Abstract
The SARS‐CoV‐2 outbreak causing the respiratory disease COVID‐19 has left many chemists in academia without an obvious option to contribute to fighting the pandemic. Some of our recent experiences indicate that there are ways to overcome this dilemma. A three‐pronged approach is proposed.
Keywords: alcohols, antivirals, disinfectants, pandemic, SARS-CoV-2
Chemists helping local communities: The SARS‐CoV‐2 outbreak causing the respiratory disease COVID‐19 has had a major impact on society. Where can chemists help in this hour of need? From preparing hand sanitizers, to working on antiviral drug candidates, and providing fact‐based outreach in collaboration with their chemical societies are options discussed in this contribution.

The current outbreak of a novel coronavirus is causing many deaths among vulnerable members of our societies and health care professionals. The viral infections can lead to a severe acute respiratory syndrome (SARS). The virus behind the outbreak was named 2019‐nCoV in an early research report,1 and SARS‐CoV‐2 by the International Committee on Taxonomy of Viruses. The disease is called corona virus disease 2019 (COVID‐19) by the World Health Organization (WHO). The virus causing it is a coronavirus (COV or CoV) of the beta‐CoV lineage. Physicians from Italy describe it as very contagious.2 The WHO Regional Director for Europe reports 1 out of 10 infections are in health care workers,3 and there is no doubt that the death toll among physicians was (and is) massive.
Many chemists in academia would like to contribute to the efforts to contain the disease, but may not be aware of opportunities to do so. After more than six weeks of involvement in efforts to produce hand sanitizers locally and more than two‐and‐a‐half decades of experience with nucleoside synthesis and active pharmaceutical ingredients, we take the liberty of sharing a few of our thoughts in this Viewpoint.
Hand Sanitizers
The interruption of infection chains through appropriate hand sanitization was pioneered in 1847 by the Hungarian physician Semmelweis Ignác Fülöp (Ignaz Philipp Semmelweis) and became what is probably the first example of evidence‐based medicine.4 Isolation or quarantine, social distancing and physical barriers like masks, are additional measures to slow the transmission of respiratory viruses. Other measures, like prophylactic coating of airways with polymers, are under development.5 Frequent handwashing proved effective against the spread of the first severe acute respiratory syndrome pandemic in 2002/20036 and of the Middle East respiratory syndrome (MERS) in 2007.7 However, this hygienic measure is not easy to implement for individuals with frequent customer or patient contact or travelers. Hand sanitizer solutions or gels are recommended as an alternative in these cases. Among the most popular sanitizers are alcoholic hand rub solutions.
While non‐enveloped viruses like norovirus or rhinoviruses have a low sensitivity towards alcoholic denaturing agents, members of the (enveloped) coronaviridae family are highly sensitive towards ethanol or isopropanol (2‐propanol). Chlorhexidine gluconate and sodium hypochlorite were found to be surprisingly inefficient.8 Repeated use of alcohols dries out the skin, and small quantities of the alcohols may be absorbed, but they are effective.9 The literature on the inactivation of previously known coronaviruses by surface disinfection procedures suggested 62–71 % ethanol to be effective against SARS‐CoV‐2 within 1 min.10 Experimental work by several laboratories on the virus itself then showed that it is even less stable toward denaturation by alcoholic solutions.11 Exposure to concentrations of just 30 % of either ethanol or isopropanol for 30 seconds fully suppressed viral infectivity. Likewise, the virucidal activity of the hand rub solutions known as WHO formulation 1, with 85 % ethanol, and WHO formulation 2, with 75 % isopropanol, against SARS‐CoV‐2 was found to be excellent, with full inactivation of the coronavirus at 40 % or 30 % concentration, respectively. While the alcohol component is the main virucide, 0.125 % v/v H2O2 is added to kill bacterial spores that may be present in the raw materials or the container. The addition of 1.45 % v/v glycerol as a humectant improves the dermatological properties and thus the acceptance of the product.12 For surface disinfection, the same formulation may be used, with no additives required, and surgical hand preparation may be achieved by three applications for a duration of 3–5 min.
