Abstract
In cardiac myocytes, action potentials are initiated by an influx of sodium (Na+) ions via voltage-gated Na+ channels. Na+ channel gain of function (GOF), arising in both inherited conditions associated with mutation in the gene encoding the Na+ channel and acquired conditions associated with heart failure, ischemia, and atrial fibrillation, enhance Na+ influx, generating a late Na+ current that prolongs action potential duration (APD) and triggering proarrhythmic early afterdepolarizations (EADs). Recent studies have shown that Na+ channels are highly clustered at the myocyte intercalated disk, facilitating formation of Na+ nanodomains in the intercellular cleft between cells. Simulations from our group have recently predicted that narrowing the width of the intercellular cleft can suppress APD prolongation and EADs in the presence of Na+ channel mutations because of increased intercellular cleft Na+ ion depletion. In this study, we investigate the effects of modulating multiple extracellular spaces, specifically the intercellular cleft and bulk interstitial space, in a novel computational model and experimentally via osmotic agents albumin, dextran 70, and mannitol. We perform optical mapping and transmission electron microscopy in a drug-induced (sea anemone toxin, ATXII) Na+ channel GOF isolated heart model and modulate extracellular spaces via osmotic agents. Single-cell patch-clamp experiments confirmed that the osmotic agents individually do not enhance late Na+ current. Both experiments and simulations are consistent with the conclusion that intercellular cleft narrowing or expansion regulates APD prolongation; in contrast, modulating the bulk interstitial space has negligible effects on repolarization. Thus, we predict that intercellular cleft Na+ nanodomain formation and collapse critically regulates cardiac repolarization in the setting of Na+ channel GOF.
Significance
In this work, we propose a mechanism for the regulation of repolarization in cardiac tissue with defective sodium channel gating. Using whole-heart optical mapping, single-cell patch clamp, and computational modeling, we find that the width of the intercellular cleft and the subcellular distribution of sodium channels critically regulate the action potential duration and the formation of arrhythmias. These results point to a novel determinant of arrhythmia formation in inherited and acquired cardiac diseases with sodium channel gain of function.
Introduction
Sodium (Na+) channel gain of function (GOF) is a pathological condition that is associated with a host of acquired and inherited cardiac disorders like ischemia and heart failure (1) or the Long-QT type 3 (LQT3) syndrome linked to mutations in the SCN5A gene encoding the cardiac voltage-gated Na+ channel (Nav1.5) (2). This GOF manifests as a late Na+ current in isolated cardiac cells that can generate proarrhythmic early afterdepolarizations (EADs) (3,4). However, LQT3 is often a concealed disease (5,6), generally requiring additional perturbations to manifest in intact tissue (7,8). We and others have demonstrated that Nav1.5 channels are preferentially localized at the intercalated disk (ID), i.e., at the cell ends, in cardiac tissue (9, 10, 11, 12, 13, 14). Critically, many computational studies have predicted that Na+ current and, thus, electrical coupling between cardiomyocytes can be modulated via Na+ nanodomain signaling localized at the ID, a mechanism also termed ephaptic coupling (EpC) (9,15, 16, 17, 18, 19).
Although there are many mechanisms of EpC (17), we consider two primary effects: extracellular electric fields and intercellular cleft Na+ depletion. These EpC mechanisms can be described as follows: Na+ influx during the cardiac action potential (AP) in a depolarizing cell reduces the extracellular electrical potential of the intercellular cleft, i.e., the narrow extracellular space between electrically coupled neighboring cells. The intercellular cleft potential reduction in turn depolarizes an apposing cell from the extracellular rather than the intracellular side of the cell membrane. Na+ current into the depolarizing cell also reduces the local concentration of extracellular Na+ exposed to Na+ channels at the ID in both the depolarizing and apposing cells. In the setting of narrow intercellular cleft separation, electric field effects elevate the apposing cell’s transmembrane potential at the ID and Na+ fluxes reduce , which in turn reduces Na+ reversal potential , both of which reduce the Na+ driving force and thus the peak Na+ current in the downstream cell. This reduction in peak Na+ current slows conduction, a mechanism termed “self-attenuation” (9,18,20).
Studies suggest that EpC and gap junction (GJ) coupling are parallel pathways of electrical communication between cardiomyocytes. The relationship between ephaptic and gap junctional coupling is likely complex. When impaired, reduced EpC can slow conduction under baseline conditions (10,21,22) and exacerbate conduction slowing when sodium channel availability or gap junctional coupling is reduced (10,20,23). Yet reducing ephaptic self-attenuation may also be an adaptive response. For example, inward rectifier potassium channel (i.e., current) inhibition can increase conduction, but perinexal expansion can counter this effect (21). Further, reducing EpC self-attenuation may support invariant conduction during early time points of metabolic ischemia (24). Taken together, adaptive and modulated Na+ channel ephaptic self-attenuation may maintain invariant and stable cardiac conduction under a variety of conditions.
In a recent study, we demonstrated in tissue simulations and isolated heart experiments that the self-attenuation mechanism could also suppress EAD formation in cardiac tissue with enhanced late Na+ current (25). We also demonstrated that expanding intercellular cleft width can prolong action potential duration (APD) and promote EADs, thus unmasking the Na+ channel GOF phenotype (25) in tissue comprising myocytes coupled via GJs and EpC. Specifically, using a simulated cardiac tissue model modified to account for subcellular distribution of Nav1.5 channels and LQT3-associated channel mutations (26), simulations predicted that narrowing the intercellular spacing promoted localized Na+ depletion of the intercellular cleft, which reduced the driving force for the late Na+ current at the ID. Importantly, these effects required preferential localization of Na+ channels at the ID. Simulations also predicted that the Na+ depletion mechanism of EpC and self-attenuation were critical for EAD suppression, whereas extracellular electric field effects were not, although these fields did modulate electrical conduction velocity. Further, reduced gap junctional coupling minimally altered APD prolongation. However, we did not specifically consider the effects of altering bulk interstitial spaces in cardiac tissue. Although we would not expect that the bulk interstitial spaces play a critical role in EpC, in particular in the setting in which Na+ channels are localized at the ID, the bulk interstitial spaces may modulate Nav1.5 channels with GOF, the associated Na+ currents along the axial cell membrane, and thus APD and EAD formation. Although the bulk interstitial space is much larger than the intercellular cleft, it is not clear whether similar local Na+ depletion also occurs in the bulk interstitial space, in particular in the context of Nav1.5 channel redistribution to the axial membrane. Further, any potential therapeutic agents targeting the extracellular space may modulate both the intercellular cleft and the bulk interstitial space, and thus it is therefore important to investigate modulation of the bulk interstitial space and the relative local effects of axial Na+ current on APD and EAD formation.
In our previous study, we experimentally probed the effects of intercellular cleft expansion in a sea anemone toxin (ATXII)-induced guinea pig model of Na+ channel GOF using mannitol (25), an osmotic agent known to cause intercellular cleft expansion and acute interstitial edema (11,24,27). In this study, we delve deeper into the mechanisms of enhanced Na+ channel GOF unmasking via simulations and experiments to determine the functional consequences of altering interstitial spaces and intercellular clefts. Osmotic agents albumin, mannitol, and dextran were chosen for the study because they are all clinically relevant blood volume expanders. Importantly, small molecules like mannitol can increase interstitial volume (27,28) and increase intercellular separation in the perinexus (10). Dextran 70 kDa on the other hand can extravasate into the interstitial space without changing interstitial osmotic pressure (29) and has an estimated diameter of 10 nm, which is on the order of intercellular separations of ∼34 nm in desmosomes (30), 25 nm in adherens junctions (31), 15 nm in perinexi (10), and 2 nm in GJs (32). Although albumin has a similar molecular weight to dextran 70 kDa, it binds to the endothelial glycocalyx and reduces capillary hydraulic conductivity (33). Further, although both dextran 70 kDa and albumin have been shown to decrease extracellular volume in ex vivo cardiac preparations (27,34), importantly, the effects of these agents on perinexal width are unknown. We additionally measure the effects of these osmotic agents on the late Na+ current and APD in isolated cells. Finally, expanding on our prior work, we also perform new in silico studies and broadly investigate the relationship between Nav1.5 preferential localization and spacing of the intercellular cleft and bulk interstitial space. We test the hypothesis that Na+ nanodomain signaling localized at the ID mediates the unmasking of the Na+ channel GOF phenotype because of regulation by intercellular cleft width and not by bulk interstitial spaces.
