Abstract
Complement is a major contributor to inflammation and graft injury. This system is especially important in ischemia-reperfusion injury/delayed graft function as well as in acute and chronic antibody-mediated rejection (AMR). The latter is increasingly recognized as a major cause of late graft loss, for which we have few effective therapies. C1 inhibitor (C1-INH) regulates several pathways which contribute to both acute and chronic graft injuries. However, C1-INH spares the alternative pathway and the membrane attack complex (C5–9) so innate antibacterial defenses remain intact. Plasma-derived C1-INH has been used to treat hereditary angioedema for more than 30 years with excellent safety. Studies with C1-INH in transplant recipients are limited, but have not revealed any unique toxicity or serious adverse events attributed to the protein. Extensive data from animal and ex vivo models suggest that C1-INH ameliorates ischemia-reperfusion injury. Initial clinical studies suggest this effect may allow transplantation of donor organs which are now discarded because the risk of primary graft dysfunction is considered too great. Although the incidence of severe early AMR is declining, accumulating evidence strongly suggests that complement is an important mediator of chronic AMR, a major cause of late graft loss. Thus, C1-INH may also be helpful in preserving function of established grafts. Early clinical studies in transplantation suggest significant beneficial effects of C1-INH with minimal toxicity. Recent results encourage continued investigation of this already-available therapeutic agent.
C1 inhibitor (C1-INH) is a serine protease inhibitor encoded by the SERPING1 gene and a member of the serpin superfamily.1,2 Most serpins target a limited range of proteases. In contrast, C1-INH inhibits multiple enzymes, including: factors XIIa and XIa in the contact and coagulation systems; kallikrein in the kinin system; plasmin in the fibrinolytic system; C1s and C1r in the classic pathway of complement; and mannan-binding lectin-associated serine proteases (MASP-1 and MASP-2) in the lectin complement pathway.1,2 Because C1s is frequently assayed spectrophotometrically by cleavage of synthetic esters, C1-INH is often referred to as “C1 esterase inhibitor”.3 The C1-INH also differs from other serpins structurally as it has a large N-terminal mucin-like domain in addition to the C-terminal domain. The classic serpin C-terminal domain contains the protease binding site and the reactive center loop. The additional domain contains 10 extra glycosylation sites, in addition to 3 in the C-terminal domain. Thus, C1-INH is one of the most heavily glycosylated proteins in serum. Half of its molecular mass of 100 kDa is composed of glycans which confer additional properties not present in other serpins.1,2,4 The C1-INH can bind and neutralize lipopolysaccharides, inhibiting both sepsis and endotoxin shock in animal models.1,2,4 In addition, because the glycans contain sialyl-Lewis-x motifs, C1-INH can block binding of leukocytes to P and E selectins at sites of inflammation.4,5 The normal serum concentration of C1-INH is 25 mg/dL, but can increase 2- to 5-fold in response to acute inflammation.6
As a major regulator of the contact and kinin systems, C1 INH plays an important role in controlling vascular permeability. Heterozygous deficiency of C1-INH leads to the condition hereditary angioedema (HAE), which is characterized by recurrent episodes of dermal and submucosal swelling.7 Although the contact, coagulation, and fibrinolytic systems are also regulated by other serpins, C1-INH is the only inhibitor of the early-acting proteases in the classic and lectin complement pathways. We will first review the roles of complement in ischemia-reperfusion injury (IRI) and in amplifying the pathology induced by antibodies during antibody-mediated rejection (AMR). Then, we will examine how C1-INH can be used to ameliorate these obstacles to transplantation.
Two forms of C1-INH are currently marketed in the United States and Europe: plasma-derived C1-INH (marketed as Berinert; CSL Behring, King of Prussia, PA; and Cinryze; Shire, St Helier, Jersey, UK)8,9 and recombinant human C1-INH from transgenic rabbits (rhC1-INH; marketed as Ruconest by Pharming Group NV, Leiden, the Netherlands).10 The purification of C1-INH from plasma includes several dedicated virus inactivation/removal steps. Berinert has an excellent safety profile in clinical use, including more than 30 years in Germany.11 However, transmission of currently unknown viral pathogens or prions remains a theoretical possibility.8,9 Clinical experience with rhC1-INH is more limited, but it has not been associated with any specific viral/prion safety concerns. The rhC1-INH has different glycans than human pdC1-INH, giving it a shorter half-life and an increased potential to cause allergic reactions.10 Plasma-derived C1-INH has been used extensively for the treatment and long-term prophylaxis of acute attacks of HAE.7,11
Comparison of C1-INH With Other Complement Inhibitors
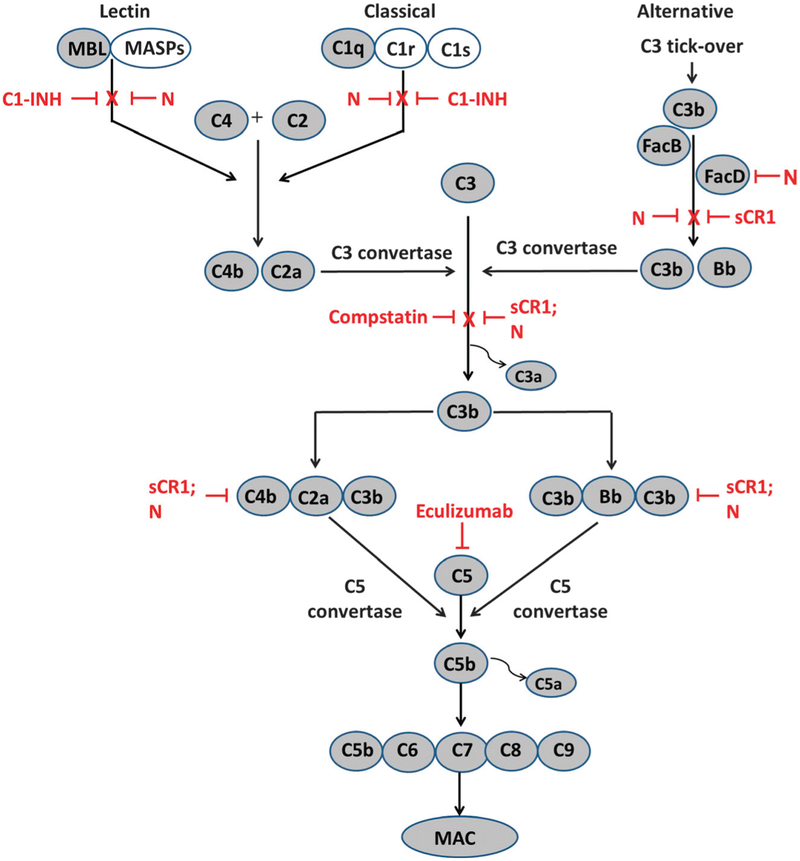
In addition to C1-INH, several other complement inhibitors have been proposed for and/or studied during transplantation.12 (Table 1 and Figure 1). These include engineered forms of complement receptor type 1 (CR1),19 synthetic inhibitors of complement convertases14,15,17 and a monoclonal antibody against C5.20 In particular, the use of molecules based on CR1, compstatin (C3 convertase inhibitor) and eculizumab (monoclonal antibody to C5) have shown promising results, mostly in preclinical models. However, these all target downstream proteins (Figure 1) which are common to all the complement pathways and may excessively increase the risk of infection. Constructs based on human CR1 (TP-10; Mirococept), which accelerate decay of C3 and C5 convertases and inactivation of C3b have been evaluated for safety in kidney and lung transplantation. This soluble CR1 analog significantly shortened time to extubation, ventilator time, and days in intensive care in a subgroup of lung transplant recipients16 Compstatin, a synthetic 13-residue peptide C3 convertase inhibitor, and nafamostat (FUT-175), a synthetic broad spectrum protease inhibitor, inhibit all 3 complement pathways and are currently under investigation in preclinical models.14,17 Eculizumab is a monoclonal antibody against C5, which blocks generation of the chemoattractant/leukocyte activator C5a and formation of the membrane attack complex (MAC). Eculizumab is licensed in the United States for treatment of paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome.20 It has been studied for prevention of AMR in HLA-sensitized and ABO-incompatible transplant recipients21,22 and is currently in clinical trials for prevention and/or treatment of AMR.23–25 It is also being studied in prevention/treatment of IRI and delayed graft function (DGF) in kidney transplantation.26,27
TABLE 1.
