Abstract
Meningitis caused by Streptococcus equi (SE) is a rare disease associated with high rates of complications. Commonly identified risk factors are regular horse contact and consumption of unpasteurized dairy products. When diagnosed promptly, this infection can be cured.
We report the case of a 73 year old woman who presented to the hospital with a sudden holocranial headaches, fever, photophobia, sonophobia, vomiting and behavioural disorders.
She lived in a rural area and regularly consumed unpasteurized milk products. She had a medical history of osteodural defect, chronic otitis, high blood pressure and pulmonary fibrosis.
We suspected bacterial meningitis associated with an ear infection. A lumbar puncture was performed. Streptococcus equi zooepidemicus(SEZ) was discovered in the CSF’s culture. Initially, the patient was treated with ceftriaxone. She had a tonic-clonic seizure 2days later. On the cerebral enhanced MRI, we found a right temporal pored cavity adjacent to a tegmen tympani bone breach. The patient received 15 days of antibiotic therapy with a good outcome. However, she was readmitted 24 h after being discharged for the same initial symptomatology. She received a total of 25 days of antibiotics and 4 days of corticoids with good results.
Only a few cases of Streptococcus equi meningitis have been documented. We reported this case to insist on the importance of considering this diagnosis in patients with risk factors. We also point out that severe complications may occur despite the early initiation of adequate treatment.
Keywords: Meningitis, Streptococcus equi, Osteodural defect
Introduction
Streptococcus equi (SE) rarely causes an infection in humans. Streptococcus equi zooepidemicus (SEZ) is part of the SE group. It is a zoonotic pathogen with adhesive and invasive properties. It can be responsible for septicemia, meningitis, arthritis and several other serious diseases.
It affects most commonly people who have been consuming unpasteurized cow and goat milk products, or having close contact with horses or pigs. Treatment is primarily medical.The absence of a performant vaccine confounds the control of SEZ infection.
Case report
We report the case of a 73 year old housewife living in a rural area. She had no contact with horses but she was regularly consuming unpasteurized milk products. She was obese, had high blood pressure, diffuse interstitial pulmonary fibrosis, asthma, obstructive sleep apnea and chronic otitis. Besides, she had been recently diagnosed with an osteodural right tegmenal defect and temporal meningoencephalocele.
The patient presented to the emergency department of La Rabta’s hospital complaining of intense and brutal holocranial headaches, fever, photophobia, sonophobia, nausea, incoercible vomiting, confusion, hallucinations and behaviaroul disorders.
On physical examination she was febrile and confused with a Glasgow Coma Scale score of 14. She was polypneic with 24 respiratory cycles per minute.
She had a soft neck, brudzinski and kernig signs were absent. She had no pyramidal nor cereberall syndrome, no motor nor sensory deficits. The examination of her cranial nerves was normal. She had chronical rhinorrachae.
The heart rate was 75 bpm with regular rhythm and no pathological murmur at the valve area. Lung auscultation was clear. There were no palpable superficial lymph nodes throughout the body. The abdomen was soft without tenderness or rebound tenderness.
The ear examination showed the presence of liquid and air behind the tympanic membrane and otomycosis. On the nasal examination we found liquid in the right tubal orifice. She also had naso-labial herpes.
The blood word revealed hyperleukocytosis with a leucocyte count of 13.82*10 < 3 of which 80 % were neutrophils, a hemoglobin rate of 11.5 g/dl and an elevated C-reactive protein of 52.6 mg/l.
Cerebrospinal fluid (CSF) analysis showed a cloudy liquid, 1850 cells /mm 3 of which 80 % were neutrophils and 20 % were lymphocytes, with a glucose rate of 0.68 g/l, a central glucose rate of 1.49 (ratio 0.48) and elevated proteins rate of 2.1 g/l.