Materials and Methods
Ex vivo guinea pig hearts
The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (35). All animal study protocols were approved by the Institutional Animal Care and Use Committee at the Virginia Polytechnic Institute and State University.
Optical mapping of Langendorff-perfused guinea pig hearts has been extensively described by the Poelzing group (10,36). Briefly, male retired breeder guinea pigs were anesthetized with isoflurane inhalation, and hearts were rapidly excised and retrogradely perfused through the aorta. The anterior epicardial surface of the left and right ventricles was mapped during pacing. Di-4-ANEPPS (15 μM) was perfused into the heart to detect the membrane potential changes (10). Blebbistatin (10 μM) was perfused to reduce the heart motion until the end of the study. Sea anemone venom ATXII (7 nM) was perfused into the heart to prolong APD, simulating the phenotype of LQT3 syndrome (25). Mannitol (26.1 g/L) was perfused to produce acute interstitial edema and expand intercellular space width adjacent to the GJ in the ID (27). Dextran 70 (40 g/L) was perfused to narrow the bulk interstitial space without changing the intercellular space width (37,38). Albumin (4 g/L) was perfused to narrow the intercellular space (37). Each heart was perfused for 15 min before pacing. These whole-heart optical mapping experiments include five groups: control (n = 13), ATXII (n = 14), ATXII + albumin (n = 7), ATXII + dextran 70 (n = 7), and ATXII + mannitol (n = 5). Left ventricular tissue was collected at the end of the optical mapping experiment and fixed as previously described (25) for transmission electron microscopy (TEM). Full details of the optical mapping and TEM are provided in the Supporting Material.
Isolated myocyte patch-clamp experiments
Myocyte isolation
Myocytes were isolated enzymatically from left ventricular free wall of guinea pig hearts using the enzymatic dispersion technique described previously (39). They were then resuspended in 10 mL of Dulbecco modified Eagle medium. All myocytes were stored at room temperature and used within 24 h of isolation.
Action potential recording
Patch technique was used to record APs under current clamp conditions. Briefly, the cells were bathed in a chamber continuously perfused with Tyrode’s solution composed of (mmol/L) NaCl 137, KCl 5.4, CaCl2 2.0, MgSO4 1.0, glucose 10, and HEPES 10 with NaOH added to achieve a pH of 7.35. Patch pipettes were pulled from borosilicate capillary glass and lightly fire-polished to a resistance of 0.9–1.5 M when filled with electrode solution composed of (in mM) aspartic acid 120, KCl 20, NaCl 10, MgCl2 2, and HEPES 5 with a pH of 7.3.
Myocytes were paced in the current clamp mode using a 1.5–2 diastolic threshold 5-ms current pulse at a basic cycle length (BCL) from 200 to 1000 ms. The experiments were performed at 35°C. Command and data acquisition were operated with an Axopatch 200B patch-clamp amplifier controlled by a personal computer using a Digidata 1200 acquisition board driven by pCLAMP 7.0 software (Axon Instruments, Foster City, CA). These AP recording studies include eight groups: control (n = 8), ATXII (n = 10), dextran 70 (n = 7), ATXII + dextran 70 (n = 10), albumin (n = 7), ATXII + albumin (8), mannitol (n = 6), and ATXII + mannitol (n = 3).
Late sodium current
Na+ currents were recorded by ruptured-patch whole-cell voltage clamp at room temperature. Microelectrodes were filled with a solution of (in mM) cesium methanesulfonate (CsMES) 120, tetraethylammonium chloride (TEACl) 20, MgCl2 2, HEPES 10, EGTA 11, MgATP 4 and brought to a pH of 7.3. Isolated myocytes were placed in the solution containing (in mmol/L) NaCl 25, N-methyl D-glucamine 120, CsCl 5.4, MgCl2 1.8, CaCl2 1.8, glucose 10, HEPES 10 and brought to a pH of 7.3. Nisodipine (1 μM) was used to block L-type calcium currents. Na+ currents were elicited from a holding potential of −80 to 0 mV for 250 ms. Tetrodotoxin (TTX,M) was used to obtain TTX-sensitive current. Late Na+ current was calculated from the percentage of the current measured at 200 ms to the peak current. These Na+ current recording studies include six groups: albumin control (n = 7), albumin (n = 8), dextran 70 control (n = 7), dextran 70 (n = 8), mannitol control (n = 10), and mannitol (n = 9).
Computational model formulation
Full details of the computational model are provided in Supporting Material. In brief, we simulated a 50-cell strand of guinea pig ventricular myocytes (40), incorporating a model of either a wild-type (WT) or LQT3-associated mutant (Y1795C) Na+ channel (Fig. 1; (26)). Na+ channel dynamics were governed by a 13-state Markov model (26,41), previously shown to produce a late Na+ current in the mutant channel, with channel states representing two modes of gating, a baseline “background” gating mode, and a non-inactivating “burst” mode (Fig. 1 C). As in prior work by us (19,25) and others (9), we account for nonuniform Na+ channel subcellular localization by spatially discretizing each cell into 12 membrane patches, 2 ID patches at the cell ends, and 10 axial patches. Cells were coupled via GJs and EpC: gap junctional coupling is represented by coupling ID nodes via a gap junctional resistance , and EpC is represented by a T-shaped junction of intercellular cleft and radial bulk resistances , which are proportional and inversely proportional to intercellular cleft width w, respectively. Intracellular nodes were coupled with a myoplasmic resistance (Fig. 1 A).
Figure 1.
Schematic of the computational model. (A) Electric circuit representation of coupled myocytes. Intracellular nodes are coupled via a myoplasmic resistance . End nodes are coupled via a gap junctional resistance . Extracellular potentials at the disk and intercellular cleft ( and , respectively) are governed by a T-shaped network of two axial resistances in the intercellular cleft and one radial resistance . (B) Shown is the Na+ concentration in diffusively coupled compartments, including intracellular Na+ in the axial and disk compartments ( and ) and extracellular Na+ in the intercellular cleft, bulk interstitial, “junctional,” and bulk spaces (, , , and ). (C) Markov model of the Na+ channel is shown. Upper (U) states represent the background mode of gating, and lower (L) states represent the burst mode with two open (O) conducting states. Rates at which the channel enters a certain state vary on tissue state (e.g., wild-type or mutant variation). To see this figure in color, go online.
We account for dynamic [Na+] in three extracellular spaces: 1) the intercellular cleft , mediated by ID Na+ current , and 2) the bulk interstitial space , mediated by axial Na+ current . These two extracellular spaces depend on intercellular cleft and bulk interstitial space volumes, respectively (proportional to intercellular cleft width w and bulk interstitial width W, respectively) (Fig. 1 B). We also represent 3) an additional “junctional” extracellular space diffusively coupled to the intercellular cleft and bulk interstitial space, in addition to a constant bulk extracellular solution . We similarly account for dynamic intracellular [Na+] in two compartments: the ID , mediated by ID Na+ current , and the axial intracellular space , mediated by axial Na+ current .
The cell strand was paced at one end with a specified BCL, and BCL was varied to determine rate dependence on APD and EAD formation for various conditions. We varied Na+ channel localization at the ID and axial cell membranes to investigate the interdependence of channel localization and extracellular volumes. We varied intercellular cleft width w and bulk interstitial width W, to mimic the osmotic agent modulation of the extracellular space volumes. In our parameter studies, we varied w from 10 to 50 nm, consistent with the perinexi width ranges in our prior work (10,25) and measures in this study. Measures of bulk interstitial width W are challenging to estimate because of the heterogeneity of the extracellular space. Using electron microscopy, Frank and Langer estimate that 36% of the cell membrane is within 200 nm of capallaries in the interstitial space (42), whereas Spach and colleagues measured an average bulk interstitial width of 2.1 μm (43). Thus, we consider a broad range and varied W from 100 nm to 2 μm.
