Abstract
Background
In phase 3 MODIFY I/II trials, bezlotoxumab significantly reduced recurrence of Clostridioides (Clostridium) difficile infection (rCDI) over 12 weeks. Choice of CDI antibacterial treatment may affect CDI-related outcomes; therefore, this prespecified analysis assessed if the magnitude of bezlotoxumab-induced rCDI reduction was influenced by the antibiotic administered.
Methods
In MODIFY I/II (NCT01241552/NCT01513239), participants received a single infusion of bezlotoxumab (10 mg/kg) or placebo during anti-CDI treatment. Using pooled data from MODIFY I/II, initial clinical cure (ICC) and rCDI were assessed in metronidazole-, vancomycin-, and fidaxomicin-treated subgroups.
Results
Of 1554 participants in MODIFY I/II, 753 (48.5%) received metronidazole, 745 (47.9%) vancomycin, and 56 (3.6%) fidaxomicin. Fewer participants receiving metronidazole had a prior CDI episode in the previous 6 months (12.9%) or ≥1 risk factor for rCDI (66.0%) vs participants receiving vancomycin (41.2% and 83.6%, respectively) and fidaxomicin (55.4% and 89.3%, respectively). ICC rates were similar in the bezlotoxumab (metronidazole, 81.0%; vancomycin, 78.5%; fidaxomicin, 86.7%) and placebo groups (metronidazole, 81.3%; vancomycin, 79.6%; fidaxomicin, 76.9%). In placebo-treated participants, the rCDI was lower in the metronidazole subgroup vs the vancomycin and fidaxomicin subgroups (metronidazole, 28.0%; vancomycin, 38.4%; fidaxomicin, 35.0%). When analyzed by subsets based on history of CDI, rCDI rates were similar in the metronidazole and vancomycin groups. rCDI rates were lower in all antibiotic subgroups for bezlotoxumab vs placebo (metronidazole: rate difference [RD], –9.7%; 95% confidence interval [CI], –16.4% to –3.1%; vancomycin: RD, –15.4%; 95% CI, –22.7% to –8.0%; fidaxomicin: RD, –11.9%; 95% CI, –38.1% to 14.3%).
Conclusion
Bezlotoxumab reduces rCDI vs placebo in participants receiving metronidazole and vancomycin, with a similar effect size in participants receiving fidaxomicin.
Keywords: antibacterial drug treatment, bezlotoxumab, Clostridioides (Clostridium) difficile infection recurrence, toxin
In recent years, Clostridioides (Clostridium) difficile has become one of the leading causes of hospital-acquired infections in the United States [1], with a marked increase in associated morbidity and mortality [2–4].
For 30 years, metronidazole and vancomycin were the main antibacterial drugs used in the treatment of C. difficile infection (CDI); metronidazole was recommended for mild to moderate CDI, whereas vancomycin was the preferred therapy for severe and recurrent infections [5]. However, studies have shown that vancomycin results in superior clinical cure rates compared with metronidazole in individuals with both nonsevere and severe CDI [6, 7]. Furthermore, treatment of an initial episode with fidaxomicin has been shown to significantly reduce the likelihood of recurrent CDI (rCDI) compared with vancomycin [8–10]. These findings have resulted in significant revisions to treatment guidelines, and in the 2017 Infectious Diseases Society of America/Society of Healthcare Epidemiology of America (IDSA/SHEA) CDI guidelines, the recommended first-line antibacterial drug treatment for severe and nonsevere CDI was vancomycin or fidaxomicin, rather than metronidazole [1].
Whereas antibiotic treatment of primary CDI is usually successful in decreasing signs and symptoms of the disease, it has been reported that ~25% of individuals will experience rCDI after initial antibiotic therapy [6, 10]. Treatment of rCDI remains challenging; following the first recurrent episode, individuals have a 38% probability of a second recurrence, despite initial episodes being treated with metronidazole or vancomycin [11]. The likelihood of additional recurrences is influenced by the choice of antibacterial drug treatment received. In treating a first recurrent episode, fidaxomicin has been associated with a reduced rate of second recurrence compared with vancomycin [9].
