, UK: 2014. pp. 157–193. [Google Scholar]
- He L., Nada K., Kasukabe Y., Tachibana S. Enhanced susceptibility of photosynthesis to low-temperature photoinhibition due to interruption of chill-induced increase of S-adenosylmethionine decarboxylase activity in leaves of spinach (Spinacia oleracea L.) Plant Cell Physiol. 2002;43:196–206. doi: 10.1093/pcp/pcf021. [DOI] [PubMed] [Google Scholar]
- Holmgren P., Jarvis P.G., Jarvis M.S. Resistances to carbon dioxide and water vapour transfer in leaves of different plant species. Physiol. Plant. 1965;18:527–573. [Google Scholar]
- Karimi S., Hossein A., Jafar M.S., Hassan M. Effects of water deficit and chitosan spraying on osmotic adjustment and soluble protein of cultivars castor bean (Ricinus communis L.) J. Stress Physiol. Biochem. 2012;8(3):160–169. [Google Scholar]
- Kofalvi S.A., Nassuth A. Influence of wheat streak mosaic virus infection phenyl propanoid metabolism and the accumulation of phenolics and lignin in wheat. Physiol. Mol. Plant Pathol. 1995;47:365–377. [Google Scholar]
- Krizek D.T., Kramer G.F., Upadhyaya A., Mirecki R.M. UV-B response to cucumber seedlings grown under metal halide and high pressure sodium/deluxe lamps. Physiol. Plant. 1993;88:350–358. [Google Scholar]
- Kumar S.G., Mattareddy A., Sudhakar C. NaCl effects on proline metabolism in two high yielding genotypes of mulberry (Morus alba L.) with contrasting salt tolerance. Plant Sci. 2003;165:1245–1251. [Google Scholar]
- Kusano T., Berberich T., Tateda C., Takahashi Y. Polyamines: essential factors for growth and survival. Planta. 2008;228:367–381. doi: 10.1007/s00425-008-0772-7. [DOI] [PubMed] [Google Scholar]
- Larcher W. fourth ed. Springer; Berlin, Germany: 2003. Physiological Plant Ecology. [Google Scholar]
- Li L., Gu W., Li C., Li W., Li C., Li J., Wei S. Exogenous spermidine improves drought tolerance in maize by enhancing the antioxidant defence system and regulating endogenous polyamine metabolism. Crop Pasture Sci. 2018;69(11):1076–1091. [Google Scholar]
- Li Z., Jing W., Peng Y., Zhang X.Q., Ma X., Huang L.K., Yan Y. Spermine alleviates drought stress in white clover with different resistance by influencing carbohydrate metabolism and dehydrins synthesis. PloS One. 2015;10(4):1–16. doi: 10.1371/journal.pone.0120708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler H.K. Chlorophyll and carotenoids pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–382. [Google Scholar]
- Liu J.H., Kitashiba H., Wang J., Ban Y., Moriguchi T. Polyamines and their ability to provide environmental stress tolerance to plants. Plant Biotechnol. 2007;24:117–126. [Google Scholar]
- Liu J.H., Wang W., Wu H., Gong X., Moriguchi T. Polyamines function in stress tolerance: from synthesis to regulation. Front. Plant Sci. 2015;6:827. doi: 10.3389/fpls.2015.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery O.H., Rosebought N.J., Far A.L., Randall R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:291–297. [PubMed] [Google Scholar]
- Madhava Rao K.V., Sresty T.V. Antioxidative parameters in seedlings of pigeon pea (Cajanus cajan L. Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000;157:113–128. doi: 10.1016/s0168-9452(00)00273-9. [DOI] [PubMed] [Google Scholar]
- Mapelli S., Brambilla I., Radyukina N., Ivanov Y.V., Kartashov A., Reggiani R. Free and bound polyamines changes in different plants as a consequence of UV–B light irradiation. Gen. Appl. Plant Physiol. 2008;34:55–66. [Google Scholar]
- Minocha R., Majumdar R., Minocha S.C. Polyamines and abiotic stress in plants: a complex relationship. Front. Plant Sci. 2014;5:175. doi: 10.3389/fpls.2014.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S., Stein W. From the laboratories of Rockefeller institute for medical research; New York: 1948. Photometric Ninhydrine Method for Use in the Chromatography of Amino Acid. [Google Scholar]
- Mukherjee S.P., Choudhuri M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983;58:166–170. [Google Scholar]
- Murty K.S., Majumder S.K. Modifications of technique for determination of chlorophyll stability index in relation to studies of drought resistance in rice. Curr. Sci. 1962;31:470–471. [Google Scholar]