Results
Ex vivo
Modulation of extracellular spaces by osmotic agents in drug-induced whole-heart model of Na+ channel gain of function
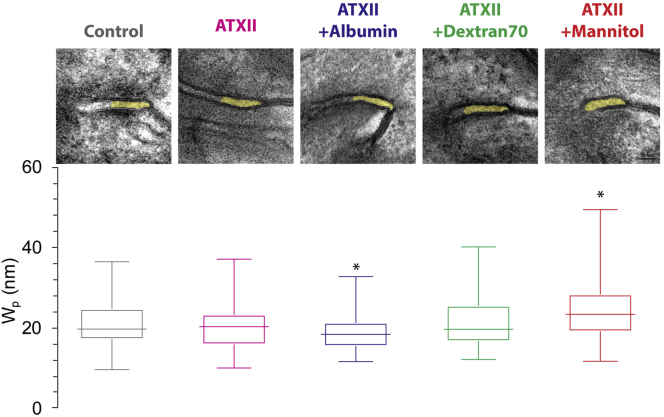
The focus of this study is to test the hypothesis that modulation of the intercellular cleft, but not bulk interstitial spaces, regulates the unmasking of the Na+ channel GOF phenotype. We first investigate a drug-induced Na+ GOF guinea pig model by adding three osmotic agents to modulate extracellular spaces. As in our previous work (25), TEM image analysis with an updated semiautomated measurement algorithm (44) shows that agent ATXII, inducing late Na+ current, does not alter width in the GJ-adjacent perinexal region , compared with control (Fig. 2). The addition of albumin significantly reduces compared with control (control: nm; ATXII: nm; ATXII + albumin: nm). In contrast, the addition of dextran 70 does not significantly alter relative to control. For comparison, we reanalyze our previously collected images from mannitol-perfused hearts with the updated measurement algorithm, and the results support our previous finding that mannitol significantly increases compared with control (25,44). Importantly, measurements in hearts with the osmotic agents in the presence of ATXII are comparable to our previous measurements in the presence of the osmotic agents alone (24,25,37). This suggests that ATXII alone neither alters extracellular spaces alone nor synergistically with osmotic agents (20,25). Thus, in a drug-induced Na+ channel GOF model, we can perturb extracellular spaces via different osmotic agents with distinct effects on intercellular cleft and bulk interstitial spaces: albumin, which narrows both intercellular cleft and bulk interstitial spaces (27); dextran 70, which minimally affects intercellular cleft and narrows bulk interstitial spaces (34); and mannitol, which expands both intercellular cleft and bulk interstitial spaces (27).
Figure 2.
Effects of osmotic agents on intercellular cleft spacing in tissue. Top: TEM images of control (gray), ATXII (magenta), ATXII + albumin (blue), ATXII + dextran 70 (green), and ATXII + mannitol (red). Bottom: summary intercellular perinexus width measurements under each condition. Asterisk indicates p < 0.05 compared with control, n = 3 hearts/condition, 10–16 images/heart. One-sample Wilcoxon test, replicates averaged within a heart, and hearts averaged for each condition. To see this figure in color, go online.
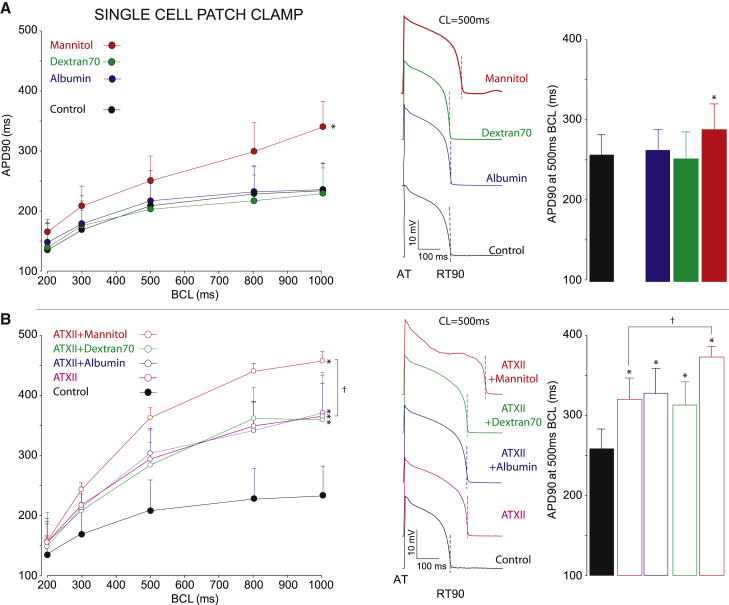
We next performed optical mapping experiments of isolated guinea pig hearts in the presence or absence of ATXII and the osmotic agents (Fig. 3). APD restitution was assessed by pacing at a fixed BCL, and steady-state values of APD were averaged over the optically mapped epicardial surface. Under control conditions, the presence of any of the osmotic agents alone does not alter APD restitution curves compared with control (Fig. 3 A, left), as in our prior work (24,25,37). APD at the bradycardia BCL of 500 ms is also not significantly different under any condition (Fig. 3 A, right), demonstrating that modulating the extracellular spaces in the absence of enhanced late Na+ has negligible effects on repolarization.
Figure 3.
Osmotic agent modulation in guinea pig heart optical mapping experiments. (A) Left: action potential duration restitution at 90% repolarization (APD90) is shown as a function of BCL without ATXII. There are no significant differences between control (gray) and control + albumin (blue), control + dextran 70 (green), or control + mannitol (red); n = 12 control, 4 ATXII + albumin, 4 ATXII + dextran 70, 4 ATXII + mannitol; p = ns (second-order polynomial regression analysis of intercept and two-way ANOVA). Middle: shown are representative optical APs at BCL = 500 ms time aligned at activation (AT) and 90% repolarization (RT90) annotated. Right: summary APD90 at BCL = 500 ms reveals no significant difference between control and any osmotic agent (p = ns, two-way ANOVA). (B) Left: APD90 restitution curves are significantly different from control under conditions of ATXII alone (magenta), ATXII + albumin (blue), ATXII + dextran 70 (green), and ATXII + mannitol (red); n = 13 control, 6 ATXII, 6 ATXII + albumin, 6 ATXII + dextran 70, 7 ATXII + mannitol; ∗p < 0.05 relative to control (two-way ANOVA and paired Student’s t-test). APD90 restitution is significantly different between ATXII + mannitol relative to ATXII alone (†p < 0.05). Middle: representative optical APs at 500 ms reveal significant APD90 prolongation, particularly at 500 ms BCL. Right: summary APD90 at BCL = 500 ms reveals significant APD prolongation between control and ATXII + the osmotic agents (∗p < 0.05, two-way ANOVA and Student’s t-test), and APD is further prolonged between with ATXII + mannitol relative to ATXII alone (†p < 0.05). Error bars denote standard deviation. To see this figure in color, go online.
As expected, ATXII significantly prolongs APD and significantly shifts the APD restitution curve upward (Fig. 3 B, left), consistent with previous work (25). APD for the ATXII + albumin and ATXII + dextran 70 cases is also significantly prolonged compared with control. However, APD restitution curves for ATXII alone, ATXII + albumin, or ATXII + dextran 70 are not significantly different. Even during bradycardia, APD is significantly prolonged with ATXII, ATXII + albumin, and ATXII + dextran 70, without significant differences measured within this group (Fig. 3 B, right). Our previous work (25) predicted that narrowing the perinexal space could mask the Na+ channel GOF phenotype by promoting Na+ intercellular cleft depletion and reducing the late Na+ current. Thus, we would expect that perinexal width narrowing due to the addition of albumin would shorten APD. However, the absence of APD shortening suggests that in healthy guinea pig hearts, the perinexal width for baseline conditions (i.e., without osmotic agents) is sufficiently narrow to mitigate the late Na+ current in the presence of the concentration of ATXII used here, such that further perinexal narrowing has minimal effects. We will consider this further in simulations.
For comparison, we reproduce APD measurements for ATXII + mannitol, and as previously shown (25), the addition of mannitol significantly alters the APD restitution curve relative to control and ATXII alone (Fig. 3 B, left), but importantly, the significant APD prolongation occurs preferentially during bradycardia (Fig. 3 B, right) compared with control and ATXII alone, which again, we note is predicted to be due to reduced intercellular cleft Na+ depletion.