Complement Inhibitors
| Complement | ||||
|---|---|---|---|---|
| Name(s) | Structure | Major targets | Pathways inhibited* | References |
| C1 INH (Berinert, Cinryze, Ruconest) | C1r, C1s, MASPs | C, L, | 1, 2, 4,5,7–11, 13 | |
| Natural or Recombinant Glycoprotein | ||||
| Nafamostat (FUT-175) | Synthetic guanidinobenzoate (broad spectrum low molecular weight serine protease inhibitor) | C1r, C1s, MASPs, B, D, C2 | A, C, L | 14 |
| Compstatin | Synthetic 13 residue cyclic peptide | C3 and C5 convertase | A, C, L | 15 |
| Mirococept/TP-10 | Recombinant soluble analogs of type 1 complement receptor | C3b, C3 and C5 convertases | A, C. L | 16 |
| Eculizumab (Soliris) | Humanized monoclonal antibody | C5 | A, C, L | 17, 18 |
A indicates alternate; C, classic; L, lectin.
FIGURE 1.
Complement activation pathways and sites of action of inhibitors (red). Note that C1 INH blocks only the first enzymes in the lectin and classic pathways: MASPs and C1r and C1s, shown in unshaded symbols. Thus, C1 INH leaves the alternative pathway intact. In contrast, Nafamostat (N) blocks proteases in all three pathways, including MASPs and C1r/C1s, and also factor D, C2a, and Bb. Soluble recombinant forms of the type 1 complement receptor (sCR1), like TP-10 and Mirococept, facilitate dissociation of C3b from convertases and its further proteolytic degradation, and hence inhibit the C3 convertases and C5 convertases which might be initiated by any of three pathways. By blocking C3 convertases, Compstatin inhibits cleavage of C3 and further activation. By binding and preventing cleavage of C5, Eculizumab inhibits formation of the MAC.
The use of C1-INH in transplantation may have several benefits compared to other complement inhibitors. C1-INH targets the first proteins activated in the complement cascade, and leaves the alternative pathway and MAC (C5–9) largely uninhibited.13 Leaving the innate antibacterial defenses of the alternative pathway intact may be especially important in preventing infection in immunosuppressed transplant patients.28,29 In fact, human plasma-derived C1-INH actually improved antibacterial defenses and prolonged survival in a primate model of severe sepsis.30 However, by preventing the classic pathway from amplifying the effects of donor-specific antibodies, C1-INH may inhibit complement’s enhancement of antibody-dependent cellular cytotoxicity as well as leukocyte attraction and activation. In addition, C1-INH likely inhibits complement’s enhancement of antigen presentation and B-cell responses.31,32 Together, these effects are expected to ameliorate AMR and promote long-term graft survival. The C1-INH also regulates the kallikreinkinin systems and can inhibit leukocyte adhesion to the endothelium, which also contributes to IRI as well as AMR.4,33 Inhibitors such as eculizumab, which target more distal complement proteins and formation of the MAC have been associated with an increased risk of infection with bacteria, such as Neisseria.18 Furthermore, eculizumab acts later in the complement pathway, beyond deposition of C3b and iC3b, which can still contribute to graft damage in a number of ways.
The Role of Complement in IRI
The IRI is a leading cause of DGF and primary graft dysfunction (PGD) in solid organ transplantation. DGF is reported to occur in 25% to 30% of kidney transplants, necessitating postoperative dialysis, prolonged hospitalization and further complications.34,35 Similarly, in liver transplantation, DGF is associated with hepatocellular injury leading to impaired tissue regeneration and significant increases in morbidity and mortality.36 The PGD occurs in approximately 8% to 25% of lung transplants, increases morbidity and mortality, and prolongs ventilator dependence.37 The PGD is associated with increases in pulmonary vascular permeability and resistance, presumably reflecting endothelial damage/dysfunction.37–39 Given the impact of IRI on tissue injury and graft dysfunction and the costs of transplant complications, it is an important clinical problem.
Although complement is most often considered part of the host defense system, it is now understood to have critical roles in normal tissue and immunologic homeostasis. Complement recognizes damage-associated molecular patterns (DAMPs) in addition to pathogen-associated molecular patterns.40–42 The DAMPs and normally intracellular antigens which can bind “natural” IgM antibodies are exposed during ischemia.43,44 Ischemia-induced membrane changes also lead to exposure of proteins which can activate C1 in the absence of antibodies,45 as well as polysaccharides which are recognized by mannan-binding lectin.46,47 Together, these tissue antigens and DAMPs can activate the classic and lectin pathways43–51 and allow for amplification by the alternative pathway.48 Colocalization of IgM, C1q, C4d, and MAC, as well as mannan-binding lectin and MASPs, have been demonstrated in animal models of IRI.43,47–51 Complement activation in models of IRI in situ has been shown to cause injury to the heart,51 lung,52 intestine,53–55 liver,56 kidney,46,48 and skeletal muscles.43,50,51 Besides direct effects in damaging and opsonizing tissues, complement activation releases C5a which attracts and can cause aggregation of neutrophils.57 Neutrophil aggregates, in turn, can obstruct capillaries and exacerbate local ischemia. These aggregates can also exert remote effects including plugging pulmonary capillaries.56–58 In addition, activation of endothelial cells by C3a, C5a, and/or the MAC leads to increased expression of adhesion molecules (eg, ICAM-1, VCAM, E-selectin) and production of proinflammatory cytokines and chemokines.57 In animal models of IRI, C1-INH decreases C4d and C3b deposition and preserves structural integrity of tissues. The C1 INH decreases release of intracellular constituents and infiltration and capillary plugging by neutrophils. Together, these effects down-modulate increased vascular permeability and ameliorate systemic hypotension.53,54,56 The IRI during transplantation is also associated with activation of the coagulation and contact systems.46,59–61 Thus, the unique ability of C1-INH to inhibit multiple deleterious pathways may contribute to its beneficial effects. In a swine kidney model, the presence of exogenous C1-INH during postischemia reperfusion reduced the deposition of C4d and the MAC.46 In mouse and rat models, C1-INH reduced IRI after transient occlusion of the superior mesenteric artery. This was demonstrated morphologically (preservation of microvilli and decreased mucosal damage), as well as by decreasing release of intracellular constituents. The decreased IRI was accompanied by decreased infiltration of leukocytes, improved microcirculation and perfusion, and improvements in systemic hypotension and shock. Together, these effects improved survival.54,55 Similarly, treatment with human C1-INH or overexpression of a C1-INH transgene protected liver from IRI and improved survival in mouse models.56,62 In an ex vivo model of pig liver reperfusion injury, C1-INH reduced complement activation, inflammation, and subsequent cellular damage.63 Also, in a lower torso ischemia-reperfusion model, C1-INH overexpression protected both the local skeletal muscle from IRI and the lungs from leukocyte aggregate-induced dysfunction.64
In addition to these models of in situ IRI, there have been several studies of C1-INH in pre-clinical transplant models. During lung transplantation in sheep, treatment with C1-INH either 10 minutes before or after reperfusion was associated with better gas exchange, lower pulmonary vascular resistance, and lower leukocyte activation and lytic enzyme release than in controls.65 Morphological assessment of lung tissue revealed edema and polymorphonuclear leukocyte infiltration in controls, whereas C1-INH preserved alveolar structure and minimized polymorphonuclear leukocyte aggregation.65 In vitro studies have shown that C1-INH is able to bind to the surface of ischemic endothelial cells while retaining its ability to inhibit complement.63 Thus, pretreating the donor organ ex vivo could reduce local complement activation after reperfusion without affecting the recipient’s host defense functions.