Unfortunately, commercial sanitizer solutions containing either alcohol can be difficult to obtain in the current health crisis. We are unaware of data for other countries, but data published by the German Federal Statistical Office (Destatis) show the severity of the problem. During the week of March 2–8, 2020, sales of disinfectants were 7.5‐fold higher than usual, and in the subsequent weeks, they dropped to half the normal sales, as the products were largely sold out.13 By comparison, the increase in sales of soap reached a peak of 3.4‐fold of the average and that of toilet paper just 2.1‐fold of the sales averaged over the preceding six months.
Ethanol is produced on a large scale on our planet. The U.S. Renewable Fuels Association reported that 85 million metric tons were produced in 2018 worldwide.14 The European renewable ethanol association (ePURE) stated that the production of the European Union in the same year was 4.6 million metric tons, while an additional 0.5 million tons were imported.15 Besides minor usage by the chemical industry and food and beverages production (9 % each), the lion's share (82 %) of the production was used as a fuel additive. Against the backdrop of reduced individual mobility during the crisis in most countries, it appears likely that enough (bio)ethanol should currently be available for the health sector.
Several factors appeared to contribute to the supply problems. As mentioned above, one factor was hoarding by consumers who bought unreasonable quantities of sanitizers while they were still available, quickly depleting what pharmacies and drug stores had in stock. Furthermore, the supply chain was partially disrupted. While truckloads of ethanol were available in Europe, the logistics of getting trucks to sites that can fill 200 L drums became more challenging due to closed or tightly controlled borders. Drums of this size then had to be transported to facilities that can either produce sanitizers or fill the smaller containers commonly used by pharmacists. Furthermore, the containers used in medical practices, as well as the dispensers used by physicians, health care workers, and consumers, became ever more difficult to obtain, prompting unconventional measures, such as refills.
On March 4, the Bundesanstalt für Arbeitsschutz und Arbeitsmedizin (BAuA),16 the relevant regulatory body in Germany, issued a decree that allowed pharmacies to produce hand sanitizer solutions locally. The decree and its update of March 6 confirmed that aqueous solutions of ethanol do not require regulatory approval to be produced for sale as biocidical agents. The first decree also explicitly mentioned aqueous 2‐propanol (70 %) and WHO formulation 2 as biocidal agents for hand hygiene.
Unfortunately, medical grade alcohol as a starting material for the local production of disinfectant solutions, including hand rub formulations, was largely sold out in Germany in the second week of March. The usual suppliers of pharmacists typically gave delivery times of several weeks at the peak of the supply crunch. This left technical grade ethanol or isopropanol of unknown purity as the only available starting material for the preparation of hand rub formulations. Without analysis, this source was considered problematic by many. Solving the analytical problem is where organic chemists in academia were able to contribute.
The BAUA decree mentions a purity of 99 % (w/w) or 99.22 % (v/v) for isopropanol to be in accordance with EU law. The WHO guideline states that analysis should be made when an analysis certificate is not available to verify the alcohol concentration and to adjust the volume used to obtain the final recommended concentration. What local pharmacists use for quality control and what the WHO guideline mentions is an alcoholmeter that measures the density of the liquid, but does not provide information on its chemical composition. Gas chromatography is mentioned in the WHO guideline for higher level quality control, but this option is largely unavailable to pharmacists in the field.
We and others then analyzed technical grade isopropanol and ethanol by 1H NMR spectroscopy. A 1 % solution of either alcohol in CDCl3 gave well‐resolved spectra with excellent signal‐to‐noise ratio, showing minimal levels of impurities. For isopropanol, acetone was detected as the most prominent trace impurity by spiking with authentic material. Due to its low toxicity and its traditional use in nail polish remover, this impurity was considered unproblematic for hand rubs, and the purity of our technical grade isopropanol was declared sufficient for producing hand sanitizer locally together with a collaborating pharmacist. As a consequence, substantial supplies of the alcohol could be freed up for local production quickly. As of mid April, over 2 metric tons of WHO formulation 2 have been delivered to physicians in the State of Baden‐Württemberg.