In vitro
Effects of osmotic agents on electrophysiology of isolated myocytes
We next performed patch-clamp studies on isolated myocytes in the presence of the individual osmotic agents to assess the respective individual contributions of the drugs to single-cell electrophysiology. Specifically, we were interested in the effects of the individual osmotic agents on late Na+ current and APD without and with ATXII-induced enhanced late Na+ currents to determine whether the agents alter either of these parameters. Importantly, for all three osmotic agents, we found no significant differences in the late Na+ current, normalized to the peak Na+ current, compared with the corresponding control (Fig. 4, A and B). The magnitudes of peak and late Na+ were also not significantly different in the presence of the osmotic agents compared with their corresponding controls (Fig. S1).
Figure 4.
Effects of osmotic agents on single-cell late Na+ current. (A) Representative Na+ current traces are shown as a function of time. (B) Shown is a summary of late Na+ current, relative to peak Na+ current, measurements. Control cases (black) are compared with three osmotic agents: albumin (blue), dextran 70 (green), and mannitol (red). n = 7 albumin control, 8 albumin; 7 dextran 70 control, 8 dextran 70; 7 mannitol control, 7 mannitol. ∗p < 0.05 compared with control (paired Student’s t-test). To see this figure in color, go online.
In the absence of ATXII, albumin and dextran 70 had a negligible effect on APD compared with control in the isolated cells for all BCL values (Fig. 5 A). However, we found that mannitol significantly prolongs APD relative to control. As expected, and consistent with optical mapping results, ATXII significantly prolongs APD and shifts the APD restitution curve upward (Fig. 5 B). Importantly, APD for ATXII + albumin and ATXII + dextran 70 are similarly prolonged, relative to control, and with values similar to ATXII alone. The addition of mannitol to ATXII in isolated myocytes further prolongs APD.
Figure 5.
Effects of osmotic agents on single-cell action potential restitution. (A) Left: shown is action potential duration restitution at 90% repolarization (APD90) as a function of BCL without ATXII. There is an increase in APD90 in control + mannitol (red) compared with control (black), control + albumin (blue), and control + dextran 70 (green). Middle: representative APs at BCL = 500 ms. Right: summary APD90 data at BCL = 500 ms. n = 8 control, 7 albumin, 7 dextran 70, 6 mannitol, ∗p < 0.05 vs. control (second-order polynomial regression analysis of intercept and two-way ANOVA). (B) Left: shown is APD90 restitution with the addition of ATXII. Control (black) cases compared with ATXII alone (pink), ATXII + albumin (blue), ATXII + dextran 70 (green), and ATXII + mannitol (red). Middle: shown are representative APs at BCL = 500 ms. Right: shown is summary APD90 data at BCL = 500 ms. n = 8 control, 10 ATXII, 8 ATXII + albumin, 10 ATXII + dextran 70, 3 ATXII + mannitol. Error bars denote standard deviation. To see this figure in color, go online.
Importantly, the observation that mannitol does not enhance late Na+ suggests that APD prolongation in isolated cells occurs via another mechanism. Further, mannitol-mediated APD prolongation in isolated cells is in contrast with optical mapping experiments that found no significant difference between APD or APD restitution (Fig. 3 A) in the presence of mannitol (but absence of ATXII), also as in our prior work (25). We hypothesize that the APD prolongation observed in isolated cells is potentially due to partial block of human Ether a-go-go Related Gene (hERG) potassium current, as prior work has previously shown a mannitol dose-dependent reduction in hERG current amplitude (45). We will later investigate the different mannitol responses between isolated cells and cardiac tissue using numerical simulations.
In silico
Predicted effects of extracellular space modulation in simulated cardiac tissue model of LQT3
We next simulate cardiac tissue comprising myocytes with either WT or mutant (Y1795C) Na+ channels and modulated intercellular cleft and bulk interstitial space widths to compare with experimental changes observed due to the addition of different osmotic agents. As we describe in the Introduction, mannitol, albumin, and dextran 70 alter intercellular spacing (24,37): mannitol widens both intercellular and bulk interstitial space, albumin narrows both intercellular and bulk interstitial space, and dextran 70 has no effect on intercellular space but narrows bulk interstitial space.
For simulated WT tissue, APD gradually increases for increasing BCL, consistent with typical APD restitution (Fig. 6 A, black). The addition of osmotic agents slightly increases APD, but no EADs are produced. For the mutant tissue, under control conditions (i.e., no simulated osmotic agents), APD is prolonged, more so for longer BCLs, but no EADs are observed, even for bradycardic conditions (Fig. 6 B, magenta). The simulated addition of dextran 70 to the mutant tissue results in nearly identical APD measurements across all BCLs (dashed green). The simulated addition of albumin to the mutant tissue slightly shortens APD (solid blue), consistent with reduced late Na+ current as a consequence of intercellular cleft narrowing. However, the APD differences between simulations with and without the simulated effects of albumin are small, which explains the lack of differences observed in optical mapping experiments because these differences are likely within experimental detection limits. Consistent with our prior work (25), the simulated addition of mannitol prolongs APD and promotes EAD formation in mutant tissue for BCLs longer than 1000 ms (solid red).
Figure 6.
Simulations of in silico effects of different osmotic agents on APD. APD (maximal value over the last three beats) is shown as a function of BCL for WT (black) and Y1795C (magenta) cardiac tissue, with the simulated addition of the osmotic agents albumin (blue), dextran 70 (green), and mannitol (red) to the Y1795C case. Parameters are as follows: control and Y1795C: intercellular cleft width w = 20 nm, bulk interstitial space width W = 1 μm; mannitol: w = 40 nm, W = 2 μm; albumin: w = 15 nm, W = 0.5 μm; dextran 70: w = 20 nm, W = 0.5 μm; all cases: Na+ ID localization = 90%. To see this figure in color, go online.
Extracellular sodium signaling in wild-type and mutant tissue
Motivated by the findings that modulation of extracellular spaces can alter APD in simulated LQT3 cardiac tissue models and, specifically, that changes in the intercellular cleft (and not bulk interstitial spaces) are critical regulators of APD changes, we more thoroughly investigate the mechanism underlying these responses by considering changes in extracellular Na+ signaling. We investigate the individual contributions of intercellular cleft and bulk interstitial spaces on Na+ signaling, considering two extreme cases of narrow and wide intercellular cleft spacing and fixed bulk interstitial spacing (Fig. 7). We first consider the case of a nominal bulk interstitial spacing of 1 μm and high (90%) preferential localization of Na+ channels at the ID. The time series for transmembrane voltage , Na+ current at the ID , and Na+ channel open probability at the ID are shown for a cell in the middle of the 50-cell chain paced at a BCL of 1000 ms. Consistent with our prior work (25), we find that WT cell strands did not produce EADs in either wide ( nm, black) or narrow ( nm, red) intercellular cleft widths. The time series for and are similar for the two different intercellular cleft widths, with channels at the ID rapidly opening and then inactivating and closing (Fig. 7, B and C). In mutant cell strands with narrow intercellular cleft width, the channel open probability is increased after the AP upstroke (Fig. 7 C, green); however, only a small late Na+ current is present at the ID, such that APD is minimally prolonged and EADs are not formed. In contrast, in mutant cell strands with wide intercellular cleft width (blue), APD is prolonged and EADs form on alternating beats, driven by a significantly enhanced late Na+ current at the ID.
Figure 7.
EADs are suppressed in mutant cardiac tissue with narrow intercellular clefts. (A) Transmembrane voltage , (B) Na+ current at the ID , and (C) Na+ channel open probability at the ID are shown as functions of time in tissue of myocytes with WT and mutant (Y1795C) Na+ channels for narrow (20 nm) and wide (35 nm) intercellular cleft widths (w). For clarity, traces are shown for cell 25 out of 50 in the one-dimensional chain of cells. Parameters are as follows: BCL = 1000 ms, bulk interstitial space width W = 1 μm, and Na+ ID localization = 90%. To see this figure in color, go online.