Bezlotoxumab is a fully human monoclonal antibody against C. difficile toxin B that is indicated to prevent the recurrence of rCDI in at-risk adults receiving antibacterial drug treatment for CDI [12, 13]. MODIFY I/II were global, randomized, double-blind, placebo-controlled trials of the efficacy of bezlotoxumab for the prevention of rCDI in adults receiving metronidazole, vancomycin, or fidaxomicin. In the MODIFY trials, bezlotoxumab administered with or without actoxumab (a fully human monoclonal antibody against C. difficile toxin A) was shown to significantly lower the rate of rCDI over 12 weeks compared with placebo. The addition of actoxumab did not improve efficacy [14].
In the MODIFY trials, the selection of metronidazole, vancomycin, or fidaxomicin to treat the presenting CDI episode was made by the treating physician [14]. The 2010 IDSA/SHEA CDI guidelines, which were in effect at the time of study enrollment, recommended metronidazole for the treatment of mild/moderate CDI and vancomycin for severe CDI and multiple rCDI [5]. Fidaxomicin was not included in the 2010 guidelines because it was approved in 2011 [15]. Fidaxomicin became available shortly before initiation of the MODIFY trials, and as it is indicated for treatment of CDI, it was allowed as an antibiotic choice for these trials. As the rate of rCDI could be influenced by the antibacterial drug treatment received for the presenting CDI episode, treatment groups were stratified at the time of randomization according to this variable in both trials.
Given the potential influence of the antibacterial drug treatment received on rCDI, this prespecified analysis examined if the administration of metronidazole, vancomycin, or fidaxomicin had an impact on efficacy outcomes in participants receiving bezlotoxumab or placebo in the MODIFY I/II trials.
METHODS
Study Design
MODIFY I (NCT01241552) and MODIFY II (NCT01513239) were randomized, double-blind, placebo-controlled, multicenter, phase 3 trials conducted between November 1, 2011, and May 22, 2015, at 322 sites in 30 countries. Full details of the trials have been published previously [14]. Briefly, participants receiving metronidazole, vancomycin, or fidaxomicin for primary or rCDI were randomized in a 1:1:1:1 ratio to receive a single infusion of actoxumab plus bezlotoxumab (10 mg/kg of body weight each), bezlotoxumab alone (10 mg/kg), actoxumab alone (10 mg/kg; MODIFY I only), or placebo (0.9% saline).
Antibacterial drug treatment for the presenting CDI episode was to be administered for a minimum of 10 days and a maximum of 14 days in an attempt to control any influence of treatment duration on CDI-related outcomes. Initial clinical cure (ICC) was imputed as failure if treatment was given longer than 14 days (defined as 16 calendar days to account for partial dosing on the first and last days). Choice of antibacterial treatment for CDI was per the treating clinician’s discretion and could not be changed during the MODIFY I/II trials. Randomization was stratified according to oral antibacterial drug treatment (metronidazole, vancomycin, or fidaxomicin) and hospitalization status (inpatient or outpatient) at the time of study randomization.
MODIFY I/II were conducted in accordance with Good Clinical Practice guidelines and the provisions of the Declaration of Helsinki. The protocols and amendments were approved by the institutional review board or independent ethics committee at each study site. Written informed consent was provided by all participants before entering the trial.
Prespecified Analysis of Pooled Data From MODIFY I/II
In this prespecified analysis of pooled data from the MODIFY I/II trials, which includes only participants who received bezlotoxumab alone or placebo, efficacy end points were assessed based on the antibacterial drug administered to treat the presenting CDI episode. The metronidazole, vancomycin, and fidaxomicin subgroups were based on the antibacterial drug that was entered by the investigator into the randomization system (ie, the stratification factor). In a few instances (62 participants of 1554 [4%] included in the modified intent-to-treat [mITT] population), the stratification factor differed from the antibacterial drug that the participant ultimately received. The results reported here are based on the stratification factor to be consistent with the original statistical analysis plan.