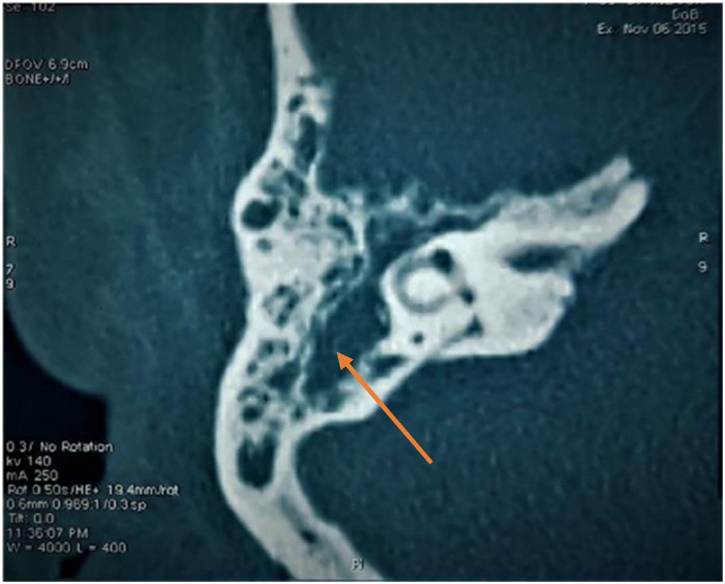
A brain CT-scan was initially performed and showed several abnormalities ; A sequelary right temporal cortico-subcortical hypodensity, a total filling of the right mastoid air cells (Fig. 1) and subtotal filling of the midlle ear, (Fig. 3) the lysis of the tegmen tympani corresponding to a cholesteatoma chronic otitis media (Fig. 2), the filling of the left ear’s wall and a thick nasopharynx wall.
Fig. 1.
Total filling of the right mastoid air cells.
Fig. 3.
Subtotal filling of the midlle ear.
Fig. 2.
The lysis of the tegmen tympani corresponding to a cholesteatoma chronic otitis media.
The diagnosis of a meningoencephalitis probably caused by Streptococcus pneumonia associated with an ear infection was suspected. Upon arrival, an empiric therapy was begun: ceftriaxone 2 g *6/jour (300 mg/kg/j)
Microbiologists were able to isolate a multi sensitive Streptococcus equi zooepidemicus in the CSF the following day. This stain had a high minimum inhibitory concentration (MIC) againt Ampicillin. Thus the same treatment including cetriaxone was carried out.
The patient was still febrile with intense headaches even after 72 h of treatment. On day 7 of antimicrobial therapy, she presented a tonic-clonic seizure.
An emergent cerebral CT scan was done and didn’t reveal recent brain damage. The patient was put on anticonvulsant therapy (phenobarbital). A cerebral MRI was also performed showing right temporal pored cavity adjacent to a tegmen tympani bone breach. We decided to do another lumbar puncture because of the persistence of symptoms. It showed no white cells, a normal rate of glucose and proteins.
She was treated for 15 days in total with ceftriaxone, with a good outcome. We obtained apyrexia on the 10th day of the treatment and she didn’t present other seizures. She was discharged after 15 days of hospitalization. She also received a pneumococcal vaccination.
She presented 24 h later to the emergency department complaining of intense headaches. She was readmitted to our department.
We suspected a cerebral abscess as a complication of the actute bacterial meningitis due to Streptococcus equi. It was later rulled out on a cerebral CT scan.
We restarted the same treatment (ceftriaxone 2 g*6 per day) with corticoids (dexamethasone 0.4 mg/kg per day) which she received for a period of 4 days.
The headaches disappeared within 3days. She had 25 days of antibiotics in total and was discharged with anticonvulsant therapy.
Three months later, the patient presented to her appointment, she was asymptomatic except rhinorrhagia. The anticonvulsant treatment was maintained for 6 months with progressive decrease in doses. An EEG and a cerebral MRI performed prior to treatment discontinuation were normal.
Discussion
Our case report and the review of the literature shows that Streptococcus Equi induced meningitis is a serious threat to humans health and may be fatal.
Hence we insist on the importance of history taking, especially animal contact when treating patients with sepsis or bacteraemia.
Very little is known about its risk factors, presenting features and outcome. The first case reported in the literature was in a 13 year old boy who had an undefined intracranial inflammatory tumour resected at 9 years old.(Elsayed, Hammerberg, Massey, & Hussain, 2003)
Infection can be endogenous or exogenous.
Endogenous infection often occurs in hosts predisposed by extreme age, alcoholism, drug abuse, diabetes mellitus, immunosuppressive therapy with corticosteroids, cytotoxic drugs, or underlying malignancy [1].
Meningitis caused by Streptococcus equi in humans is rare. It was primarily described as a veterinary pathogen. Rarely, It can be caused secondary to close contact with infected domestic animals (via respiratory tract) or by the consumption of their unpasteurized products (via digestive tract).
These organisms are described as either commensals or pathogens in many domestic animal species. They cause common diseases like strangles and other upper respiratory infections in horses.
In humans, they are recognized as occasional pathogens that might colonize the nasopharynx, the skin, gastrointestinal and genital tracts [2].
Streptococcus equi is a β-hemolytic, Lancefield group C streptococcal bacterium, that possesses Lancefield's Group-C carbohydrate.
5 different subspecies are forming the group C streptococci (GCS): Streptococcus dysgalactiae subsp. dysgalactiae, S. dysgalactiae subsp. equisimilis, Streptococcus equi subsp. equi, S. equi subsp. zooepidemicus and S. equi subsp. ruminatorum.