In Fig. 8, we investigate the dynamics of extracellular Na+ and associated changes in the Na+ current driving force for these four cases. For simulated WT tissue with a narrow intercellular cleft width (Fig. 8 A, red), rapidly depletes during the AP upstroke, resulting in a transiently decreased Na+ reversal potential at the ID, , which in turn reduces Na+ driving force. For a wide intercellular cleft width (black), is reduced to a smaller extent. However, in simulated WT tissue, for both intercellular cleft widths, recovers to near baseline levels before the next beat. In simulated mutant tissue with narrow intercellular cleft width, as in the WT strands, rapidly depletes during the AP upstroke (Fig. 8, green). The increased mutant channel open probability produces a small-amplitude late Na+ current (Fig. 7 B, green); however, this current drives a secondary depletion in , which further reduces the and the driving force and thus prevents EAD formation. As we describe in our prior work (25), this secondary depletion introduces a negative feedback, which can be interpreted physiologically as a protective mechanism that suppresses EAD formation. In contrast, in simulated mutant tissue with a wide intercellular cleft width, is depleted to a lesser extent (Fig. 8, blue), which maintains an elevated Na+ reversal potential at the ID, a larger driving force and, thus, a persistent late Na+ current, which facilitates EAD formation.
Figure 8.
Intercellular cleft sodium depletion drives EAD suppression in mutant cardiac tissue with narrow intercellular clefts. [Na+] in the (A) intercellular cleft, , and (B) bulk interstitial space, , and the reversal potential at the (C) ID and at the (D) axial membrane are shown as functions of time in tissue of myocytes with wild-type (WT) and mutant (Y1795C) Na+ channels for narrow (20 nm) and wide (35 nm) intercellular cleft widths (w). For clarity, traces are shown for cell 25 out of 50 in the one-dimensional chain of cells. Parameters are the same as Fig. 7. To see this figure in color, go online.
We next investigate the dynamics of [Na+] in the bulk interstitial space and the Na+ reversal potential on the axial membrane (Fig. 8, B and D). We find essentially no differences between the four cases: there is negligible depletion of and thus minimal change in throughout the AP. Thus, our model predicts that for a nominal bulk interstitial space and high Na+ channel localization at the ID, [Na+] changes in bulk interstitial space have little effect on EAD formation; in contrast, [Na+] changes in the intercellular cleft are a significant regulator of EADs. The lack of changes in is not unexpected, because of both low channel localization on the axial membrane and larger volume of the bulk interstitial space (relative to the intercellular cleft). Thus, we next performed a thorough investigation of the interdependence of Na+ channel localization, the intercellular cleft, and bulk interstitial spaces on APD and EAD formation in the simulated LQT3 mutant tissue model.
Interdependence of sodium channel localization, intercellular cleft, and bulk interstitial space
As noted above, experimental investigation using osmotic agents in general varied both the intercellular cleft and bulk interstitial space in conjunction. In silico, we can vary these extracellular spaces independently and further investigate to what extent the relationship with these spaces also depends on Na+ channel ID localization.
We first consider the role of Na+ channel localization at the ID on APD and EAD formation for a nominal bulk interstitial width of 1 μm (Fig. 9). Localization of Na+ channels at the ID was varied from 10 to 90%, with the remaining channels uniformly distributed on the axial cell membrane. Consistent with our previous study (25), changes in intercellular cleft width or Na+ channel localization minimally affect APD in WT tissues (Fig. 9 A). In mutant cell strands with high Na+ ID localization, APD increases as intercellular cleft width w increases (Fig. 9 B, green, magenta), as in Fig. 7. In general, APD increases as Na+ ID localization is reduced, such that EADs form for narrower intercellular clefts. For Na+ channel ID localization of 50% or less, EADs are predicted for all intercellular cleft widths (black, red, blue). These results can be explained as follows: lower Na+ channel ID localization reduces , which results in less intercellular cleft Na+ depletion during both the AP upstroke and subsequent late Na+ current. Further, the driving force for Na+ channels on the axial membrane remains high throughout the AP because of negligible depletion of Na+ for the bulk interstitial space, as in Fig. 8 B.
Figure 9.
APD dependence on intercellular cleft width and Na+ channel localization in WT and mutant tissue. (A) In WT tissue, APD negligibly depends on intercellular cleft width and Na+ channel localization. (B) In mutant tissue, decreasing Nav1.5 localization prolongs APD. Jumps in APD, corresponding with longer EADs, occur for smaller intercellular cleft width, as Na+ channel localization decreases. Parameters: BCL = 1000 ms. To see this figure in color, go online.
We next studied the interdependence of intercellular cleft and bulk interstitial widths and Na+ channel localization on APD in mutant cell strands (Fig. 10). We considered a wide range of bulk interstitial widths, from 100 nm to 2 μm. For most levels of Na+ channel ID localization levels, APD did not depend on bulk interstitial width for all intercellular cleft widths (Fig. 10, A, C, and D). That is, for high Na+ channel ID localization, APD and EAD formation strictly depends only on intercellular cleft width, whereas for low Na+ channel ID localization, EADs form for all intercellular cleft widths. Interestingly, our model does predict a specific regime for wide intercellular cleft and moderately high Na+ channel ID localization of 70% (Fig. 10 B) in which narrow bulk interstitial space prolongs APD; however, this only occurs for the case in which intercellular cleft and bulk interstitial space are comparable in size (50 and 100 nm, respectively), which would be considered an extreme nonphysiological condition. In Supporting Material, we illustrate a broader visualization of the intercellular cleft and bulk interstitial space parameter space (Fig. S2). As in Fig. 10, bulk interstitial space in general negligibly alters APD regardless of intercellular cleft width or Na+ channel ID localization, except marginally for a few conditions. We further illustrate that the absence of APD dependence on the bulk interstitial space arises because of the lack of local Na+ depletion, a consequence of both the large bulk interstitial space volume and distribution of Na+ channels along the larger surface area of the axial membrane. In Fig. S3, we consider the case of a narrow (100 nm) bulk interstitial space and low ID localization of 10% (i.e., high axial membrane localization) and find that, even for these conditions that promote local Na+ depletion to the largest extent, [Na+] in the bulk interstitial space varies by less than 1 mM throughout the simulation, leading to a negligible decrease in Na+ driving force and thus sustained late Na+ current and EAD formation.
Figure 10.
Bulk interstitial space width negligibly alters APD in mutant cardiac tissue. APD is shown as a function of bulk interstitial space width for varying intercellular cleft widths for Na+ channel ID localization of (A) 90%, (B) 70%, (C) 50%, and (D) 10% (uniform distribution) in mutant (Y1795C) tissue. Parameters: BCL = 1000 ms. To see this figure in color, go online.
Simulations predict the differential mannitol response in whole-heart experiments and isolated cells
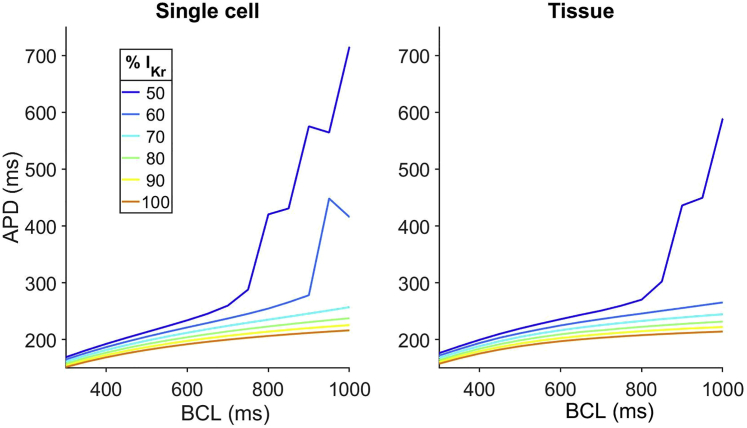
Finally, we return to the seemingly inconsistent experimental result that, in the absence of ATXII, mannitol prolongs APD in isolated myocytes (but not via enhanced late Na+ current) but does not prolong APD in intact whole hearts. We hypothesize that the differences may occur because of mannitol-induced partial block of the hERG potassium channel that manifests differently in isolated cells versus whole hearts. We thus performed simulations in both single-cell and tissue WT models in which the maximal conductance for the current was varied (Fig. 11). In single-cell simulations, reducing conductance by 40% (i.e., increasing block of the hERG channel) significantly prolongs APD and promotes EADs at long BCLs. However, in simulated WT tissue, the hERG blocking effects are mitigated, such that significant APD prolongation and EAD formation requires additional block.