Antiviral Therapy
Another area where chemists may contribute is the development of antiviral drugs. Small molecule drugs have been game changers in the fight against other viruses. Among them are AZT (ZDV, Zidovudin, Retrovir),17 a nucleoside reverse transcriptase inhibitor and the first compound approved to treat AIDS. Another break‐through antiviral drug is sofosbuvir (1, trade name Sovaldi) shown in Figure 1,18 an inhibitor of the RNA‐dependent RNA polymerase of hepatitis C virus (HCV) that is able to cure patients of the vicious disease caused by this virus. Like SARS‐CoV‐2, HCV is an enveloped, single‐stranded, positive‐sense RNA virus.
Figure 1.
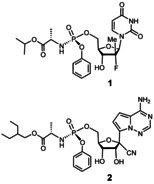
The structures of sofosbuvir (1) and remdesivir (2).
While SARS‐CoV‐2 is commonly described as a “novel” virus, genetically, it is closely related to the SARS coronavirus (SARS‐CoV or SARS‐CoV‐1).1 The RNA‐dependent RNA polymerase (RdRp) is 96 % homologous for the two viruses. This polymerase is a prime target for antiviral therapy, as human cells do not contain such an enzyme that copies RNA sequences into RNA. Two other drug targets are the main proteinase (3CLpro) and the spike protein of the viral envelope, with a much lower level of sequence identity. A review of this and the avenues it offers for drug intervention can be found in a recent paper by Liu and colleagues.19 In this context, it is worth mentioning that opportunities for therapy were identified for SARS‐CoV‐1 more than 15 years ago.20, 21, 22 Another betacoronavirus that is related to SARS‐CoV‐2 is the virus causing MERS. Again, this virus has been studied in detail, and options for therapeutic intervention have been identified.23, 24
Successful RNA polymerase inhibitors, such as sofosbuvir,18 work by incorporating modified nucleosides into the viral genome that halt viral replication. This approach has been very successful in the treatment of hepatitis C, which can be cured within a short treatment period. The drug sofosbuvir is a phosphoramidate prodrug or ProTide, a type of compound pioneered by Chris McGuigan.25 It is metabolized in the liver in several steps into its active triphosphate form, which is recognized and incorporated as an analog of uridine‐5′‐triphosphate by the hepatitis‐C virus polymerase. One of the frontrunner drug candidates for the treatment of COVID‐19, remdesivir (2), is a similar prodrug construct. It is a C‐nucleoside mimic of adenosine monophosphate26 in ProTide phenyl phosphoramidate form. Remdesivir was developed by Gilead Sciences to combat Ebola and related filoviruses, but did not have sufficient activity against these targets. In 2017, it was shown in vitro and in animals that it can inhibit MERS‐CoV and SARS‐CoV‐1 replication.27 In the replication processes, remdesivir, in its triphosphate form, competes with its natural counterpart, adenosine‐triphosphate (ATP), inducing RNA chain termination.
Based on the high sequence similarity between SARS‐CoV‐1 and the causal agent of the COVID‐19 pandemic, homology modeling was used to predict the 3D structures of the RdRp and other important viral proteins of the latter. Out of the more than 20 viral proteins encoded in the SARS‐CoV‐2 genome, proteases 3CLpro, mentioned above, and PLpro represent potential targets for antiviral drugs. The 3‐chymotrypsin‐like protease (3CLpro) of SARS‐CoV‐2 differs in only 12 out its 306 amino acid residues from its ortholog in SARS‐CoV‐1. Its structure was quickly predicted in silico and this was used for the virtual docking of a collection of 7173 commercial drugs in the search for candidates for drug repurposing.28 Among the top‐scoring candidates, for which the highest binding affinities were predicted, were the two antivirals velpatasvir and ledipasvir. Both compounds are FDA‐approved inhibitors of the NS5A protein of the hepatitis C virus, but so far, the predicted action against the SARS‐CoV‐2 3CLpro has not been confirmed experimentally. A similar approach on the same enzyme was employed by another team, and led to different candidate structures,29 while a team from China and Saudi Arabia chose to identify phytochemicals as potential inhibitors in similar fashion using in silico methods.30 While modeling is fast, it does not produce a new drug, and experimental work is needed to validate those predictions. A recent crystal structure of 3CLpro, also referred to as Mpro, complexed with an α‐ketoamide inhibitor shows how quickly encouraging results can be obtained experimentally.31 A crystal structure of the RdRp of SARS‐CoV‐2 has also been published in the last few days.32