In liver transplantation, the shortage of donors has led to the increasing use of organs from “marginal donors,” for example, with steatotic compared to lean livers. However, IRI occurs in over 30% of steatotic livers. A study in mice showed that complement activation was increased in steatotic as compared with nonsteatotic livers but could be reduced by C1-INH, improving survival.66 Thus, C1-INH could increase the number of donor organs available for transplantation.
Clinical Studies of C1-INH in IRI During Transplantation
Data on the use of C1-INH to prevent and/or treat IRI in human transplantation is already accumulating. Clinicaltrials.gov lists 7 studies of C1 INH in transplantation (Table 2). In lung transplant recipients with poor gas exchange (PaO2/FiO2 <100) immediately on reperfusion, oxygenation improved rapidly on administration of C1-INH.69 Patients who received C1-INH in the operating room and for the next 72 hours required significantly less time on ventilators (105 vs 483 hours, P < 0.03) and less time in intensive care than those who developed a similar grade of PGD later in their course.69 This C1-INH treatment was associated with reduced hospital costs compared to nontreated severe PGD patients.69 This result recapitulates the findings of an earlier randomized, blinded, prospective study in which a single dose of a soluble CR1 analog (TP-10, Mirococept) in the OR before reperfusion significantly increased the number of patients extubated within 24 hours (50% with TP-10 vs 19% with placebo; P < 0.01).16 The TP-10 also significantly reduced the days on the ventilator among patients who required cardiopulmonary bypass during surgery.
TABLE 2.
Trials of C1 INH in transplantation listed in clinicaltrials.gova
| Title | Phase | NCT Number | Type | Sponsor | Status |
|---|---|---|---|---|---|
| C1 INH Preoperative and post-kidney transplant to prevent DGF and IRI | I | 02134314 | R, PC, DB | Cedars-Sinai Medical Center, Los Angeles, CA | Recruiting |
| Safety and tolerability of Berinert (C1 inhibitor) therapy to prevent AMR in HLA sensitized kidney transplant recipients | I | 01134510 | R, PC, DB | Cedars-Sinai Medical Center | Completed67 |
| Recombinant human C1 INH for the treatment of early AMR in renal transplantation | II | 01035593 | R, OL | Shire, Lexington, MA | Withdrawnb |
| A pilot study to evaluate the use of C1 INH (human) in patients with acute (kidney) AMR | II | 01147302 | R, PC, DB | Shire, Lexington, MA | Completed68 |
| Combined drug approach to prevent IRI during transplantation of livers | I | 01886443 | SB | Universitaire Ziekenhuisen Leuven, Belgium | Completed |
| Combined drug approach to prevent IRI during transplantation of livers (CAPITL) | II | 02251041 | R, SB | Universitaire Ziekenhuisen Leuven, Belgium | Recruiting |
| Cinryze as a donor pretreatment strategy in kidney recipients of KDPI>85% organs | I | 02435732 | R, PC, | University of Wisconsin Madison, Wl | Not yet recruiting |
No trials are listed in EUdraCT which are not listed here.
Sponsor’s comment: “Withdrawn due to Improvements in Clinical Practice (which) Have Reduced The Apparent Incidence of AMR.”
R indicates randomized; PC, placebo controlled; DB, double blinded; SB, single blinded (subject); OL, open label.
In a pilot study in kidney transplantation, C1-INH was given to prevent acute AMR in desensitized patients with anti-HLA antibodies (see below). Administration of C1-INH intraoperatively and 3 days later reduced the incidence of DGF and the requirement for dialysis from 40% in the placebo group to 10%.67 A 70 subject randomized, double-blinded, placebo-controlled study of C1-INH to prevent DGF due to IRI in recipients of kidneys from extended criteria and deceased due to cardiac death donors is now underway. The requirement for dialysis within the first week after transplantation is its primary endpoint.70 Eculizumab is also in clinical trials for treatment of IRI in kidney transplantation.26,27 A major hypothesis in these studies is that by decreasing IRI and DGF, complement inhibition will allow the use of organs which are currently discarded. This should increase the number of transplants. Besides these trials in kidney transplantation, a group in Leuven, Belgium is exploring the use of a cocktail including C1 INH and 8 other plasma proteins and antioxidants to prevent IRI during liver transplantation (see Table 2, NCT 01886443 and 02251041).
Complement in AMR
Although improvements in tissue typing and immunosuppressive regimens have improved control of cell-mediated rejection, we have fewer effective tools against AMR.71,72 The type and severity of AMR depend on the characteristics of antigraft antibodies and the kinetics of their formation and binding. Antibodies to donor HLA antigens (DSA) are major culprits in most cases of AMR. However, antibodies against ABO blood group antigens, angiotensin receptors (anti-angiotensin receptor type 1), and other non-HLA endothelial cell antigens can also participate.73 Hyperacute rejection (HAR) occurs within minutes to hours if large amounts of preformed antibodies bind as soon as the graft comes in contact with the recipient’s circulation.74 This is particularly likely in highly sensitized patients and is a major barrier to ABO-incompatible transplants, despite attempts to remove antiblood group antibodies.75 Acute AMR can result from either preformed or de novo DSA and is characterized by graft dysfunction in the days after transplantation. Acute AMR also occurs whenever antibody titers rise because immunosuppression is reduced or adherence lapses. In recent years, graft loss in the first year has markedly decreased as acute AMR has been better controlled with improved immunosuppression, careful monitoring, and prompt treatment with corticosteroids and IVIG ± plasmapheresis.76–79
In contrast to this success, chronic AMR remains problematic, and there is increasing appreciation that it causes many forms of late graft pathology.79–81 Thus, “transplant glomerulopathy,”80–82 “bronchiolitis obliterans syndrome,” and other forms of chronic lung allograft dysfunction,83 as well as accelerated transplant coronary artery vasculopathy,84,85 may all represent organ-specific forms of chronic AMR. The clinical, laboratory, and pathologic features of AMR all suggest that complement is a major contributor. Classic pathway activation amplifies antibody-induced injury and promotes the pathology.83–88 C1q-binding DSA have been associated with increased deposition of C4d, increased rejection and markedly increased risk of kidney allograft loss.89,90 These studies clearly emphasize the contribution of complement to graft damage and loss induced by DSA. The avidity, concentration, epitope specificity, and subclass of anti-HLA antibodies are all important in determining the extent of complement activation on antibody binding. Binding of C1q to patients’ anti-HLA antibodies which have bound to single-antigen beads (Luminex) is 1 method for confirming in vitro the ability of these antibodies to activate the classic pathway.89 Some recent studies have shown that C1q positivity in single-antigen bead assays correlates strongly with the titer of antidonor HLA IgG as measured by the mean fluorescence intensity. In particular, some studies suggest that high titer anti-DSA tends to be dominated by the complement-activating subclasses IgG1 and IgG3.89 However, other studies have reported discrepant results,90–92 probably due to differences in handing of the sera and technical features of different assays. Binding of IgG1 and IgG3 DSAs to endothelial cells can activate the classic complement pathway. This, in turn, releases C3a and C5a, which increase infiltration and adherence of inflammatory cells (eg, neutrophils, monocyte/macrophages). The C3a and C5a also activate adjacent endothelial cells, leading to increased expression of adhesion molecules and increased synthesis of proinflammatory cytokines and chemokines.93–95 In addition, complement attack on parenchymal cells can lead to formation of the MAC (C5b-9) and membrane vesiculation or cell lysis.40,41,61 Inhibition of complement activation by C1-INH likely decreases all of these damaging effects. Furthermore, binding of IgG to class I HLA antigens on endothelial cells increases membrane expression of P-selectin, even without complement activation.95–97 Increased P-selectin enhances neutrophil and macrophage attachment and damage to the endothelial cells, but selectin binding can also be directly blocked by the glycans on C1-INH.5
Deposition of C4d in graft tissue is often a key feature of both acute and chronic AMR. C4d staining is included, along with evidence of circulating DSA, in the Banff criteria.98,99 Correlations between DSA level, C4d staining in biopsies, and severity of tissue damage have been reported in some, but not all studies.87 Increased C4d deposition is associated with increased inflammation 3 months and 1 year after kidney transplantation100,101 and greater risk of late graft loss.100–103 However, not all DSAs are equally damaging, and not all antibodies which cause graft damage/rejection are anti-HLA. Antibodies to angiotensin receptors and major histocompatibility complex MHC class 1-related chain A are also important in some patients.73 Nevertheless, the ability of DSA to bind complement (C1q) seems to be one important indicator of the likelihood of complement deposition in the graft, and that in turn, seems to be a major determinant of the outcome.89,90,100 Most investigators agree that complement-binding DSA which arise de novo after transplantation carry the worst prognosis.90,103–105 Cell death and/or turnover of membrane can result in negative staining for C4d without meaning that classic pathway complement activation did not occur and did not contribute to graft damage. Since classic pathway activation is involved in acute as well as chronic AMR, C1-INH is a particularly attractive candidate for amelioration of graft damage/loss.