Figure 11.
Simulations of the effects of hERG block on WT single cells and cardiac tissue. APD is shown as a function of BCL for different levels of current. Reducing current in single cells prolongs APD to a greater extent than in tissue. Tissue parameters: intercellular cleft width w = 20 nm, bulk interstitial space width m, and Na+ ID localization = 90%. To see this figure in color, go online.
Discussion
In this study, we demonstrate in cardiac tissue with gain-of-function Na+ channel activity that intercellular cleft Na+ nanodomain signaling critically regulates cardiac repolarization and formation of EADs. Simulations predict that Na+ channel localization and intercellular cleft width modulate the temporal dynamics of intercellular cleft [Na+] and thus regulate a negative-feedback mechanism that can suppress or promote EAD formation. In contrast, our model predicts that Na+ in the bulk interstitial space minimally regulates APD. These results are a consequence of both the high channel density and small volume at the intercellular cleft, which can drive rapidly occurring formation and collapse of Na+ nanodomains in the intercellular cleft space. Even in the setting of low Na+ channel ID localization and thus high Na+ channel density on the axial membrane, bulk interstitial space [Na+] is minimally altered because of both the large bulk interstitial space volume and Na+ channels distributed along the entire length of the cell. Na+ pumps and exchangers are also present on the axial membrane, critically maintaining high [Na+] levels in the bulk interstitial space. Although there is some evidence for Na+ pumps and exchangers also present at the ID (46), the densities are, to our knowledge, not well known (47).
Optical mapping experiments showed that the addition of osmotic agents to a drug-induced model of Na+ channel GOF with enhanced late Na+ current can alter APD dramatically. Importantly, we demonstrate that the three osmotic agents investigated separately did not enhance late Na+ current. In the presence of ATXII-induced enhanced late Na+ current, narrowing the intercellular cleft or the bulk interstitial space with albumin and dextran 70, respectively, did not further reduce APD prolongation, which differs from the small APD shortening predicted in silico but is consistent with experimentally measurable differences. Our prior work (25) showed that the addition of mannitol widened the intercellular cleft space and prolonged APD. Here, isolated cell patch-clamp experiments demonstrate that mannitol does not separately enhance late Na+ current, and thus APD prolongation, in isolated hearts, and the presence of ATXII and mannitol can be attributed to intercellular cleft expansion, with likely additional contributions from partial hERG block as well. Simulations of partial hERG block reproduce the differences observed in APD measurements between isolated cell patch-clamp recordings and optical mapping experiments under control conditions with mannitol, demonstrating that the effects of hERG block are mitigated in tissue; Pueyo and colleagues observed similar differences in EAD formation between single-cell and tissue simulations and experiments after hERG block (48). However, we caution that the different mannitol responses may be due to several factors, including cellular remodeling that occurs because of differences in the preparations, tissue digestion, etc.
Many prior computational studies have demonstrated that EpC can play a critical role in regulating electrical conduction in the heart (9,15,16,19,49). As noted above, EpC can arise via several mechanisms; in general, electrical field effects that arise in the intercellular cleft, and subsequent modulation of peak Na+ current, have been shown to be the primary mechanism responsible for conduction changes. In our prior work (25), we demonstrated that electrical field effects play a minimal role in regulating changes in repolarization in the setting of an Na+ channel gain of function, whereas Na+ ion depletion in the extracellular space is the key regulating factor. Here, we expand on this mechanism and demonstrate that extracellular Na+ ion depletion specifically in the intercellular cleft and not bulk interstitial space is critical, regardless of Na+ channel localization. Interestingly, the detailed model used by Mori and colleagues accounted for intracellular and extracellular Na+, and they demonstrate local intercellular cleft depletion after the AP upstroke (16); however, they note that this depletion plays a minimal role in modulating conduction under normal conditions. In conjunction with our work, this suggests that different mechanisms underlying EpC can play different modulatory and protective roles in both normal and pathological settings.
Prior experimental work from our group has demonstrated that modulating EpC is complex and highly dependent on physiological conditions. For example, we showed under high extracellular Na+ concentrations that albumin and associated intercellular cleft narrowing increased conduction, and mannitol and associated intercellular cleft expansion slowed conduction (27), consistent with predictions of EpC enhancing conduction. However, under reduced extracellular Na+ conditions, intercellular cleft narrowing was associated with conduction slowing (23). Under metabolic ischemic conditions, we previously found that regulation by intercellular cleft narrowing/expansion was enhanced (23). Further, in contrast with the result stated above, albumin facilitated ischemia-induced conduction slowing, whereas mannitol-induced perinexi expansion preserved conduction. Although seemingly contradictory, our experimental findings are consistent with theoretical models of EpC, which have demonstrated that the relationship between intercellular cleft width and conduction is biphasic, in which narrowing the intercellular cleft can increase conduction velocity due to enhanced EpC but also decrease conduction velocity due to self-attenuation, as described above (9,16).
Critically, models predict that the relationship between EpC and intercellular cleft width is also highly dependent on Na+ channel localization, gap junctional coupling, and extracellular Na+ perfusate (9,15,18), all of which may be altered under physiological or disease conditions. For example, during development, both the voltage-gated Na+ channel and GJ protein connexin-43 redistribute from primarily diffuse or lateral membrane localization during prenatal stages to the ID during early postnatal and adult stages (50). In contrast, abnormal localization of ID proteins has been demonstrated in arrhythmogenic cardiomyopathy, with reduced Na+ channel expression at the ID arising because of dysfunctional trafficking (51,52). Reduced Na+ channel ID localization has also been associated with a Brugada syndrome variant with a loss of expression of the ID protein plakophilin-2 (53). Our work here has demonstrated similarly complex regulation by EpC and Na+ channel localization on late Na+ current in the setting of Na+ channel GOF, and we speculate that altering EpC (e.g., by reducing Na+ current or altering bulk extracellular Na+) will also modulate APD prolongation and EAD formation.
Currently, patients with LQT3 are treated with drugs including β-blockers, mexiletine, and ranolazine, as well as implantation of implantable cardioverter-defibrillators (54). Although the presence of a late Na+ current is consistent across all LQT3-associated mutations, the mechanism underlying the Na+ channel gain of function can vary substantially between different genetic mutation variants. Zhu and colleagues recently showed that drug efficacy can vary substantially across different LQT3 variants (55), suggesting that the efficacy of therapeutics directly targeting late Na+ currents is highly dependent on the individual patient. In contrast, the mechanism underlying late Na+ current suppression via intercellular cleft narrowing is independent of the specific genetic mutation, because current is reduced by reducing the current driving force, not the channel conductivity. Note that we further demonstrate this genotype invariance in our prior work (25). Thus, our study suggests that therapeutics narrowing intercellular cleft width may provide an alternative approach to reduce arrhythmic burden in LQT3 patients.
Inherently, computational models are simplifications of complex physiological systems. Although our modeling approach incorporates significant subcellular details, including intercellular fluxes and changes in Na+ channel localization within the ID, and perturbations to the intercellular cleft width, this representation is still a simplification of the physiological intercellular cleft and ID geometry. The ID geometry is highly heterogeneous, with intercellular spacing varying throughout, whereas our model assumes a simplified cellular geometry and a single value for the intercellular spacing. Although these differences likely account for quantitative differences between model and experiments, our simulations consistently predict qualitative trends, thus demonstrating potential novel mechanisms for regulation of Na+ channel gain of function.
Conclusions
In studies using isolated cardiomyocytes, isolated hearts, and detailed computational models, we demonstrate that intercellular cleft width, but not bulk interstitial spacing, is the critical factor in EAD formation in the setting of Na+ channel gain of function. Narrow intercellular cleft widths prevent EAD formation due to Na+ depletion near the ID, ultimately preventing the late Na+ current generation. Additionally, the localization of Na+ channels is crucial in governing APD; higher ID Na+ channel localization shortens APD. Our work suggests that modulation of the extracellular space may be a novel therapeutic target for inherited or acquired Na+ channel gain of function, specifically targeting agents that modulate intercellular cleft spacing.