Efficacy End Points and Statistical Methods
The primary end point was rCDI, which was defined as a new episode of diarrhea (≥3 unformed stools in 24 hours) associated with a positive stool test for toxigenic C. difficile within 12 weeks of bezlotoxumab or placebo infusion in participants who had achieved ICC of the baseline CDI episode (clinical cure population). ICC was an exploratory end point, which was defined as no diarrhea during the 2 consecutive days after completion of ≤14 days of antibacterial drug treatment. Rates of all-cause 30-day and 90-day mortality were estimated in the all-patients-as-treated (APaT) population. The rates of all-cause and CDI-related rehospitalization within 30 days of antibiotic treatment were assessed in participants who were inpatients at the time of randomization. An additional analysis using only data from placebo participants is presented here. This analysis explores the impact of a confounding variable (prior history of CDI) on the assessment of rCDI in each of the antibacterial drug cohorts.
The analysis population for ICC was the mITT population, which included all randomized participants in the overall population of the MODIFY I/II trials who received the study infusion, had a positive baseline stool test for toxigenic C. difficile, and initiated antibacterial drug treatment before, or within 1 day after, the study infusion [14].
Observed rates of ICC and rCDI are presented, along with rate differences between the bezlotoxumab and placebo groups and their 95% confidence intervals (CIs). The 95% CIs are based on the Miettinen and Nurminen method without stratification [16].
RESULTS
Participants
The integrated mITT population from MODIFY I/II consisted of 1554 participants, of whom 781 received bezlotoxumab and 773 received placebo. The majority of participants received oral metronidazole or oral vancomycin. Across both treatment groups, 753 (48.5%) participants were in the metronidazole stratum, 745 (47.9%) were in the vancomycin stratum, and 56 (3.6%) were in the fidaxomicin stratum. By design, the proportions of participants receiving bezlotoxumab and placebo were similar in each antibacterial drug treatment subgroup (Table 1).
Table 1.
Administered Antibacterial Drug Treatment for CDI According to Treatment Group (mITT Population)
| Bezlotoxumab (N = 781), n (%) | Placebo (N = 773), n (%) | |
|---|---|---|
| Metronidazole stratum (48.5% of participants) | 379 | 374 |
| Oral metronidazole alone | 361 (95.3) | 344 (92.0) |
| Oral metronidazole with IV metronidazole | 7 (1.8) | 2 (0.5) |
| Othera | 11 (2.9) | 28 (7.5) |
| Days on antibacterial treatment before infusionb | ||
| Mean (SD) | 3.4 (2.1) | 3.1 (2.1) |
| Median (range) | 3 (0 to 13) | 3 (0 to 11) |
| Total days on antibacterial treatmentb,c | ||
| Mean (SD) | 13.4 (3.5) | 13.7 (4.9) |
| Median (range) | 14 (2 to 37) | 13 (5 to 62) |
| Vancomycin stratum (47.6% of participants) | 372 | 373 |
| Oral vancomycin alone | 318 (85.5) | 300 (80.4) |
| Oral vancomycin with IV metronidazole | 44 (11.8) | 52 (13.9) |
| Othera | 10 (2.7) | 21 (5.6) |
| Days on antibacterial treatment before infusionb | ||
| Mean (SD) | 3.2 (2.2) | 3.3 (2.1) |
| Median (range) | 3 (0 to 14) | 3 (–1 to 13) |
| Total days on antibacterial treatmentb,c | ||
| Mean (SD) | 14.7 (7.7) | 14.3 (5.1) |
| Median (range) | 14 (4 to 87) | 14 (3 to 67) |
| Fidaxomicin stratum (3.8% of participants) | 30 | 26 |
| Oral fidaxomicin alone | 26 (86.7) | 24 (92.3) |
| Oral fidaxomicin with IV metronidazole | 1 (3.3) | 1 (3.8) |
| Othera | 3 (10.0) | 1 (3.8) |
| Days on antibacterial treatment before infusionb | ||
| Mean (SD) | 3.0 (2.9) | 3.0 (2.2) |
| Median (range) | 2 (0 to 12) | 3 (0 to 8) |
| Total days on antibacterial treatmentb,c | ||
| Mean (SD) | 11.8 (2.3) | 12.0 (2.3) |
| Median (range) | 11 (10 to 20) | 11 (10 to 21) |
Abbreviations: CDI, Clostridioides difficile infection; IV, intravenous; mITT, modified intent-to-treat.