One of the most detailed theoretical studies thus far used homology modeling on 19 different SARS‐CoV‐proteins and on the human ACE2 receptor protein, the putative molecular entry vehicle for the virus into human cells, for virtual drug screening.33 For each viral protein and for the ACE2 receptor, several drugs or natural products were identified as potential binders. Notably, remdesivir was also found to bind to the RdRp enzyme of SARS‐CoV‐2 in silico. Experimentally, activity against SARS‐CoV‐2 was also demonstrated in vitro.34 In the same experimental study, antiviral activity was found for the antimalarial drug chloroquine, which is unlikely to act on either RdRp or proteases. Chloroquine and hydroxychloroquine35 act on several targets and can have severe side effects. Their anti‐inflammatory effect may reduce the level of pro‐inflammatory cytokine IL‐6, mobilized by the immune system of COVID‐19 patients in order to kill infected cells before too many copies of the virus are made. Sometimes this defense mechanism overreacts, resulting in a life‐threatening condition.36 Both remdesivir and chloroquine, are currently among the compounds undergoing clinical trials with COVID‐19 patients.
The synthesis of compounds like remdesivir is far from trivial, though, not least because the amino acid ester and aryloxy group make the phosphorus of the phosphoramidate a stereogenic center. Synthesizing chiral phosphoramidates of this level of complexity is a synthetic challenge,37 and so is the synthesis of other drug candidates for treating the virus that will be identified by homology modeling and target‐based virtual ligand screening or conventional medicinal chemistry approaches. Developing efficient syntheses for such compounds and making them available to laboratories that test them is a second opportunity for chemists who wish to contribute to fighting SARS‐CoV‐2.
Outreach
There is a third opportunity for chemists in the current crisis: reaching out to others through our scientific societies. When a virus spreads and employees stay home, running an efficient operation becomes difficult. As soon as an employee gets infected and obtains a positive test result, entire divisions may be shut down to prevent the spreading of the virus. Colleagues in quarantine must be provided with food and other supplies, and homeschooling ties up significant resources. As a consequence, many members of our societies are experiencing unprecedented levels of stress or are too overwhelmed by emails and calls to remain fully operational. This problem affects the supply chain from drivers to executives, but it is not limited to logistics and production. It also affects authorities. The apparent suicide of Dr. Thomas Schäfer, the minister of finance of the German State of Hesse, is a tragic example of what can happen to those put under ever increasing pressure in the current pandemic. Trying to spread useful information through governmental channels can thus add to the stress level of administrators.
We feel that the network our scientific societies, such as the German Chemical Society (GDCh), offer can and should be used to reach out to each other. Social media can be very useful, but are also the source of unfiltered anger and accusations. Emails to a select group of colleagues, such as the members of the Liebig‐Vereinigung, the organic division of the GDCh, well maintained and checked by the officers of the society, can be a way to disseminate information that does not suffer from said disadvantages. Another option is posting useful information on the newly installed corona webpage of the GDCh.38
Chemists have contributed very significantly already, not least by synthesizing the necessary amounts of primers and dNTP′s for test kits, but there are countless challenges that lie ahead. Broader testing for viral infections,39 as well as serological testing to confirm immunity, a task that proved difficult, even for the relatively confined MERS outbreak,40, 41 is just one of them. Improving the social acceptance of face masks in some Western countries,42 and producing such masks in sufficient quantities, is another.
Whether it is instructions for producing buffers required for test kits, or simple face masks for protection against infectious droplets, useful information can be shared. The same is true for funding opportunities or legal issues that affect society members. Chemists are resourceful people, and scientific societies are exceptionally experienced in sorting through information submitted to their journals. Here lies a third opportunity for chemists to contribute.
When we almost ran out of isopropanol for our hand sanitizer solutions, a non‐public email call led to a company in the area offering larger quantities of the solvent. The company was willing to offer it well below its current inflated market price, and our university was willing to support us with logistics and the free use of facilities. At the end of a busy day, one of the clinical pharmacologists who helped with the local production of WHO formulation 2 looked at our less than attractive facility and said: “There is still an important role for good old chemistry in our society.”