Acknowledgments
This study was supported by funding from the National Institutes of Health, grant number R01HL138003 (S.H.W., S.P., and I.D.).
Editor: Eric Sobie.
Footnotes
Seth H. Weinberg and Steven Poelzing contributed equally to this work.
Supporting Material can be found online at https://doi.org/10.1016/j.bpj.2020.04.014.
Contributor Information
Seth H. Weinberg, Email: weinberg.147@osu.edu.
Steven Poelzing, Email: poelzing@vtc.vt.edu.
Supporting Citations
References (56, 57, 58, 59, 60, 61) appear in the Supporting Material.
Author Contributions
M.B.N. performed computational studies, performed statistical analysis, and wrote the original manuscript draft. A.G.-S. performed optical mapping and TEM experiments. X. Wan performed single-cell patch-clamp experiments and contributed to the manuscript draft. X. Wu contributed to the manuscript draft. S.P., S.H.W., and I.D. designed simulations and experiments, provided funding, and contributed to the manuscript draft.
Supporting Material
References
- 1.Belardinelli L., Giles W.R., Shryock J.C. Cardiac late Na+ current: proarrhythmic effects, roles in long QT syndromes, and pathological relationship to CaMKII and oxidative stress. Heart Rhythm. 2015;12:440–448. doi: 10.1016/j.hrthm.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Wang Q., Shen J., Keating M.T. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80:805–811. doi: 10.1016/0092-8674(95)90359-3. [DOI] [PubMed] [Google Scholar]
- 3.Burashnikov A., Antzelevitch C. Acceleration-induced action potential prolongation and early afterdepolarizations. J. Cardiovasc. Electrophysiol. 1998;9:934–948. doi: 10.1111/j.1540-8167.1998.tb00134.x. [DOI] [PubMed] [Google Scholar]
- 4.Clancy C.E., Rudy Y. Linking a genetic defect to its cellular phenotype in a cardiac arrhythmia. Nature. 1999;400:566–569. doi: 10.1038/23034. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg I., Horr S., Zhang L. Risk for life-threatening cardiac events in patients with genotype-confirmed long-QT syndrome and normal-range corrected QT intervals. J. Am. Coll. Cardiol. 2011;57:51–59. doi: 10.1016/j.jacc.2010.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barsheshet A., Dotsenko O., Goldenberg I. Genotype-specific risk stratification and management of patients with long QT syndrome. Ann. Noninvasive Electrocardiol. 2013;18:499–509. doi: 10.1111/anec.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimizu W., Antzelevitch C. Differential effects of beta-adrenergic agonists and antagonists in LQT1, LQT2 and LQT3 models of the long QT syndrome. J. Am. Coll. Cardiol. 2000;35:778–786. doi: 10.1016/s0735-1097(99)00582-3. [DOI] [PubMed] [Google Scholar]
- 8.de Lange E., Xie Y., Qu Z. Synchronization of early afterdepolarizations and arrhythmogenesis in heterogeneous cardiac tissue models. Biophys. J. 2012;103:365–373. doi: 10.1016/j.bpj.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kucera J.P., Rohr S., Rudy Y. Localization of sodium channels in intercalated disks modulates cardiac conduction. Circ. Res. 2002;91:1176–1182. doi: 10.1161/01.res.0000046237.54156.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veeraraghavan R., Lin J., Poelzing S. Sodium channels in the Cx43 gap junction perinexus may constitute a cardiac ephapse: an experimental and modeling study. Pflugers Arch. 2015;467:2093–2105. doi: 10.1007/s00424-014-1675-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veeraraghavan R., Gourdie R.G. Stochastic optical reconstruction microscopy-based relative localization analysis (STORM-RLA) for quantitative nanoscale assessment of spatial protein organization. Mol. Biol. Cell. 2016;27:3583–3590. doi: 10.1091/mbc.E16-02-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leo-Macias A., Agullo-Pascual E., Delmar M. Nanoscale visualization of functional adhesion/excitability nodes at the intercalated disc. Nat. Commun. 2016;7:10342. doi: 10.1038/ncomms10342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agullo-Pascual E., Lin X., Delmar M. Super-resolution imaging reveals that loss of the C-terminus of connexin43 limits microtubule plus-end capture and NaV1.5 localization at the intercalated disc. Cardiovasc. Res. 2014;104:371–381. doi: 10.1093/cvr/cvu195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhett J.M., Ongstad E.L., Gourdie R.G. Cx43 associates with Na(v)1.5 in the cardiomyocyte perinexus. J. Membr. Biol. 2012;245:411–422. doi: 10.1007/s00232-012-9465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin J., Keener J.P. Modeling electrical activity of myocardial cells incorporating the effects of ephaptic coupling. Proc. Natl. Acad. Sci. USA. 2010;107:20935–20940. doi: 10.1073/pnas.1010154107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mori Y., Fishman G.I., Peskin C.S. Ephaptic conduction in a cardiac strand model with 3D electrodiffusion. Proc. Natl. Acad. Sci. USA. 2008;105:6463–6468. doi: 10.1073/pnas.0801089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sperelakis N. An electric field mechanism for transmission of excitation between myocardial cells. Circ. Res. 2002;91:985–987. doi: 10.1161/01.res.0000045656.34731.6d. [DOI] [PubMed] [Google Scholar]
- 18.Hichri E., Abriel H., Kucera J.P. Distribution of cardiac sodium channels in clusters potentiates ephaptic interactions in the intercalated disc. J. Physiol. 2018;596:563–589. doi: 10.1113/JP275351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinberg S.H. Ephaptic coupling rescues conduction failure in weakly coupled cardiac tissue with voltage-gated gap junctions. Chaos. 2017;27:093908. doi: 10.1063/1.4999602. [DOI] [PubMed] [Google Scholar]
- 20.George S.A., Bonakdar M., Poelzing S. Extracellular sodium dependence of the conduction velocity-calcium relationship: evidence of ephaptic self-attenuation. Am. J. Physiol. Heart Circ. Physiol. 2016;310:H1129–H1139. doi: 10.1152/ajpheart.00857.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veeraraghavan R., Lin J., Poelzing S. Potassium channels in the Cx43 gap junction perinexus modulate ephaptic coupling: an experimental and modeling study. Pflugers Arch. 2016;468:1651–1661. doi: 10.1007/s00424-016-1861-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veeraraghavan R., Hoeker G.S., Gourdie R.G. The adhesion function of the sodium channel beta subunit (β1) contributes to cardiac action potential propagation. eLife. 2018;7:e37610. doi: 10.7554/eLife.37610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.George S.A., Sciuto K.J., Poelzing S. Extracellular sodium and potassium levels modulate cardiac conduction in mice heterozygous null for the Connexin43 gene. Pflugers Arch. 2015;467:2287–2297. doi: 10.1007/s00424-015-1698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.George S.A., Hoeker G., Poelzing S. Modulating cardiac conduction during metabolic ischemia with perfusate sodium and calcium in guinea pig hearts. Am. J. Physiol. Heart Circ. Physiol. 2019;316:H849–H861. doi: 10.1152/ajpheart.00083.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greer-Short A., George S.A., Weinberg S.H. Revealing the concealed nature of long-QT type 3 syndrome. Circ. Arrhythm. Electrophysiol. 2017;10:e004400. doi: 10.1161/CIRCEP.116.004400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clancy C.E., Tateyama M., Kass R.S. Insights into the molecular mechanisms of bradycardia-triggered arrhythmias in long QT-3 syndrome. J. Clin. Invest. 2002;110:1251–1262. doi: 10.1172/JCI15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veeraraghavan R., Salama M.E., Poelzing S. Interstitial volume modulates the conduction velocity-gap junction relationship. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H278–H286. doi: 10.1152/ajpheart.00868.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rangel-Castilla L., Gopinath S., Robertson C.S. Management of intracranial hypertension. Neurol. Clin. 2008;26:521–541, x. doi: 10.1016/j.ncl.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arakawa M., Jerome E.H., Staub N.C. Effects of dextran 70 on hemodynamics and lung liquid and protein exchange in awake sheep. Circ. Res. 1990;67:852–861. doi: 10.1161/01.res.67.4.852. [DOI] [PubMed] [Google Scholar]
