Summary
With the developing COVID‐19 pandemic, patients with inherited anaemias require specific advice regarding isolation and changes to usual treatment schedules. The National Haemoglobinopathy Panel (NHP) has issued guidance on the care of patients with sickle cell disease, thalassaemia, Diamond Blackfan anaemia (DBA), congenital dyserythropoietic anaemia (CDA), sideroblastic anaemia, pyruvate kinase deficiency and other red cell enzyme and membrane disorders. Cascading of accurate information for clinicians and patients is paramount to preventing adverse outcomes, such as patients who are at increased risk of fulminant bacterial infection due to their condition or its treatment erroneously self‐isolating if their fever is mistakenly attributed to a viral cause, delaying potentially life‐saving antibiotic therapy. Outpatient visits should be minimised for most patients, however some, such as first transcranial dopplers for children with sickle cell anaemia should not be delayed as known risk of stroke will outweigh the unknown risk from COVID‐19 infection. Blood transfusion programmes should be continued, but specific changes to usual clinical pathways can be instituted to reduce risk of patient exposure to COVID‐19, as well as contingency planning for possible reductions in blood available for transfusions. Bone marrow transplants for these disorders should be postponed until further notice. With the current lack of evidence on the risk and complications of COVID‐19 infection in these patients, national data collection is ongoing to record outcomes and eventually to identify predictors of disease severity, particularly important if further waves of infection travel through the population.
With the developing COVID‐19 pandemic (caused by the novel zoonotic SARS‐CoV‐2 coronavirus), the UK population are urged to follow Government advice on managing symptoms and reducing viral transmission. 1 Unusually, patients with sickle cell anaemia (HbSS genotype) have been explicitly mentioned in this as a group at increased risk due to their non‐functioning spleen. 2 Unfortunately, there is no specific advice for patients with sickle cell disease (SCD) of other genotypes, thalassaemia and other inherited anaemias. Many of these are at increased risk of fulminant bacterial infection, and therefore may erroneously self‐isolate if their fever is mistakenly attributed to a viral cause, delaying potentially life‐saving antibiotic therapy. Blanket advice cannot be expected to cover all eventualities and a risk–benefit assessment will frequently be needed. For patients with rare diseases such as SCD, thalassaemia, Diamond–Blackfan anaemia (DBA), congenital dyserythropoietic anaemia (CDA), sideroblastic anaemia, pyruvate kinase deficiency and other red cell enzyme and membrane disorders, the rarity of their condition, and lack of access to specialised advice and care can put patients at risk. The timely generation and distribution of accurate information for clinicians and patients is paramount to preventing adverse outcomes.
In the UK, NHS England (NHSE) has recently re‐organised care provided for patients with inherited anaemias, and while the bulk of these patients have haemoglobinopathies, patients with the rare inherited anaemias are also explicitly included under the remit of the newly formed National Haemoglobinopathy Panel (NHP). 3 The new scheme is hierarchical but highly linked for effective delivery of needed expertise and illustrated in Fig 1. Networks are organised at the level of Specialist Haemoglobinopathy Teams (SHTs), consisting of a central teaching hospital and a number of local care providers. SHTs are grouped into Haemoglobinopathy Coordinating Centres (HCCs), themselves represented in the NHP. The NHP also has representatives from the Clinical Reference Group (CRG), and the National Haemoglobinopathy Registry and patient representatives. The remit of the NHP is to oversee the activities of the HCCs, to carry out national multidisciplinary team meetings (MDTs), and to provide general guidance so that care can be equitable and co‐ordinated across the UK.
Fig 1.
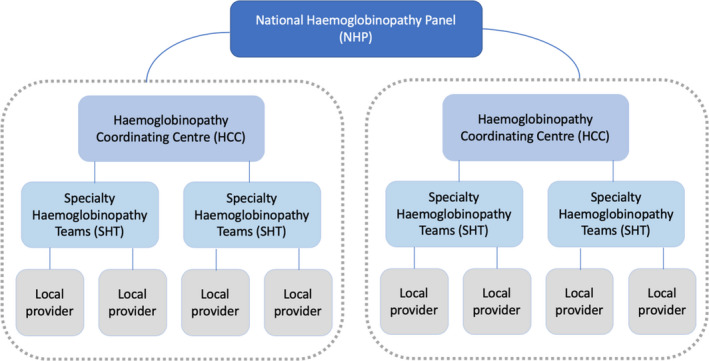
New NHSE service organisation for haemoglobinopathy disorders and rare inherited anaemias. [Colour figure can be viewed at http://www.wileyonlinelibrary.com/]
Immediately upon the emergence of the pandemic, the NHP instituted the formation of a COVID‐19 Working Group, which combined membership of the CRG, NHP and key members of the blood service, and provides weekly guidance to clinicians on advice that can be given to patients, as well as specific changes to usual clinical pathways that can be instituted to reduce risk of patient exposure to COVID‐19. In addition it has suggested areas for consistency in government advice, as well as contingency planning for possible reductions in blood available for transfusions.