This group of Streptococci can be differentiated from other species by their hemolysis pattern and their ability to ferment sorbitol and trehalose.
It has been suggested that S. zooepidemicus is potentially more virulent than other subspecies of the group [1]. Previous studies have demonstrated that S. zooepidemicus carries antigens with antiphagocytic characteristics similar to the M proteins expressed in Lancefield group A and G streptococcal. It is named the M-like protein (SzP). SzP is a cell surface-anchored protein that confers phagocytosis resistance [3].
SEZ induce meningitis in humans and animals by breaking through the blood-brain barrier (BBB) of the host. Some SEZ virulence factors may interact with brain microvascular endothelial cells and contribute to SEZ binding to the endothelium of the BBB. High bacteremia is necessary to contribute to the development of meningitis.
Literature research identified 34 studies describing 33 episodes of S. equi meningitis, occurring between 1978 and 2015. The median age was 61 years, with a slight masculine predominance; 20 % of the patients were immunocompromised. Generally, they had underlying comorbid conditions such as cardiovascular insufficiency or malignancy.
When a source of infection was reported it was mainly regular horse contact (66 %) and the consumption of unpasteurized dairy products (28 %) [4].
The infection source of these outbreaks was mostly the cow’s milk [5]. Meningitis that was secondary to S. equi subsp. zooepidemicus ha a mortality rate of 24 %. Among survivors, only 38 % made a total recovery [6].
The clinical presentation is most likely acute. Nevertheless, the emergence of the symptoms could be subacute. However, respiratory signs or gastrointestinal complaints can prevail in the early stage of the disease in some patients. This can result in a misdiagnosis and therefore delays the institution of the appropriate therapy [2].
The classic meningitis symptoms are seen in more than half of the patients including headaches, fever, neck stiffness, vomiting, photophobia, sonophobia and altered consciousness. If not treated rapidly patients can develop severe symptoms.
The cerebrospinal fluid examination mostly shows a pleocytosis with neutrophilic preponderance, hypoglycorrhachia and an increased proteinorrhagia. These results correspond to classic bacterial meningitis features. About 50 % of the isolated bacterias are Gram-positive [7].
We must keep in mind that GCS are Gram-variable rather than Gram-positive when viewed in the CSF [2].
The majority of the group C streptococci produces β-hemolysis on sheep blood agar but all types of hemolysis have been reported.
Using the Kirby-Bauer disk which contains trimethoprim-sulfamethoxazole may facilitate the antimicrobial screening. Trimethoprim-Sulfamethoxazole is a drug to which group C bacterias are sensitive and group A ones are resistant [1]. S. equi subsp zooepidemicus has slightly different characteristics.
It possesses a hemolysin which is not the streptolysin O neither the streptolysin S and does not produce streptokinase. It ferments sorbitol, but not trehalose.
We must complete the microbiological study by obtaining blood cultures. Streptococcus equi subsp zooepidemicus can also be isolated from blood cultures in meningitis cases.
Complications in Streptococcus equi meningitis are frequent. They occur in about half of the patients. The bacterial gene-encoded extracellular hyaluronate lyase facilitates the infection’s spreading [8].
S. equi zooepidemicus can be responsible for a variety of infections in different localisation; endocarditis, sinusitis, septic arthritis, osteomyelitis, pericarditis, a streptococcal toxic shock syndrome and also endogenous endophthalmitis.
Endogenous endophthalmitis is a serious complication reported in about 10.7 % of all published S. equi zooepidemicus meningitis cases. This suggests that ophthalmological damage occurs more frequently in S. zooepidemicus meningitis than in meningitis caused by other microorganisms [9].
We also report other rare localisations such as cavernous sinus thrombosis; a life-threatening infection which requires urgent treatment including antibiotics and sometimes surgical drainage [10].
Musculoskeletal localisations are also a very uncommon localisation. The first case of osteomyelitis due to S. zooepidemicus has been reported in 2012. In that case, the infection was widely disseminated involving the bloodstream, spine‘s seeding and the peritoneal cavity. The starting point of the infection was probably the skin.
The first line used as antimicrobial agent for group C β-hemolytic streptococci is penicillin G. Other groups of antibiotics with good in vitro activity including amoxicillin, ureidopenicillins, cefazolin, cefotaxime, vancomycin are not efficient.
Moreover, therapeutic trials including other antimicrobial agents than penicillin are restricted beside experiences with the vancomycin.
A recent study of 20 isolates of group C streptococci demonstrated 30 % resistance rate to tetracycline, 25 % resistance to erythromycin, and 10 % had resistance to ciprofloxacin. Two of these isolates had intermediate susceptibility to clindamycin.