- Nabi R.B.S., Tayade R., Hussain A., Kulkarni K.P., Imran Q.M., Mun B.G., Yun B.W. Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environ. Exp. Bot. 2019;161:120–133. [Google Scholar]
- Nahar K., Hasanuzzaman M., Alam M.M., Rahman A., Mahmud J.-A., Suzuki T., Fujita M. Insights into spermine-induced combined high temperature and drought tolerance in mung bean: osmoregulation and roles of antioxidant and glyoxalase system. Protoplasma. 2017;254:445–460. doi: 10.1007/s00709-016-0965-z. [DOI] [PubMed] [Google Scholar]
- Nawaz F., Ahmad R., Waraich E.A., Naeem M.S., Shabbir R.N. Nutrient uptake, physiological responses and yield attributes of wheat (Triticum aestivum L.) exposed to early and late drought stress. J. Plant Nutr. 2012;35:961–974. [Google Scholar]
- Pál M., Szalai G., Janda T. Speculation: polyamines are important in abiotic stress signaling. Plant Sci. 2015;237:16–23. doi: 10.1016/j.plantsci.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Pandey S., Ranade S.A., Nagar P.K., Kumar N. Role of polyamines and ethylene as modulators of plant senescence. J. Biosci. 2000;25:291–299. doi: 10.1007/BF02703938. [DOI] [PubMed] [Google Scholar]
- Podlešáková K., Ugena L., Spíchal L., Doležal K., De Diego N. Phytohormones and polyamines regulate plant stress responses by altering GABA pathway. New Biotechnol. 2019;48:53–65. doi: 10.1016/j.nbt.2018.07.003. [DOI] [PubMed] [Google Scholar]
- Premachuandra G.S., Saneoka A.H., Fujta K., Ogata S. Leaf water relations, osmotic adjustment, cell membrane stability, epicuticular wax load and growth as affected by increasing water deficits in Sorghum. J. Exp. Bot. 1992;43:1569–1576. [Google Scholar]
- Prieto P., Pineda M., Aguilar M. Spectrophotometric quantification of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application of vitamin E. Anal. Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan R., Lee I.J. Spermine promotes acclimation to osmotic stress by modifying antioxidant, abscisic acid, and jasmonic acid signals in soybean. J. Plant Growth Regul. 2013;32:22–30. [Google Scholar]
- Reddy A.R., Chaitanya K.V., Vivekanandan M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004;161:1189–1202. doi: 10.1016/j.jplph.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Richards L.A. Pressure-membrane apparatus: construction and use. Agric. Eng. 1947;24:451–454. [Google Scholar]
- Richards R.A. Defining selection criteria to improve yield under drought. Plant Growth Regul. 1996;20:57–166. [Google Scholar]
- Romero F.M., Maiale S.J., Rossi F.R., Marina M., Ruíz O.A., Gárriz A. Polyamine metabolism responses to biotic and abiotic stress. In: Alcázar R., Tiburcio A.F., editors. Vol. 1694. Springer Business Media; New York, NY: 2018. pp. 37–49. (Polyamines: Methods and Protocols. Methods in Molecular Biology). [DOI] [PubMed] [Google Scholar]
- Roychoudhury A., Ban S., Sengupta D.N. Amelioration of salinity stress by exogenously applied spermidine and spermine in three varieties of indice rice differing in their level of salt tolerance. J. Plant Physiol. 2011;168:317–328. doi: 10.1016/j.jplph.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Sallam A., Alqudah A.M., Dawood M.F., Baenziger P.S., Börner A. Drought stress tolerance in wheat and barley: advances in physiology, breeding and genetics research. Int. J. Mol. Sci. 2019;20:31–37. doi: 10.3390/ijms20133137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallam A., Amro A., Elakhdar A., Dawood M.F., Moursi Y.S., Baenziger P.S. Marker–trait association for grain weight of spring barley in well-watered and drought environments. Mol. Biol. Rep. 2019;46(3):2907–2918. doi: 10.1007/s11033-019-04750-6. [DOI] [PubMed] [Google Scholar]