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
We thank R. Linderer, S. Mahmutovic, M. Bechthold, H. Griesser, D. Göhringer, D. Zeidler, P. Seckler, M. Landa, O. Doppleb, O. Bernhard, E. Wuckert, T. Göckler, R. Larosa, N. Metke, W. Ressel, M. Rannenberg, M. Dellian, W. Koch, R. O. F. Scharr, and B. Schittenhelm for supporting our efforts to produce hand sanitizers and improvised face masks, and M. Egli for sharing information on polymerase structures.
T. Opatz, J. Senn-Bilfinger, C. Richert, Angew. Chem. Int. Ed. 2020, 59, 9236.
Dedicated to the health care professionals who lost their lives fighting SARS‐CoV‐2
References
- 1. Chan J. F.-W., Kok K.-H., Zhu Z., Chu H., To K. K.-W., Yuan S., Yuen K.-Y., Emerging Microbes Infect. 2020, 9, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nacoti M., Ciocca A., Giupponi A., Brambillasca P., Lussana F., Pisano M., Goisis G., Bonacina D., Fazzi F., Naspro R., Longhi L., Cereda M., Montaguti C., NEJM Catalyst 2020, https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0080. [Google Scholar]
- 3.World Health Organization, http://www.euro.who.int/en/media-centre/sections/statements/2020/statement-physical-and-mental-health-key-to-resilience-during-covid-19-pandemic.
- 4. Best M., Neuhauser D., Qual. Saf. Health Care 2004, 13, 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kyluik D. L., Sutton T. C., Le Y., Scott M. D. in Progress in Molecular and Environmental Bioengineering (Ed.: A. Carpi), IntechOpen, London, 2011, pp. 167–190. [Google Scholar]
- 6. Jefferson T., Del Mar C., Dooley L., Ferroni E., Al-Ansary L. A., Bawazeer G. A., van Driel M. L., Foxlee R., Rivetti A., BMJ 2009, 339, b3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang J., Park E.-C., Lee S. A., Lee S. G., Health Educ. Behav. 2019, 46, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saknimit M., Inatsuki I., Sugiyama Y., Yagami K.-i., Exp. Anim. 1988, 37, 341. [DOI] [PubMed] [Google Scholar]
- 9. Lachenmeier D. W., J. Occup. Med. Toxicol. 2008, 3, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kampf G., Todt D., Pfaender S., Steinmann E., J. Hosp. Infect. 2020, 104, 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kratzel A., Todt D., V′kovski P., Steiner S., Gultom M. L., Thao T. T. N., Ebert N., Holwerda M., Steinmann J., Niemeyer D., Dijkman R., Kampf G., Drosten C., Steinmann E., Thiel V., Pfaender S., bioRxiv preprint, 2020, 10.1101/2020.03.10.986711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization, https://www.who.int/gpsc/5may/Guide_to_Local_Production.pdf.
- 13.DESTATIS, press release No. 112 of 25 March 2020, https://www.destatis.de/EN/Press/2020/03/PE20_112_61.html.
- 14.Renewable Fuels Association, https://ethanolrfa.org/statistics/annual-ethanol-production.
- 15.ePURE, https://epure.org/media/1920/190828-def-data-statistics-2018-infographic.pdf.
- 16.Bundesanstalt für Arbeitsschutz und Arbeitsmedizin, https://www.baua.de/DE/Angebote/Aktuelles/Meldungen/2020/2020-03-04-Desinfektionsmittel.html.