The addition of gentamicin or rifampin to a β-lactam or vancomycin confers a bactericidal activity against group C streptococci [1].
In experimental models of meningitis, very few outcomes were linked to the degree of subarachnoid inflammation, which has prompted investigations of the potential role of adjunctive corticosteroids in conjunction with systemic antibiotics. Corticosteroids should be given just before or, at the very latest, with the first dose of antibiotics.
Multiple studies showed that the C5a Peptidase (SCP) of some streptococcus species is an important virulence factor and a vaccine target having enzymatic adhesive and invasive activities are being studied. This could confer significant protection against SEZ infections. [11].
Conclusion
Streptococcus equi subspecies zooepidemicus is a zoonotic pathogen, especially for people in contact with horses. It is still underdiagnosed although infections associated with S. zooepidemicus are often severe in humans.
The early diagnosis will facilitate appropriate medical treatment and timely epidemiologic surveillance and finally, prevent the spread of a potentially lifethreatening infection.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Corresponding author: study design data collections, analysis, writing, literature review.
Ammari Lamia: Study design, data collections, writing.
Other authors: writing, literature review.
Funding
No funding received
Declaration of Competing Interest
The authors have no competing interests to declare.
References
- 1.Elston D.M., Gibson L.E., Kutzner H. Handbook of practical immunohistochemistry: frequently asked questions. 2015. Infectious diseases; pp. 641–663. [Google Scholar]
- 2.Rajasekhar A., Clancy C.J. Meningitis due to group C Streptococcus: a case report and review of the literature. Scand J Infect Dis. 2010 doi: 10.3109/00365541003754428. [DOI] [PubMed] [Google Scholar]
- 3.Zhe M., Jie P., Hui Z., Bin X., Xiaomeng P., Huixing L. SILAC and LCMS/MS identification of Streptococcus equi ssp. zooepidemicus proteins that contribute to mouse brain microvascular endothelial cell infection. Appl Microbiol Biotechnol. 2016;100(16):7125–7136. doi: 10.1007/s00253-016-7579-4. [DOI] [PubMed] [Google Scholar]
- 4.van Samkar A., Brouwer M.C., van der Ende A., van de Beek D. 2016. Streptococcus equi meningitis. Clinical microbiology and infection. [DOI] [PubMed] [Google Scholar]
- 5.Bordes-Benítez A., Sánchez-Oñoro M., Suárez-Bordón P., García-Rojas A.J., Saéz-Nieto J.A., González-García A. Outbreak of Streptococcus equi subsp. zooepidemicus infections on the island of Gran Canaria associated with the consumption of inadequately pasteurized cheese. Eur J Clin Microbiol Infect Dis. 2006 doi: 10.1007/s10096-006-0119-x. [DOI] [PubMed] [Google Scholar]
- 6.Pelkonen S., Lindahl S.B., Suomala P., Karhukorpi J., Vuorinen S., Koivula I. Transmission of streptococcus equi subspecies zooepidemicus infection from horses to humans. Emerg Infect Dis. 2013 doi: 10.3201/eid1907.121365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.2019. In C E S LRM, B R O W N 2 A N D PJ, Dk L. Human meningitis from Streptococcus equi subsp. zooepidemicus acquired as zoonoses. Available from: [DOI] [PubMed] [Google Scholar]
- 8.Singh S.K., Bharati A.P., Singh N., Pandey P., Joshi P., Singh S.K. The prophage-encoded hyaluronate lyase has broad substrate specificity and is regulated by the N-terminal domain. J Biol Chem. 2014 doi: 10.1074/jbc.M113.507673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madžar D., Hagge M., Möller S., Regensburger M., Lee D.H., Schwab S. Endogenous endophthalmitis complicating Streptococcus equi subspecies zooepidemicus meningitis: a case report neurology. BMC Res Notes. 2015 doi: 10.1186/s13104-015-1133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke M., Eenuh H., Ssaverimuttu J., Nfonoyim J. Streptococcus group C meningitis with cavernous sinus thrombosis. Infect Drug Resist. 2013;6(August):79–81. doi: 10.2147/IDR.S49690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei Z., Fu Q., Chen Y., Li M., Cong P., Mo D. Streptococcus equi ssp. zooepidemicus C5a peptidase, a putative invasin, induces protective immune response in mice. Res Vet Sci [Internet] 2013;95(2):444–450. doi: 10.1016/j.rvsc.2013.03.026. https://linkinghub.elsevier.com/retrieve/pii/S0034528813001264 Oct [cited 2019 Aug 21], Available from: [DOI] [PubMed] [Google Scholar]