In addition to developing weekly updated guidance for clinicians and patients, a crucial role of the COVID‐19 Working Group is to ensure that this information is cascaded in a timely manner. The new structure of care devised by the NHSE ensures clear roles and lines of responsibility, and a dedicated centralised web portal. Guidance produced by the COVID‐19 Working Group is cascaded to the HCCs, and then to the SHTs and their local care providers. By establishing these clear lines of communication, this ensures that all clinicians across the UK who look after patients with inherited anaemias are sent the correct information within 24 h of expert national advice.
Specific risks for patients with inherited red cell disorders
Sickle cell disease is the commonest inherited anaemia in the UK. Altogether, there are an estimated 14 000 patients living with homozygous SCD, HbSC and other compound heterozygote conditions. They are all at increased risk of bacterial infections partly due to functional asplenia. 4 There is a risk of missing the diagnosis of a severe bacterial infection, and mistakenly attributing symptoms to COVID‐19 infection, resulting in patients being advised to self‐isolate, thereby delaying access to life‐saving antibiotics. Patients must therefore make contact with their specialist teams as soon as they develop symptoms, to ensure they receive specialised care and advice. This may well require medical review in hospital. Patients should be encouraged to carry on taking their hydroxycarbamide to ensure they stay well and out of hospital. If they develop COVID‐19 infection, there is a theoretical risk that they are at increased risk of viral pneumonia and hypoxia precipitating vaso‐occlusive crisis or acute chest syndrome requiring a blood transfusion. While this has been reported anecdotally, there are no publications of COVID‐19 infection in SCD, highlighting the urgency of developing evidence for this group of patients.
Other groups at high risk of infection are those with thalassaemia, DBA and patients with other inherited anaemias receiving iron chelation therapy. There are specific adverse effects of chelator drugs that need to be considered. In the case of deferiprone, there is a small risk of reversible agranulocytosis (1–2% of patients, generally within the first 6 months of initiating therapy) and a higher risk of milder neutropenia. For desferrioxamine, the main consideration is an increased risk of certain potentially overwhelming bacterial infections (notably Yersinia and Klebsiella). For deferasirox, there may be an increased risk of acute kidney injury, especially during episodes of infection. It is therefore recommended that upon development of a fever, chelation therapy is stopped (interrupted) until the patient undergoes a medical assessment at an expert centre. In patients who are poorly chelated with cardiac iron overload there is the additional risk of developing cardiac failure and arrythmias in the context of sepsis, which requires specialist advice and careful consideration of their chelation.
For patients with DBA who are not transfused, the mainstay of treatment is corticosteroids, which carries an additional risk of immunosuppression. Few patients are on doses of corticosteroids that cause hypoadrenalism, but susceptibility to infection is increased in this patient group. 5
Patients with inherited red cell enzyme and membrane deficiencies are at risk of excess haemolysis with infection and may require blood transfusion. For those who have had splenectomy, the risks of infection are increased, although not as high as for haemoglobinopathy patients, who have a broader spectrum of infections.
The effect of interferon α, which is used in congenital dyserythropoietic anaemia type 1 (CDA‐1), on patients infected with COVID‐19 is not known. Interferon β has shown a potential protective effect in patients infected with COVID‐19. 6
Outpatient appointments and investigations
The importance of social distancing, as well as hospitals’ need to reduce footfall and cancel routine investigations, has implications for this group of patients. Blood test monitoring for hydroxycarbamide or iron chelating drugs may need to be done less frequently, possibly 4–6 monthly, and telephone clinics are now replacing face‐to‐face visits. Many departments are arranging for home delivery of medications, including hydroxycarbamide. Some patients will still require review in person, for new diagnosis and evaluation of new symptoms. Similarly, some patients will require essential investigations, for instance certain children with SCD who still need trans‐cranial Doppler screening to detect those that require blood transfusion therapy to prevent stroke.