Use of C1-INH for the Treatment of Different Types of AMR
As HAR predominately results from incompatible donorrecipient matches,74 its incidence has been significantly reduced by improvements in pretransplant crossmatching, and it is now rare.106,107 When a positive crossmatch is the only available option, plasmapheresis/immunoadsorption to reduce antibody titers and high-dose IVIG treatment to block antibody-mediated effector functions may reduce the severity of HAR,106,108 but C1-INH may be a useful adjunct, as well.
Use of C1-INH in Acute AMR
As with HAR, there are few randomized controlled trials of complement inhibitors in acute AMR. An open-label study of eculizumab in highly sensitized patients compared patients transplanted between 2008 and 2010 with historical controls transplanted between 2005 and 2007. In the earlier cohort, the incidence of acute AMR was 41.2% in the first 3 months after transplantation.22 That study suggested that eculizumab reduced AMR episodes to 7%. However, recent controlled studies have reported incidences of AMR only in the range of 10% to 20% in placebo groups and failed to show significant effects of treatment.67,109 Animal models, including a recent study in which rhC1-INH treatment delayed acute AMR in alloimmunized baboons,110 certainly support a role for complement in AMR. Vo et al67 has shown that C1-INH inhibits DSA-induced complement-dependent cytotoxicity in vitro in a concentration-dependent manner, with higher inputs of C1-INH being necessary when patients had higher DSA titers. A recent randomized, blinded study of C1-INH (20 U/kg) given intraoperatively then twice a week for a total of 8 doses in desensitized DSA+ kidney recipients reported 0 episodes of AMR in the treated group versus 10% within the first month in the placebo group. However, in this small study, the difference was not significant.67 Serum samples obtained just before each dose of C1-INH documented the increased circulating functional and antigenic C1-INH levels from day 4 through the end of the treatment period. Interestingly, C4 and C3 levels, which were not different between the groups at the time of transplant, subsequently dropped in the placebo patients but were maintained in the treatment group, suggesting that active complement consumption by the classic and/or lectin pathways occurred in the placebo patients but was inhibited in the C1-INH group.67 Another randomized, controlled study used 2 weeks of alternate day C1-INH or placebo in addition to plasmapheresis, IVIG ± rituximab for treatment of acute AMR in kidney recipients. All patients in both groups recovered, although there was a trend toward more sustained improvement in renal function in the C1-INH group. There were no apparent differences in histopathology 1 week after the C1-INH treatment. However, on protocol biopsies 3 to 6 months after treatment, transplant glomerulopathy was observed in 3 of 7 placebo patients versus 0 of 7 in the treatment group. Interestingly, the 3 patients who developed transplant glomerulopathy had the lowest C1-INH (antigen) levels.68 Importantly, no serious adverse effects attributable to C1-INH were reported in either study.
In the study reported by Vo et al,67 the levels of antibodies to donor class II antigens decreased after transplantation for all but 1 patient in the C1-INH group and remained low after C1-INH treatment was concluded. In contrast, positive DSAs were maintained by all subjects in the placebo group. In all C1q+ patients treated with C1-INH, C1q+ binding became undetectable or reduced after treatment (n = 5). By comparison, no decrease in C1q+ anti-HLA antibodies was seen in placebo patients (n = 3). These results are consistent with the hypothesis that C1-INH inhibited antigen presentation and/or C3 enhancement of B-cell responses.31,32 These and other potential long-term beneficial effects of perioperative and early posttransplant treatment with C1-INH should be investigated in future studies.
Involvement of Complement in Chronic AMR/Late Graft Loss Suggests a Role for C1 INH Therapy
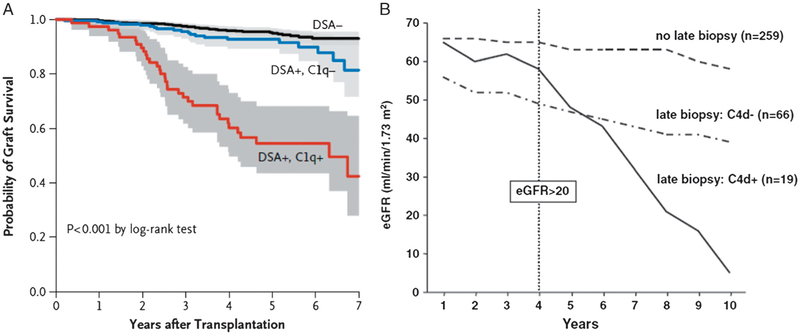
Improvements in long-term graft survival have not paralleled improvements in 1-year graft survival.76,103 Chronic AMR progresses slowly with damage to the allograft accumulating over several years,80 but it is now the leading cause of late loss of kidney allografts.76,90,103 Early insults including pretransplant ischemia, DGF, episodes of acute rejection, and viral infection all increase the risk of chronic AMR and late graft dysfunction/loss.111–113 Increasingly, transplant glomerulopathy and transplant-specific pathologies in other organs are also becoming recognized as manifestations of chronic AMR.82,111–116 As shown in Figure 2, 2 main lines of evidence suggest major involvement of complement in chronic AMR/late graft loss. These include a strong association with circulating C1q-binding DSA and the presence of C4d deposits in the tissue. In a recent report of long-term follow-up of 1016 renal transplants in France, Loupy and colleagues90 showed that the presence of de novo (absent at the time of transplantation but detectable by Luminex SBA any time afterwards) C1q+ DSA was associated with a nearly 12-fold increased risk of graft loss. Similarly, Gaston et al, Eskandary et al, and others100–103 have shown that positive staining for C4d in the graft is associated with a markedly increased risk of late graft loss. Furthermore, the presence of DSA, especially C1q-binding DSA, confers a significantly higher risk for C4d in the graft.112,113 This is consistent with classic pathway activation. Reports that C1q+ DSAwas also significantly associated with higher Banff scores for microvascular inflammation, transplant glomerulopathy, and interstitial inflammation/tubulitis suggest that these are all manifestations of chronic AMR90,99,101 This evidence for antibody-mediated activation of the classic pathway strongly suggests the possibility that C1-INH augmentation may be beneficial.
FIGURE 2.
Prognostic significance of A: C1q+ DSA90 and B: C4d deposition102 in late kidney allograft dysfunction/loss, presumably due to chronic AMR. A, reprinted with permission from Loupy A, Lefaucheur C, Vernerey D, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med. 2013;369:1215–1226. B, reproduced under the CC BY 4.0 licence provided by BioMedCentral and relating to the original publication by Eskandary F, Bond G, Schwaiger E, et al. Bortezomib in late antibody-mediated kidney transplant rejection (BORTEJECT Study): study protocol for a randomized controlled trial. Trials. 2014;15:107. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4014747/pdf/1745-6215-15-107.pdf.