aThe actual antibacterial drug treatment for CDI on the day of infusion differed from the stratification antibacterial drug treatment for CDI.
bData represent the number of calendar days.
cData for total days on antibacterial treatment reflect the days on therapy for all antibiotics given to treat the CDI episode under treatment at the time of randomization and include days before, the day of, and days after administration of study medication.
The mean number of calendar days of antibacterial treatment before infusion with the study agent was similar between subgroups (metronidazole, 3.3 days; vancomycin, 3.2 days; fidaxomicin, 3.0 days), as was the mean total duration of antibacterial treatment (metronidazole, 13.6 days; vancomycin, 14.5 days; fidaxomicin, 11.9 days). Across all subgroups, the number of days on antibacterial treatment before infusion ranged from 0 to 14 days and the total number of treatment days ranged from 2 to 87 days. (Table 1).
Although many participant characteristics were generally similar between the metronidazole, vancomycin, and fidaxomicin subgroups, there were some notable differences. Compared with participants who received metronidazole, participants who received vancomycin were of an older age (median age [range], 62 [49–75] years vs 67 [56–78] years; ≥65 years: 45.6% vs 57.1%), more likely to have ≥1 episode of CDI in the past 6 months (12.9% vs 41.2%), more likely to have ≥1 predefined risk factor for rCDI (66.0% vs 83.6%), more likely to be an inpatient at the time of infusion (64.8% vs 71.7%), and more likely to have severe disease at baseline (Zar Score ≥2: 13.3% vs 18.7%) (Table 2). Compared with the metronidazole and vancomycin subgroups, participants in the fidaxomicin subgroup were more likely to have ≥1 episode of CDI ever (metronidazole, 16.9%; vancomycin, 46.0%; fidaxomicin, 69.6%). Notably, 21.4% of participants in the fidaxomicin subgroup had ≥3 prior CDI episodes, compared with 2.3% and 9.1% of metronidazole- and vancomycin-treated participants, respectively (Table 2).
Table 2.
Baseline Demographics and Clinical Characteristics in Treatment Groups (mITT Population)
| Metronidazole Stratum (N = 753), n (%) | Vancomycin Stratum (N = 745), n (%) | Fidaxomicin Stratum (N = 56), n (%) | |
|---|---|---|---|
| Age, years | |||
| Mean (SD) | 60.6 (18.4) | 64.9 (16.8) | 61.3 (18.4) |
| Median (IQR) | 62 (49–75) | 67 (56–78) | 63 (54–72) |
| ≥65 years | 343 (45.6) | 426 (57.2) | 26 (46.4) |
| ≥75 years | 194 (25.8) | 244 (32.7) | 10 (17.9) |