Blood transfusions
Patients receiving blood transfusions are at additional risk. Regular visits to the hospital for cross‐matching and transfusion require contact with healthcare professionals and the public, leading to an overall increased risk of virus transmission, but also those on regular transfusion often have a more severe clinical phenotype. There are systems in place to reduce the incidence of transfusion transmitted infection including a 2‐week deferral of patients who have had a recent infectious illness and processes to quarantine stock in donors who become symptomatic after transfusion. There is also a risk that blood stocks will reduce as donors are deferred due to a recent infection or may feel anxiety about attending donor sessions.
There are multiple co‐ordinated strategies within the nation’s blood services to ensure sufficient stocks of blood. Nevertheless, individual clinicians may need to alter current transfusion regimens for their patients and the COVID‐19 Working Group has prepared guidance and practical advice for the optimal use of blood products and methods of mitigating blood use in patients with inherited red cell disorders who may require blood transfusion during this period. Decisions should be made on an individual patient basis. Clinical safety must guide decision‐making and the understanding of the indication for transfusion is of particular importance – a patient who is on a chronic transfusion regimen due to a previous overt cerebrovascular event would be considered at higher risk of adverse outcome from reduced‐intensity transfusion compared to a patient for whom transfusion was started to reduce the frequency of vaso‐occlusive crises. Strategies to optimise transfusion practice could include lengthening the transfusion interval, altering transfusion parameters, or relaxing criteria on age of blood for transfusion. Simple (top‐up) transfusions can be used in some patients, as they involve significantly fewer blood units than exchange transfusion. Hydroxycarbamide is an oral SCD‐modifying therapy, which could be considered as an alternative to chronic transfusion therapy in certain patients. These cases require expert clinical evaluation, discussion with the patient/parent and a planned transition from one therapy to the other, and for any amendment to their current transfusion regimen. The extent to which these options are implemented will depend on the evolution of the pandemic in each country and its effect on blood stocks and are set out in a document approved by the COVID‐19 Working Group and cascaded to all HCCs.
Escalation of treatment
National Institute for Health and Care Excellence (NICE) guidance is available for decision‐making regarding escalation of treatment and intensive care 7 :
https://www.nice.org.uk/guidance/ng159/resources/critical-care-admission-algorithm-pdf-8708948893
Patient prioritisation is based upon the Clinical Frailty Score, 8 which would place a significant proportion of high‐functioning adults with SCD aged <50 years in a high‐risk group. Appropriate interpretation of these scores in this group of patients is essential to ensure they are given equitable access to escalation of care (Fig 2).
Fig 2.
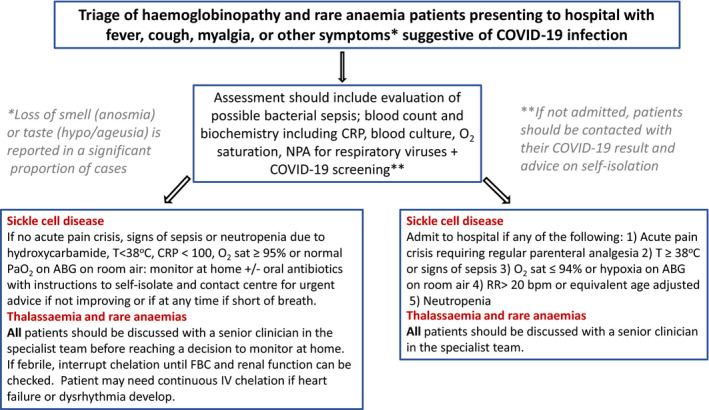
Algorithm for assessment of patients with symptoms suggestive of COVID‐19 infection. [Colour figure can be viewed at http://www.wileyonlinelibrary.com/]
Case registry and research
With the current lack of evidence on the risk and complications of COVID‐19 infection in these patients, projects to collect national data are ongoing. These are planned to record outcomes and eventually to identify predictors of disease severity. Urgent research is needed to answer key clinical questions, e.g. which patients with SCD require early blood transfusion on presenting with respiratory symptoms, and what other treatments are beneficial to avoid progressive respiratory and multi‐system deterioration. In addition, caution is needed in the rapid instigation of clinical trials, to avoid exposing patients to unintended adverse effects. For example, the possibility of haemolytic crisis due to glucose‐6‐phosphate dehydrogenase (G6PD) deficiency in trials of chloroquine and hydroxychloroquine. The COVD‐19 epidemic also creates a challenge for patients enrolled in clinical trials of novel therapies or about to enter extension phases of ongoing trials. It will be important that in the wish to minimise exposure of patients to COVID‐19 that patients are not put at risk by withdrawal of effective trial therapy.