The importance of complement in late dysfunction/loss of other organ grafts is also suggested by deposition of C4d in association with circulating DSA. In lung transplants, both alloantibodies and autoantibodies can bind C1q and lead to classic pathway activation. However, in lungs, C4d staining has been less reproducible and reliable as an indicator of AMR.115,116 Some follow-up studies have shown that bronchiolitis obliterans syndrome is clearly exacerbated by DSA116 and C4d deposition,117 resulting in reduced median survival from 5.2 years to 1.5 years.116 Similar findings have been reported in heart recipients. A 2005 study of 665 endomyocardial biopsies in 165 patients, most of whom had well-functioning grafts, reported that the 66% of the patients had significant linear capillary IgG immunofluorescence positivity (≥2+), but few had positive staining for C4d or C3d. In contrast, of the 20 patients with positive staining for C4d or C3d in addition to IgG, 40% (8) developed coronary artery vasculopathy, and 25% (5) had graft dysfunction resulting in hemodynamic compromise. The graft dysfunction developed 56 to 163 months after transplantation.118 This study suggests that the antibodies themselves seemed to have little pathogenic activity unless they activate complement, in contrast to the data of the groups of Reed,72 Sis,87,119 and others86. A more recent study examined nearly 11 000 endomyocardial biopsies obtained from 398 consecutive heart transplant recipients over 15 years. Significant associations were observed between C4d and/or C3d deposition, DSA positivity, microcirculatory injury and higher-grade coronary artery vasculopathy in patients showing very late rejection.120 Conversely, serum DSAs were absent from patients without late rejection.
The poor prognosis for chronic AMR is due in part to the lack of available treatments. Conventional therapies for acute AMR, such as plasmapheresis or high dose IVIG, ± high-dose corticosteroids, are not practical over long periods.77–79 The efficacy of rituximab is limited because it does not inhibit antibody production by plasma cells. The growing recognition of chronic AMR as a major cause of late graft loss delineates a need for new treatment options. Identification of C1q-binding DSAs and C4d deposition as indicators of increased risk of late graft loss suggest that complement inhibition may be a useful strategy for long-term allograft preservation. However, assessing endpoints in chronic AMR studies may be difficult.121 The long-term safety of C1-INH in HAE suggests it may be suitable for prolonged use in transplant patients, but data in this setting are needed. Although the need for IV administration of C1 INH is an obstacle to its chronic use, development of new preparations which are more highly concentrated and can be easily injected subcutaneously by the patient at home would greatly facilitate long-term use.122
CONCLUSIONS AND FUTURE DIRECTIONS
The C1 INH is a naturally occurring inhibitor of the classic and lectin pathways of complement and other plasma protease cascades involved in inflammation and endothelial cell function. Several properties of C1 INH suggest it may have great value in transplantation. First, as an acute phase reactant, its concentration can increase physiologically without adverse effects, suggesting that augmentation should be well tolerated. Plasma-derived C1-INH has been used clinically for over 30 years, and it has an excellent safety record in both acute and chronic prophylactic therapies for HAE. Although data in transplant patients are limited, no unique adverse effects attributable to C1-INH have emerged. Because C1-INH does not affect the alternative pathway of complement activation, it can inhibit antibody-mediated processes without interfering with complement’s innate immune functions. In addition, because C1 INH does not inhibit formation of the MAC (C5–9), it may be free from the increased risk of Neisseria infections which accompanies the use of C5 inhibitors.
The art and science of transplantation have advanced remarkably in recent years, but 2 particular areas of unmet medical need have come into sharp focus. One is the shortage of available donor organs, and the other is the increasing recognition of chronic AMR as the major cause of late graft loss. C1-INH may be potentially important in ameliorating both of these problems. First, by inhibiting IRI, C1-INH may be able to moderate or prevent DGF. Reducing that risk may allow more extended criteria and deceased-after-cardiac-death donor organs to be successfully transplanted. Second, the growing recognition of the involvement of C1q-binding DSAs and C4d in late graft loss strongly implicate the classic complement pathway and therefore suggest that C1-INH may be able to promote graft survival. We are thus encouraged that ongoing and future studies will lead to C1-INH becoming an important addition to our therapeutic armamentarium in the next few years.
ACKNOWLEDGMENT
Technical assistance with preparation of the manuscript was provided by Meridian HealthComms Ltd, with funding by CSL Behring.
M.B. is an employee of CSL Behring and holder of equity. W.M.B. is supported by NIH grant no 1P01 AI087586 and has received Berinert from CSL Behring for laboratory experiments. S.C.J. has received research grants from CSL Behring, Genentech, and Hansa Medical; and consulting fees from CSL Behring, Genentech and Alexion.
Footnotes
The authors declare no conflicts of interest.
Off Label/Investigational Use: uses of C1 inhibitor in transplantation are investigational/off-label only.
REFERENCES
- 1.Davis AE 3rd, Lu F, Mejia P. C1 inhibitor, a multi-functional serine protease inhibitor. Thromb Haemost. 2010;104:886–893. [DOI] [PubMed] [Google Scholar]
- 2.Wagenaar-Bos IG, Hack CE. Structure and function of C1-inhibitor. Immunol Allergy Clin North Am. 2006;26:615–632. [DOI] [PubMed] [Google Scholar]
- 3.Ratnoff OD, Lepow IH. Some properties of an esterase derived from preparations of the first component of complement. J Exp Med. 1957; 106:327–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis AE 3rd, Cai S, Liu D. C1 inhibitor: biologic activities that are independent of protease inhibition. Immunobiology. 2007;212:313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai S, Dole VS, Bergmeier W, et al. A direct role for C1 inhibitor in regulation of leukocyte adhesion. J Immunol. 2005;174:6462–6466. [DOI] [PubMed] [Google Scholar]
- 6.Zuraw BL, Lotz M. Regulation of the hepatic synthesis of C1 inhibitor by the hepatocyte stimulating factors interleukin 6 and interferon gamma. J Biol Chem. 1990;265:12664–12670. [PubMed] [Google Scholar]
- 7.Kaplan AP. Enzymatic pathways in the pathogenesis of hereditary angioedema: the role of C1 inhibitor therapy. J Allergy Clin Immunol. 2010;126: 918–925. [DOI] [PubMed] [Google Scholar]
- 8.CSL Behring, Berinert Prescribing Information. http://labeling.cslbehring.com/PI/US/Berinert/EN/Berinert-Prescribing-Information.pdf, 2015.
- 9.Shire, Cinryze Prescribing Information. https://shared.salix.com/shared/pi/ruconest-pi.pdf, 2014.
- 10.Pharming, Ruconest Prescribing Information. https://shared.salix.com/shared/pi/ruconest-pi.pdf, 2015.