- 30.Stahley S.N., Bartle E.I., Mattheyses A.L. Molecular organization of the desmosome as revealed by direct stochastic optical reconstruction microscopy. J. Cell Sci. 2016;129:2897–2904. doi: 10.1242/jcs.185785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Troyanovsky S. Adherens junction assembly. Subcell. Biochem. 2012;60:89–108. doi: 10.1007/978-94-007-4186-7_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodenough D.A., Paul D.L. Gap junctions. Cold Spring Harb. Perspect. Biol. 2009;1:a002576. doi: 10.1101/cshperspect.a002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Berg B.M., Vink H., Spaan J.A. The endothelial glycocalyx protects against myocardial edema. Circ. Res. 2003;92:592–594. doi: 10.1161/01.RES.0000065917.53950.75. [DOI] [PubMed] [Google Scholar]
- 34.Fleischhauer J., Lehmann L., Kléber A.G. Electrical resistances of interstitial and microvascular space as determinants of the extracellular electrical field and velocity of propagation in ventricular myocardium. Circulation. 1995;92:587–594. doi: 10.1161/01.cir.92.3.587. [DOI] [PubMed] [Google Scholar]
- 35.US National Institutes of Health . Eighth Edition. National Academies Press; Washington, DC: 1996. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- 36.Radwański P.B., Veeraraghavan R., Poelzing S. Cytosolic calcium accumulation and delayed repolarization associated with ventricular arrhythmias in a guinea pig model of Andersen-Tawil syndrome. Heart Rhythm. 2010;7:1428–1435.e1. doi: 10.1016/j.hrthm.2010.03.044. [DOI] [PubMed] [Google Scholar]
- 37.Raisch T., Poelzing S. Osmotically narrowing the perinexus improves cardiac conduction. Circulation. 2018;138:A12147. [Google Scholar]
- 38.Maningas P.A., DeGuzman L.R., Bellamy R.F. Small-volume infusion of 7.5% NaCl in 6% dextran 70 for the treatment of severe hemorrhagic shock in swine. Ann. Emerg. Med. 1986;15:1131–1137. doi: 10.1016/s0196-0644(86)80852-6. [DOI] [PubMed] [Google Scholar]
- 39.Wan X., Laurita K.R., Rosenbaum D.S. Molecular correlates of repolarization alternans in cardiac myocytes. J. Mol. Cell. Cardiol. 2005;39:419–428. doi: 10.1016/j.yjmcc.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Livshitz L.M., Rudy Y. Regulation of Ca2+ and electrical alternans in cardiac myocytes: role of CAMKII and repolarizing currents. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H2854–H2866. doi: 10.1152/ajpheart.01347.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clancy C.E., Tateyama M., Kass R.S. Non-equilibrium gating in cardiac Na+ channels: an original mechanism of arrhythmia. Circulation. 2003;107:2233–2237. doi: 10.1161/01.CIR.0000069273.51375.BD. [DOI] [PubMed] [Google Scholar]
- 42.Frank J.S., Langer G.A. The myocardial interstitium: its structure and its role in ionic exchange. J. Cell Biol. 1974;60:586–601. doi: 10.1083/jcb.60.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spach M.S., Heidlage J.F., Barr R.C. Extracellular discontinuities in cardiac muscle: evidence for capillary effects on the action potential foot. Circ. Res. 1998;83:1144–1164. doi: 10.1161/01.res.83.11.1144. [DOI] [PubMed] [Google Scholar]
- 44.Raisch T., Khan M., Poelzing S. Quantifying intermembrane distances with serial image dilations. J. Vis. Exp. 2018;138:58311. doi: 10.3791/58311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yabuuchi F., Beckmann R., Heubach J.F. Reduction of hERG potassium currents by hyperosmolar solutions. Eur. J. Pharmacol. 2007;566:222–225. doi: 10.1016/j.ejphar.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 46.McDonough A.A., Zhang Y., Frank J.S. Subcellular distribution of sodium pump isoform subunits in mammalian cardiac myocytes. Am. J. Physiol. 1996;270:C1221–C1227. doi: 10.1152/ajpcell.1996.270.4.C1221. [DOI] [PubMed] [Google Scholar]
- 47.Vermij S.H., Abriel H., van Veen T.A. Refining the molecular organization of the cardiac intercalated disc. Cardiovasc. Res. 2017;113:259–275. doi: 10.1093/cvr/cvw259. [DOI] [PubMed] [Google Scholar]
- 48.Pueyo E., Corrias A., Rodríguez B. A multiscale investigation of repolarization variability and its role in cardiac arrhythmogenesis. Biophys. J. 2011;101:2892–2902. doi: 10.1016/j.bpj.2011.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei N., Mori Y., Tolkacheva E.G. The dual effect of ephaptic coupling on cardiac conduction with heterogeneous expression of connexin 43. J. Theor. Biol. 2016;397:103–114. doi: 10.1016/j.jtbi.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 50.Vreeker A., van Stuijvenberg L., van Veen T.A. Assembly of the cardiac intercalated disk during pre- and postnatal development of the human heart. PLoS One. 2014;9:e94722. doi: 10.1371/journal.pone.0094722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noorman M., Hakim S., van Veen T.A. Remodeling of the cardiac sodium channel, connexin43, and plakoglobin at the intercalated disk in patients with arrhythmogenic cardiomyopathy. Heart Rhythm. 2013;10:412–419. doi: 10.1016/j.hrthm.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Asimaki A., Kapoor S., Saffitz J.E. Identification of a new modulator of the intercalated disc in a zebrafish model of arrhythmogenic cardiomyopathy. Sci. Transl. Med. 2014;6:240ra74. doi: 10.1126/scitranslmed.3008008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cerrone M., Lin X., Delmar M. Missense mutations in plakophilin-2 cause sodium current deficit and associate with a Brugada syndrome phenotype. Circulation. 2014;129:1092–1103. doi: 10.1161/CIRCULATIONAHA.113.003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pérez-Riera A.R., Barbosa-Barros R., de Abreu L.C. The congenital long QT syndrome type 3: an update. Indian Pacing Electrophysiol. J. 2018;18:25–35. doi: 10.1016/j.ipej.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu W., Mazzanti A., Silva J.R. Predicting patient response to the antiarrhythmic mexiletine based on genetic variation. Circ. Res. 2019;124:539–552. doi: 10.1161/CIRCRESAHA.118.314050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Swift L.M., Asfour H., Sarvazyan N. Properties of blebbistatin for cardiac optical mapping and other imaging applications. Pflugers Arch. 2012;464:503–512. doi: 10.1007/s00424-012-1147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Entz M., II, George S.A., Poelzing S. Heart rate and extracellular sodium and potassium modulation of gap junction mediated conduction in guinea pigs. Front. Physiol. 2016;7:16. doi: 10.3389/fphys.2016.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Despa S., Bers D.M. Na/K pump current and [Na](i) in rabbit ventricular myocytes: local [Na](i) depletion and Na buffering. Biophys. J. 2003;84:4157–4166. doi: 10.1016/S0006-3495(03)75140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hodgkin A.L., Keynes R.D. The mobility and diffusion coefficient of potassium in giant axons from Sepia. J. Physiol. 1953;119:513–528. doi: 10.1113/jphysiol.1953.sp004863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith, G. D. 2005. Modeling intracellular calcium: diffusion, dynamics, and domains. In Modeling in the Neurosciences: From Biological Systems to Cognitive Robotics, G. N. Reeke, R. R. Poznanski, K. A. Lindsay, J. Rosenberg, and O. Sporns, eds. Foundations of analytical neuroscience, (Taylor & Francis).
- 61.Decker K.F., Heijman J., Rudy Y. Properties and ionic mechanisms of action potential adaptation, restitution, and accommodation in canine epicardium. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H1017–H1026. doi: 10.1152/ajpheart.01216.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.