Workforce
There are a significant, but undocumented number of doctors, nurses, allied health practitioners (AHPs), pharmacists and clinical scientists with SCD and other red cell disorders who work for the NHS and other front‐line services. Those with a role as front‐line staff must be appropriately redeployed so that their expertise can continue to be used, while prioritising their health. In some cases this will involve remote working, but where this is not possible these individuals are advised to remain at home.
The role of non‐profit organisations and social media
Patient organisations such as the Thalassaemia International Foundation, UK Thalassaemia Society and the Sickle Cell Society and DBA(UK), are in communication with large numbers of patients and families/carers and are a trusted source of information. For this reason it is vital that the information they provide is accurate, consistent, and up to date. These organisations have medical advisors who are on the NHP and have ensured that the information on these organisations’ websites is relevant, accurate and updated frequently. In addition, the British Society of Haematology is hosting the latest updated versions of all the documents produced by the NHP:
Advice is also available on the NHSE website and EuroBloodNet, the European Rare Disease Network:
Accurate information is critical, especially in the light of incorrect or incomplete information that circulates widely on social media. In particular, fears over the use of ibuprofen and angiotensin‐converting enzyme (ACE) inhibitors have been raised, and incorrect or non‐validated information is circulating. Guidance has been produced by other bodies on these:
Summary
For patients living with inherited anaemias and their healthcare workers, clear guidance and communication is required in this current COVID‐19 pandemic. The new NHSE‐directed structure of care for these patients has provided clinicians with a robust method of cascading information in a timely manner. In addition, non‐profit organisations are playing a key role in disseminating this information.
To save lives, it is critical not only that patients with COVID‐19 infection are appropriately identified and treated, but also that patients who have other pathologies, including bacterial infection, are rapidly recognised and appropriately treated. Also, a fine balance must be drawn between cancelling non‐urgent procedures to reduce unnecessary contact with the hospital, and minimising risk of viral transmission, and providing necessary treatment for the underlying condition.
Finally, both national and international efforts must be made to study the effects of COVID‐19 infection in these patient groups and to develop the evidence upon which treatment of future patients can be optimised.
Conflict of interest
No authors have relevant disclosures to declare.
Acknowledgements
Noémi B.A. Roy, Paul Telfer, Perla Eleftheriou, Josu de la Fuente, Emma Drasar, Farrukh Shah, David Roberts, Wale Atoyebi, Sara Trompeter, D. Mark Layton, Sanne Lugthart, Sara Stuart‐Smith, Subarna Chakravorty, Josh Wright, John Porter, Baba Inusa, and Jo Howard equally contributed to the development of the advice that forms this manuscript. Noémi B.A. Roy conceived the article and drafted the original text. All authors were involved in revisions and approved the final draft.
References
- 1. Bedford J, Enria D, Giesecke J, Heymann DL, Ihekweazu C, Kobinger G, et al COVID‐19: towards controlling of a pandemic. Lancet. 2020;395:1015–8. DOI: 10.1016/S0140-6736(20)30673-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Available from: https://www.gov.uk/government/publications/guidance-on-shielding-and-protecting-extremely-vulnerable-persons-from-covid-19/guidance-on-shielding-and-protecting-extremely-vulnerable-persons-from-covid-19 Accessed March 25, 2020.
- 3.Available from: https://www.england.nhs.uk/publication/specialist-haemoglobinopathy-services-specialist-haemoglobinopathy-teams/ Accessed March 25, 2020.
- 4. Brousse V, Buffet P, Rees D. The spleen and sickle cell disease: the sick(led) spleen. Br J Haematol. 2014;166:165–76. DOI: 10.1111/bjh.12950 [DOI] [PubMed] [Google Scholar]
- 5. Iskander D, Roberts I, Rees C, Szydlo R, Alikian M, Neale M, et al Impaired cellular and humoral immunity is a feature of Diamond‐Blackfan anaemia; experience of 107 unselected cases in the United Kingdom. Br J Haematol. 2019;186:321–6. [DOI] [PubMed] [Google Scholar]
- 6. Martinez MA. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob Agents Chemother. 2020. [Epub ahead of print]. DOI: 10.1128/AAC.00399-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Available from: https://www.nice.org.uk/guidance/ng159/resources/critical-care-admission-algorithm-pdf-8708948893 Accessed March 25, 2020.
- 8. Devaux CA, Rolain JM, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID‐19? Int J Antimicrob Agents. 2020. 10.1016/j.ijantimicag.2020.105938 [DOI] [PMC free article] [PubMed] [Google Scholar]