- 11.De Serres J, Groner A, Lindner J. Safety and efficacy of pasteurized C1 inhibitor concentrate (Berinert P) in hereditary angioedema: a review. Transfus Apher Sci. 2003;29:247–254. [DOI] [PubMed] [Google Scholar]
- 12.Touzot M, Obada EN, Beaudreuil S, et al. Complement modulation in solid-organ transplantation. Transplant Rev (Orlando). 2014;28:119–125. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen EW, Waage C, Fure H, et al. Effect of supraphysiologic levels of C1-inhibitor on the classical, lectin and alternative pathways of complement. Mol Immunol. 2007;44:1819–1826. [DOI] [PubMed] [Google Scholar]
- 14.Mastellos DC, Yancopoulou D, Kokkinos P, et al. Compstatin: a C3-targeted complement inhibitor reaching its prime for bedside intervention. Eur J Clin Invest. 2015;45:423–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emlen W, Li W, Kirschfink M. Therapeutic complement inhibition: new developments. Semin Thromb Hemost. 2010;36:660–668. [DOI] [PubMed] [Google Scholar]
- 16.Keshavjee S, Davis RD, Zamora MR, et al. A randomized, placebo-controlled trial of complement inhibition in ischemia-reperfusion injury after lung transplantation in human beings. J Thorac Cardiovasc Surg. 2005;129:423–428. [DOI] [PubMed] [Google Scholar]
- 17.Pfeifer PH, Kawahara MS, Hugli TE. Possible mechanism for in vitro complement activation in blood and plasma samples: futhan/EDTA controls in vitro complement activation. Clin Chem. 1999;45(8 Pt 1): 1190–1199. [PubMed] [Google Scholar]
- 18.Keating GM, Lyseng-Williamson KA, McKeage K. Eculizumab: a guide to its use in paroxysmal nocturnal hemoglobinuria. BioDrugs. 2012;26:125–130. [DOI] [PubMed] [Google Scholar]
- 19.Weisman HF, Bartow T, Leppo MK, et al. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science. 1990;249: 146–151. [DOI] [PubMed] [Google Scholar]
- 20.Alexion, Soliris Prescribing Information. http://www.soliris.net/sites/default/files/assets/soliris_pi.pdf, 2014.
- 21.Legendre C, Sberro-Soussan R, Zuber J, et al. Eculizumab in renal transplantation. Transplant Rev (Orlando). 2013;27:90–92. [DOI] [PubMed] [Google Scholar]
- 22.Stegall MD, Diwan T, Raghavaiah S, et al. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant. 2011;11:2405–2413. [DOI] [PubMed] [Google Scholar]
- 23.Clinicaltrials.gov, Safety & Efficacy Of Eculizumab In The Prevention Of AMR In Sensitized Recipients Of A Kidney Transplant From A Deceased Donor. http://www.clinicaltrials.gov/ct2/show/NCT01567085?term=NCT01567085&rank=1. Accessed on 28th October 2014.
- 24.Clinicaltrials.gov, Safety & Efficacy of Eculizumab to Prevent AMR in Living Donor Kidney Transplant Recipients Requiring Desensitization. http://www.clinicaltrials.gov/ct2/show/NCT01399593?term=NCT01399593&rank=1. Accessed on 28th October 2014.
- 25.Clinicaltrials.gov, Efficacy and Safety of Eculizumab for Treatment of Antibody-mediated Rejection Following Renal Transplantation. https://www.clinicaltrials.gov/ct2/results?term=01895127&Search=Search. Accessed on July 28, 2015.
- 26.Clinicaltrials.gov, Eculizumab for Prevention and Treatment of Kidney Graft Reperfusion Injury. http://www.clinicaltrials.gov/ct2/show/NCT01756508?term=NCT01756508&rank=1. Accessed on 28th October 2014.
- 27.Clinicaltrials.gov, A Study of the Activity of Eculizumab for Prevention of Delayed Graft Function In Deceased Donor Kidney Transplant. http://www.clinicaltrials.gov/ct2/show/NCT01403389?term=NCT01403389&rank=1. Accessed on 28th October 2014.
- 28.Oberbarnscheidt MH, Zecher D, Lakkis FG. The innate immune system in transplantation. Semin Immunol. 2011;23:264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kemper C, Atkinson JP, Hourcade DE. Properdin: emerging roles of a pattern-recognition molecule. Annu Rev Immunol. 2010;28:131–155. [DOI] [PubMed] [Google Scholar]
- 30.Jansen PM, Eisele B, de Jong IW, et al. Effect of C1 inhibitor on inflammatory and physiologic response patterns in primates suffering from lethal septic shock. J Immunol. 1998;160:475–484. [PubMed] [Google Scholar]
- 31.Carroll MC, Isenman DE. Regulation of humoral immunity by complement. Immunity. 2012;37:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rickert RC. Regulation of B lymphocyte activation by complement C3 and the B cell coreceptor complex. Curr Opin Immunol. 2005; 17:237–243. [DOI] [PubMed] [Google Scholar]
- 33.Calvey CR, Toledo-Pereyra LH. Selectin inhibitors and their proposed role in ischemia and reperfusion. J Invest Surg. 2007;20:71–85. [DOI] [PubMed] [Google Scholar]
- 34.Cavaille-Coll M, Bala S, Velidedeoglu E, et al. Summary of FDA workshop on ischemia reperfusion injury in kidney transplantation. Am J Transplant. 2013;13:1134–1148. [DOI] [PubMed] [Google Scholar]
- 35.Salvadori M, Rosso G, Bertoni E. Update on ischemia-reperfusion injury in kidney transplantation: pathogenesis and treatment. World J Transplant. 2015;5:52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stockmann M, Lock JF, Malinowski M, et al. How to define initial poor graft function after liver transplantation?—a new functional definition by the LiMAx test. Transpl Int. 2010;23:1023–1032. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki Y, Cantu E, Christie JD. Primary graft dysfunction. Semin Respir Crit Care Med. 2013;34:305–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diamond JM, Porteous MK, Cantu E, et al. Elevated plasma angiopoietin-2 levels and primary graft dysfunction after lung transplantation. PLoS One. 2012;7:e51932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abrahimi P, Liu R, Pober JS. Blood vessels in allotransplantation. Am J Transplant. 2015;15:1748–1754. [DOI] [PubMed] [Google Scholar]
- 40.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344: 1058–1066. [DOI] [PubMed] [Google Scholar]
- 41.Walport MJ. Complement. Second of two parts. N Engl J Med. 2001; 344:1140–1144. [DOI] [PubMed] [Google Scholar]
- 42.Kataoka H, Kono H, Patel Z, et al. Evaluation of the contribution of multiple DAMPs and DAMP receptors in cell death-induced sterile inflammatory responses. PLoS One. 2014;9:e104741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiser MR, Williams JP, Moore FD Jr, et al. Reperfusion injury of ischemic skeletal muscle is mediated by natural antibody and complement. J Exp Med. 1996;183:2343–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan RK, Verna N, Afnan J, et al. Attenuation of skeletal muscle reperfusion injury with intravenous 12 amino acid peptides that bind to pathogenic IgM. Surgery. 2006;139:236–243. [DOI] [PubMed] [Google Scholar]
- 45.Paidassi H, Tacnet-Delorme P, Verneret M, et al. Investigations on the C1q-calreticulin-phosphatidylserine interactions yield new insights into apoptotic cell recognition. J Mol Biol. 2011;408:277–290. [DOI] [PubMed] [Google Scholar]
- 46.Castellano G, Melchiorre R, Loverre A, et al. Therapeutic targeting of classical and lectin pathways of complement protects from ischemia-reperfusion-induced renal damage. Am J Pathol. 2010;176:1648–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farrar CA, Asgari E, Schwaeble WJ, et al. Which pathways trigger the role of complement in ischaemia/reperfusion injury? Front Immunol. 2012;3:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCullough JW, Renner B, Thurman JM. The role of the complement system in acute kidney injury. Semin Nephrol. 2013;33:543–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gorsuch WB, Chrysanthou E, Schwaeble WJ, et al. The complement system in ischemia-reperfusion injuries. Immunobiology. 2012;217: 1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Banz Y, Rieben R. Role of complement and perspectives for intervention in ischemia-reperfusion damage. Ann Med. 2012;44:205–217. [DOI] [PubMed] [Google Scholar]