- Scholander P.F., Bradstreet E.D., Hammel H.T., Hamingsen E.A. Sap concentration in halophytes and some other plants. Plant Physiol. 1966;41:529–532. doi: 10.1104/pp.41.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifi H.S., Shelp B.J. Spermine differentially refines plant defense responses against biotic and abiotic stresses. Front. Plant Sci. 2019;10:117. doi: 10.3389/fpls.2019.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequera-Mutiozabal M.I., Erban A., Kopka J., Atanasov K.E., Bastida J., Fotopoulos V. Global metabolic profiling of Arabidopsis polyamine oxidase 4 (AtPAO4) loss-of-function mutants exhibiting delayed dark-induced senescence. Front. Plant Sci. 2016;7:173. doi: 10.3389/fpls.2016.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini-Fracassini D., Di Sandro A., Del Duca S. Spermine delays leaf senescence in Lactuca sativa and prevents the decay of chloroplast photosystems. Plant Physiol. Biochem. 2010;48:602–611. doi: 10.1016/j.plaphy.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Shallan M.A., Hassan H.M.M., Namich A.A.M., Ibrahim A.A. Effect of sodium, nitroprusside, putrescine and glycine betaine on alleviation of drought stress in cotton plant. Am. Eurasian J. Agr. Environ. Sci. 2012;12:1252–1265. [Google Scholar]
- Sharma A., Shahzad B., Kumar V., Kohli S.K., Sidhu G.P.S., Bali A.S., Handa N., Kapoor D., Bhardwaj R., Zheng B. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules. 2019;9(7):285. doi: 10.3390/biom9070285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Fu X.Z., Peng T., Huang X.S., Fan Q.J., Liu J.H. Spermine pretreatment confers dehydration tolerance of citrus in vitro plants via modulation of antioxidative capacity and stomatal response. Tree Physiol. 2010;30:914–922. doi: 10.1093/treephys/tpq030. [DOI] [PubMed] [Google Scholar]
- Slatyer R.O. Academic press Inc.; New York: 1967. Plant-water Relationships. [Google Scholar]
- Tanou G., Ziogas V., Belghazi M., Christou A., Filippou P., Job D., Fotopoulos V., Molassiotis A. Polyamines reprogram oxidative and nitrosative status and the proteome of citrus plants exposed to salinity stress. Plant Cell Environ. 2014;37:864–885. doi: 10.1111/pce.12204. [DOI] [PubMed] [Google Scholar]
- Tian J., Wang L.P., Yang Y.J., Sun J., Guo S.R. Exogenous spermidine alleviates the oxidative damage in cucumber seedlings subjected to high temperature. J. Am. Soc. Hort. Sci. 2012;137:11–19. [Google Scholar]
- Turner N.C. Techniques and experimental approaches for the measurement of the plant water status. Plant Soil. 1981;58:339–366. [Google Scholar]
- Walter H. Die kryoskopische Bestimmung des osmotischen Wertes bei Pfanzen. In: Abderhalden E., editor. Handbuch der Biologischen Arbeitsmethoden. Urban, Schwarzenberg; Berlin, Vienna: 1931. pp. 533–571. [Google Scholar]
- Walter H. Die hydratur der Pfanze und ihre physiologisch- Ökologische Bedeutung. In: Fischer G., editor. Untersuchungen uX ber den Osmotischen Wert. Springer Verlag; Jena: 1931. [Google Scholar]
- Walter H. Tabellen zur Berechnung des osmotischen Werter von Pfanzenpressa$ften, ZuckerlÖsungen und einigen SalzlÖsungen. Berichte der Deutschen Botanischen Gesellschaft. 1936;54:328–339. [Google Scholar]
- Walter H. 1949. Gundlagen der flanzen verlientung. Eintubring, in die pflanzengeographie-Fur studierends der hocholen, standorstlehre. Stuttgart, Ulmer. [Google Scholar]
- Walter H., Thren R. Die Berechnung des osmotischen Wertes auf grund von kryoskopicshen Messungen und der vergleich mit Saugkraftbestimmungen. Jahrbuch fűr Wissenschaft der Botanik. 1934;80:20–35. [Google Scholar]
- Xu X., Guoxın S., Chunxıai D., Ye X., Juan Z., Haıyan Y. Regulation of exogenous spermidine on the reac-tive oxygen species level and polyamine metabolism in Alternanthera philoxeroides (Mart.) Griseb under copper stress. Plant Growth Regul. 2011;63:251–258. [Google Scholar]
- Yin Z.P., Li S., Ren J., Song X.S. Role of spermidine and spermine in alleviation of drought-induced oxidative stress and photosynthetic inhibition in Chinese dwarf cherry (Cerasus humilis) seedlings. Plant Growth Regul. 2014;74:209–218. [Google Scholar]
- Younes N.A., Hassan H.S., Elkady M.F., Hamed A.M., Dawood M.F. Impact of synthesized metal oxide nanomaterials on seedlings production of three Solanaceae crops. Heliyon. 2020;6(1) doi: 10.1016/j.heliyon.2020.e03188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W.C., Li H., Zhao H.J., Han Y.L., Tan J.F. Effects of exogenous polyamine osmotica regulation in wheat seedling leaves under drought stress. Chinese Agr. Sci. Bull. 2009;25:148–151. [Google Scholar]