- 17. Mitsuya H., Weinhold K. J., Furman P. A., St Clair M. H., Lehrman S. N., Gallo R. C., Bolognesi D., Barry D. W., Broder S., Proc. Natl. Acad. Sci. USA 1985, 82, 7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sofia M. J., Bao D., Chang W., Du J., Nagarathnam D., Rachakonda S., Reddy P. G., Ross B. S., Wang P., Zhang H. R., Bansal S., Espiritu C., Keilman M., Lam A. M., Steuer H. M., Niu C., Otto M. J., Furman P. A., J. Med. Chem. 2010, 53, 7202–7218. [DOI] [PubMed] [Google Scholar]
- 19. Morse J. S., Lalonde T., Xu S., Liu W. R., ChemBioChem 2020, 21, 730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cinatl J., Michaelis M., Hoever G., Preiser W., Doerr H. W., Antiviral Res. 2005, 66, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chan P. K. S., Tang J. W., Hui D. S. C., Clin. Sci. 2006, 110, 193. [DOI] [PubMed] [Google Scholar]
- 22. Haagmans B. L., Osterhaus A. D. M. E., Antiviral Res. 2006, 71, 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Momattin H., Al-Ali A. Y., Al-Tawfiq J. A., Trav. Med. Infect. Dis. 2019, 30, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zumla A., Chan J. F. W., Azhar E. I., Hui D. S. C., Yuen K.-Y., Nat. Rev. Drug Discovery 2016, 15, 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Slusarczyk M., Serpi M., Pertusati F., Antiviral Chem. Chemother. 2018, 26, 2040206618775243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cho A., Saunders O. L., Butler T., Zhang L., Xu J., Vela J. E., Feng J. Y., Ray A. S., Kim C. U., Bioorg. Med. Chem. Lett. 2012, 22, 2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sheahan T. P., Sims A. C., Graham R. L., Menachery V. D., Gralinski L. E., Case J. B., Leist S. R., Pyrc K., Feng J. Y., Trantcheva I., Bannister R., Park Y., Babusis D., Clarke M. O., Mackman R. L., Spahn J. E., Palmiotti C. A., Siegel D., Ray A. S., Cihlar T., Jordan R., Denison M. R., Baric R. S., Sci. Transl. Med. 2017, 9, eaal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen Y., Yiu C., Wong K., F1000Research 2020, 10.12688/f1000research.22457.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu X., Wang X.-J., J. Genet. Genom. 2020, 47, 119–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. ul Qamar M. T., Alqahtani S. M., Alamri M. A., Chen L.-L., J. Pharm. Anal. 2020, 10.1016/j.jpha.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L., Becker S., Rox K., Hilgenfeld R., Science 2020, 368, 409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gao Y., Yan L., Huang Y., Liu F., Zhao Y., Cao L., Wang T., Sun Q., Ming Z., Zhang L., Ge J., Zheng L., Zhang Y., Wang H., Zhu Y., Zhu C., Hu T., Hua T., Zhang B., Yang X., Li J., Yang H., Liu Z., Xu W., Guddat L. W., Wang Q., Lou Z., Rao Z., Science 2020, 10.1126/science.abb7498. [DOI] [Google Scholar]
- 33. Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X., Zheng M., Chen L., Li H., Acta Pharm. Sinica B 2020, 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G., Cell Res. 2020, 30, 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M., Cell Discovery 2020, 6, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Conti P., Ronconi G., Caraffa A., Gallenga C. E., Ross R., Frydas I., Kritas S. K., J. Biol. Regul. Homeost. Agents. 2020, 34, 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 37. Cini E., Barreca G., Carcone L., Manetti F., Rasparini M., Taddei M., Eur. J. Org. Chem. 2018, 2622. [Google Scholar]
- 38. http://www.gdch.de/corona.
- 39. Mahony J. B., Petrich A., Smieja M., Crit. Rev. Clin. Lab. Sci. 2011, 48, 217. [DOI] [PubMed] [Google Scholar]
- 40. Chan J. F. W., Sridhar S., Yip C. C. Y., Lau S. K. P., Woo P. C. Y., J. Microbiol. 2017, 55, 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Al Kahlout R. A., Nasrallah G. K., Farag E. A., Wang L., Lattwein E., Müller M. A., El Zowalaty M. E., Al Romaihi H. E., Graham B. S., Al Thani A. A., Yassine H. M., J. Immunol. Res. 2019, 1386740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Feng S., Shen C., Xia N., Song W., Fan M., Cowling B. J., Lancet Respir. Med. 2020, 10.1016/S2213-2600(20)30134-X. [DOI] [PMC free article] [PubMed] [Google Scholar]