- 51.Syriga M, Mavroidis M. Complement system activation in cardiac and skeletal muscle pathology: friend or foe? Adv Exp Med Biol. 2013;735: 207–218. [DOI] [PubMed] [Google Scholar]
- 52.Weyker PD, Webb CA, Kiamanesh D, et al. Lung ischemia reperfusion injury: a bench-to-bedside review. Semin Cardiothorac Vasc Anesth. 2013;17:28–43. [DOI] [PubMed] [Google Scholar]
- 53.Karpel-Massler G, Fleming SD, Kirschfink M, et al. Human C1 esterase inhibitor attenuates murine mesenteric ischemia/reperfusion induced local organ injury. J Surg Res. 2003;115:247–256. [DOI] [PubMed] [Google Scholar]
- 54.Lauterbach M, Horstick G, Plum N, et al. C1-esterase inhibitor reverses functional consequences of superior mesenteric artery ischemia/reperfusion by limiting reperfusion injury and restoring microcirculatory perfusion. Shock. 2007;27:75–83. [DOI] [PubMed] [Google Scholar]
- 55.Lu F, Chauhan AK, Fernandes SM, et al. The effect of C1 inhibitor on intestinal ischemia and reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1042–G1049. [DOI] [PubMed] [Google Scholar]
- 56.Inderbitzin D, Beldi G, Avital I, et al. Local and remote ischemia-reperfusion injury is mitigated in mice overexpressing human C1 inhibitor. Eur Surg Res. 2004;36:142–147. [DOI] [PubMed] [Google Scholar]
- 57.Li K, Zhou W. Anaphylatoxins in organ transplantation. Semin Immunol. 2013;25:20–28. [DOI] [PubMed] [Google Scholar]
- 58.Borg T, Gerdin B, Hallgren R, et al. The role of polymorphonuclear leucocytes in the pulmonary dysfunction induced by complement activation. Acta Anaesthesiol Scand. 1985;29:231–240. [DOI] [PubMed] [Google Scholar]
- 59.Chen G, Chen S, Chen X. Role of complement and perspectives for intervention in transplantation. Immunobiology. 2013;218:817–827. [DOI] [PubMed] [Google Scholar]
- 60.Duehrkop C, Rieben R. Ischemia/reperfusion injury: effect of simultaneous inhibition of plasma cascade systems versus specific complement inhibition. Biochem Pharmacol. 2014;88:12–22. [DOI] [PubMed] [Google Scholar]
- 61.Gustafson EK, Elgue G, Hughes RD, et al. The instant blood-mediated inflammatory reaction characterized in hepatocyte transplantation. Transplantation. 2011;91:632–638. [DOI] [PubMed] [Google Scholar]
- 62.Saidi RF, Rajeshkumar B, Shariftabrizi A, et al. Human C1 inhibitor attenuates liver ischemia-reperfusion injury and promotes liver regeneration. J Surg Res. 2014;187:660–666. [DOI] [PubMed] [Google Scholar]
- 63.Bergamaschini L, Gobbo G, Gatti S, et al. Endothelial targeting with C1-inhibitor reduces complement activation in vitro and during ex vivo reperfusion of pig liver. Clin Exp Immunol. 2001;126:412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duehrkop C, Banz Y, Spirig R, et al. C1 esterase inhibitor reduces lower extremity ischemia/reperfusion injury and associated lung damage. PLoS One. 2013;8:e72059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scherer M, Demertzis S, Langer F, et al. C1-esterase inhibitor reduces reperfusion injury after lung transplantation. Ann Thorac Surg. 2002;73: 233–238. [DOI] [PubMed] [Google Scholar]
- 66.He S, Atkinson C, Evans Z, et al. A role for complement in the enhanced susceptibility of steatotic livers to ischemia and reperfusion injury. J Immunol. 2009;183:4764–4772. [DOI] [PubMed] [Google Scholar]
- 67.Vo AA, Zeevi A, Choi J, et al. A phase I/II placebo-controlled trial of C1-inhibitor for prevention of antibody-mediated rejection in HLA Sensitized Patients. Transplantation. 2015;99:299–308. [DOI] [PubMed] [Google Scholar]
- 68.Montgomery R, Orandi B, Racusen L, et al. Human plasma-derived C1 esterase inhibitor for the treatment of acute antibofy mediated rejection in kidney transplantation in American Journal of Transplantation San Franscisco, USA: Wiley; 2014:129. [Google Scholar]
- 69.Sommer W, Tudorache I, Kuhn C, et al. C1-esterase-inhibitor for primary graft dysfunction in lung transplantation. Transplantation. 2014;97: 1185–1191. [DOI] [PubMed] [Google Scholar]
- 70.Clinicaltrials.gov, C1INH Inhibitor Preoperative and Post Kidney Transplant to Prevent DGF & IRI https://clinicaltrials.gov/ct2/show/NCT02134314. Accessed on 18th February 2015.
- 71.Terasaki P, Mizutani K. Antibody mediated rejection: update 2006. Clin J Am Soc Nephrol. 2006;1:400–403. [DOI] [PubMed] [Google Scholar]
- 72.Valenzuela NM, McNamara JT, Reed EF. Antibody-mediated graft injury: complement-dependent and complement-independent mechanisms. Curr Opin Organ Transplant. 2014;19:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dragun D, Catar R, Philippe A. Non-HLA antibodies in solid organ transplantation: recent concepts and clinical relevance. Curr Opin Organ Transplant. 2013;18:430–435. [DOI] [PubMed] [Google Scholar]
- 74.Mengel M, Husain S, Hidalgo L, et al. Phenotypes of antibody-mediated rejection in organ transplants. Transpl Int. 2012;25:611–622. [DOI] [PubMed] [Google Scholar]
- 75.Muramatsu M, Gonzalez HD, Cacciola R, et al. ABO incompatible renal transplants: good or bad? World J Transplant. 2014;4:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2011 Annual Data Report: kidney. Am J Transplant. 2013;13Suppl 1:11–46. [DOI] [PubMed] [Google Scholar]
- 77.Jordan SC, Reinsmoen N, Peng A, et al. Advances in diagnosing and managing antibody-mediated rejection. Pediatr Nephrol. 2010;25:2035–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bartel G, Schwaiger E, Bohmig GA. Prevention and treatment of alloantibody-mediated kidney transplant rejection. Transpl Int. 2011;24: 1142–1155. [DOI] [PubMed] [Google Scholar]
- 79.Djamali A, Kaufman DB, Ellis TM, et al. Diagnosis and management of antibody-mediated rejection: current status and novel approaches. Am J Transplant. 2014;14:255–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Loupy A, Hill GS, Jordan SC. The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol. 2012;8: 348–357. [DOI] [PubMed] [Google Scholar]
- 81.Loupy A, Vernerey D, Tinel C, et al. Subclinical rejection phenotypes at 1 year post-transplant and outcome of kidney allografts. J Am Soc Nephrol. 2015;26:1721–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Remport A, Ivanyi B, Mathe Z, et al. Better understanding of transplant glomerulopathy secondary to chronic antibody-mediated rejection. Nephrol Dial Transplant. 2014. [DOI] [PubMed] [Google Scholar]
- 83.Budding K, van de Graaf EA, Otten HG. Humoral immunity and complement effector mechanisms after lung transplantation. Transpl Immunol. 2014;31:260–265. [DOI] [PubMed] [Google Scholar]
- 84.Frank R, Molina MR, Goldberg LR, et al. Circulating donor-specific antihuman leukocyte antigen antibodies and complement C4d deposition are associated with the development of cardiac allograft vasculopathy. Am J Clin Pathol. 2014;142:809–815. [DOI] [PubMed] [Google Scholar]
- 85.Pober JS, Jane-wit D, Qin L, et al. Interacting mechanisms in the pathogenesis of cardiac allograft vasculopathy. Arterioscler Thromb Vasc Biol. 2014;34:1609–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smith RN, Colvin RB. Chronic alloantibody mediated rejection. Semin Immunol. 2012;24:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14:272–283. [DOI] [PubMed] [Google Scholar]
- 88.Cravedi P, Heeger PS. Complement as a multifaceted modulator of kidney transplant injury. J Clin Invest. 2014;124:2348–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sutherland SM, Chen G, Sequeira FA, et al. Complement-fixing donor-specific antibodies identified by a novel C1q assay are associated with allograft loss. Pediatr Transplant. 2012;16:12–17. [DOI] [PubMed] [Google Scholar]
- 90.Loupy A, Lefaucheur C, Vernerey D, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med. 2013; 369:1215–1226. [DOI] [PubMed] [Google Scholar]
- 91.Schaub S, Honger G, Koller MT, et al. Determinants of C1q binding in the single antigen bead assay. Transplantation. 2014;98:387–393. [DOI] [PubMed] [Google Scholar]
- 92.Freitas MC, Rebellato LM, Ozawa M, et al. The role of immunoglobulin-G subclasses and C1q in de novo HLA-DQ donor-specific antibody kidney transplantation outcomes. Transplantation. 2013;95:1113–1119. [DOI] [PubMed] [Google Scholar]
- 93.Zeevi A, Lunz J, Feingold B, et al. Persistent strong anti-HLA antibody at high titer is complement binding and associated with increased risk of antibody-mediated rejection in heart transplant recipients. J Heart Lung Transplant. 2013;32:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zeevi A, Lunz JG 3rd, Shapiro R, et al. Emerging role of donor-specific anti-human leukocyte antigen antibody determination for clinical management after solid organ transplantation. Hum Immunol. 2009;70: 645–650. [DOI] [PubMed] [Google Scholar]
- 95.Thomas KA, Valenzuela NM, Reed EF. The perfect storm: HLA antibodies, complement, FcgammaRs, and endothelium in transplant rejection. Trends Mol Med. 2015;21:319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.von Rossum A, Laher I, Choy JC. Immune-mediated vascular injury and dysfunction in transplant arteriosclerosis. Front Immunol. 2015;5:684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Valenzuela NM, Trinh KR, Mulder A, et al. Monocyte recruitment by HLA IgG-activated endothelium: the relationship between IgG Subclass and FcgammaRIIa polymorphisms. Am J Transplant. 2015;15: 1502–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Racusen LC, Colvin RB, Solez K, et al. Antibody-mediated rejection criteria—an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3:708–714. [DOI] [PubMed] [Google Scholar]
- 99.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008; 8:753–760. [DOI] [PubMed] [Google Scholar]
- 100.Zeevi A Chronic antibody-mediated rejection: new diagnostic tools—clinical significance of C4d deposition and improved detection and characterization of human leucocyte antigen antibodies. Clin Exp Immunol. 2014;178Suppl 1:52–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Loupy A, Hill GS, Suberbielle C, et al. Significance of C4d Banff scores in early protocol biopsies of kidney transplant recipients with preformed donor-specific antibodies (DSA). Am J Transplant. 2011;11:56–65. [DOI] [PubMed] [Google Scholar]
- 102.Eskandary F, Bond G, Schwaiger E, et al. Bortezomib in late antibody-mediated kidney transplant rejection (BORTEJECT Study): study protocol for a randomized controlled trial. Trials. 2014;15:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gaston RS, Cecka JM, Kasiske BL, et al. Evidence for antibody-mediated injury as a major determinant of late kidney allograft failure. Transplantation. 2010;90:68–74. [DOI] [PubMed] [Google Scholar]
- 104.Kaneku H, O’Leary JG, Banuelos N, et al. De novo donor-specific HLA antibodies decrease patient and graft survival in liver transplant recipients. Am J Transplant. 2013;13:1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Berger M, Zeevi A, Farmer DG, et al. Immunologic challenges in small bowel transplantation. Am J Transplant. 2012;12Suppl 4:S2–S8. [DOI] [PubMed] [Google Scholar]
- 106.Jordan SC, Choi J, Vo A. Kidney transplantation in highly sensitized patients. Br Med Bull. 2015;114:113–125. [DOI] [PubMed] [Google Scholar]
- 107.Singh N, Djamali A, Lorentzen D, et al. Pretransplant donor-specific antibodies detected by single-antigen bead flow cytometry are associated with inferior kidney transplant outcomes. Transplantation. 2010;90: 1079–1084. [DOI] [PubMed] [Google Scholar]
- 108.Vo AA, Choi J, Cisneros K, et al. Benefits of rituximab combined with intravenous immunoglobulin for desensitization in kidney transplant recipients. Transplantation. 2014;98:312–319. [DOI] [PubMed] [Google Scholar]
- 109.Alexion, Alexion Provides Update on Phase 2 Clinical Trial with Eculizumab in Antibody Mediated Rejection (AMR) in Living-Donor Kidney Transplant Recipients. http://news.alexionpharma.com/press-release/company-news/alexion-provides-update-phase-2-clinical-trial-eculizumab-antibody-mediat, 2015. Accessed 16th April 2015.
- 110.Tillou X, Poirier N, Le Bas-Bernardet S, et al. Recombinant human C1-inhibitor prevents acute antibody-mediated rejection in alloimmunized baboons. Kidney Int. 2010;78:152–159. [DOI] [PubMed] [Google Scholar]
- 111.Harimoto N, Ikegami T, Nakagawara H, et al. Chronic immune-mediated reaction syndrome as the cause of late graft mortality in living-donor liver transplantation for primary biliary cirrhosis. Transplant Proc. 2014;46: 1438–1443. [DOI] [PubMed] [Google Scholar]
- 112.Regele H, Bohmig GA, Habicht A, et al. Capillary deposition of complement split product C4d in renal allografts is associated with basement membrane injury in peritubular and glomerular capillaries: a contribution of humoral immunity to chronic allograft rejection. J Am Soc Nephrol. 2002;13:2371–2380. [DOI] [PubMed] [Google Scholar]
- 113.Worthington JE, McEwen A, McWilliam LJ, et al. Association between C4d staining in renal transplant biopsies, production of donor-specific HLA antibodies, and graft outcome. Transplantation. 2007;83:398–403. [DOI] [PubMed] [Google Scholar]
- 114.Banan B, Xu Z, Gunasekaran M, et al. Role of alloimmunity and autoimmunity in allograft rejection. Clin Transpl. 2013: 325–32. [PMC free article] [PubMed] [Google Scholar]
- 115.Hachem RR, Tiriveedhi V, Patterson GA, et al. Antibodies to K-alpha 1 tubulin and collagen V are associated with chronic rejection after lung transplantation. Am J Transplant. 2012;12:2164–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lobo LJ, Aris RM, Schmitz J, et al. Donor-specific antibodies are associated with antibody-mediated rejection, acute cellular rejection, bronchiolitis obliterans syndrome, and cystic fibrosis after lung transplantation. J Heart Lung Transplant. 2013;32:70. [DOI] [PubMed] [Google Scholar]
- 117.Westall GP, Snell GI, McLean C, et al. C3d and C4d deposition early after lung transplantation. J Heart Lung Transplant. 2008;27:722–728. [DOI] [PubMed] [Google Scholar]
- 118.Rodriguez ER, Skojec DV, Tan CD, et al. Antibody-mediated rejection in human cardiac allografts: evaluation of immunoglobulins and complement activation products C4d and C3d as markers. Am J Transplant. 2005;5:2778–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sis B, Halloran PF. Endothelial transcripts uncover a previously unknown phenotype: C4d-negative antibody-mediated rejection. Curr Opin Organ Transplant. 2010;15:42–48. [DOI] [PubMed] [Google Scholar]
- 120.Loupy A, Cazes A, Guillemain R, et al. Very late heart transplant rejection is associated with microvascular injury, complement deposition and progression to cardiac allograft vasculopathy. Am J Transplant. 2011;11:1478–1487. [DOI] [PubMed] [Google Scholar]
- 121.Sandal S, Zand MS. Rational clinical trial design for antibody mediated renal allograft injury. Front Biosci (Landmark Ed). 2015;20:743–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Clinicaltrials.gov, A study to evaluate the long-term clinical safety and efficacy of subcutaneously administered C1-esterase inhibitor in the prevention of hereditary angioedema. https://clinicaltrials.gov/ct2/show/NCT02316353. Accessed on 8th April 2015.