| Female | 433 (57.5) | 422 (56.6) | 36 (64.3) |
| Inpatient at the time of randomization | 488 (64.8) | 534 (71.7) | 28 (50.0) |
| Immunocompromiseda | 165 (21.9) | 155 (20.8) | 11 (19.6) |
| ≥1 of 5 predefined risk factors for rCDIb | 497 (66.0) | 623 (83.6) | 50 (89.3) |
| Charlson Comorbidity Index ≥3 | 304 (40.4) | 288 (38.7) | 30 (53.6) |
| Renal impairmentc | 113 (15.0) | 113 (15.2) | 7 (12.5) |
| Hepatic impairmentd | 43 (5.7) | 47 (6.3) | 3 (5.4) |
| ≥1 CDI episodes in past 6 months | 97 (12.9) | 307 (41.2) | 31 (55.4) |
| ≥1 CDI episode ever | 127 (16.9) | 342 (45.9) | 39 (69.6) |
| Number of prior CDI episodes | |||
| 0 | 613 (81.4) | 388 (52.1) | 17 (30.4) |
| 1 | 93 (12.4) | 178 (23.9) | 11 (19.6) |
| 2 | 17 (2.3) | 96 (12.9) | 16 (28.6) |
| ≥3 | 17 (2.3) | 68 (9.1) | 12 (21.4) |
| Severe CDI (Zar Scoree ≥2) | 100 (13.3) | 139 (18.7) | 8 (14.3) |
| Participants with a positive culture | 517 (68.7) | 431 (57.9) | 28 (50.0) |
| Ribotype 027, 078, or 244 | 89 (11.8) | 121 (16.2) | 7 (12.5) |
| Ribotype 027 | 72 (9.6) | 111 (14.9) | 6 (10.7) |
Abbreviations: ALT, alanine aminotransferase; CDI, Clostridioides difficile infection; IQR, interquartile range; mITT, modified intent-to-treat; rCDI, recurrent C. difficile infection; ULN, upper limit of normal; WBC, white blood cell.
aDefined as the basis of a participant’s medical history or use of immunosuppressive therapy.
bPredefined risk factors include CDI history in the past 6 months, severe CDI at baseline (per Zar score), age ≥65 years, having a hypervirulent strain (027, 078, or 244 ribotypes) at baseline, and being immunocompromised.
cSerum creatinine ≥1.5 mg/dL.
dBased on 2 or more of the following: (1) albumin ≤3.1 g/dL; (2) ALT ≥2X ULN; (3) total bilirubin ≥1.3X ULN; or (4) mild, moderate, or severe liver disease (as reported on the Charlson comorbidity index).
eBased on the following: (1) age >60 years (1 point); (2) body temperature >38.3˚C (>100˚F; 1 point); (3) albumin level <2.5 mg/dL (1 point); (4) peripheral WBC count >15 000 cells/mm3 within 48 hours (1 point); (5) endoscopic evidence of pseudomembranous colitis (2 points); and (6) treatment in intensive care unit (2 points).
Initial Clinical Cure
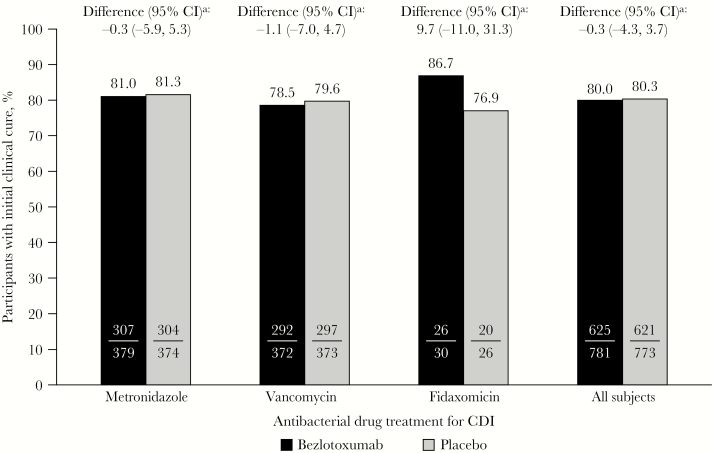
ICC rates were similar across antibiotic strata in the placebo group (metronidazole, 81.3%; vancomycin, 79.6%; fidaxomicin, 76.9%) and the bezlotoxumab group (metronidazole, 81.0%; vancomycin, 78.5%; fidaxomicin, 86.7%). There was no difference in ICC rates between participants receiving bezlotoxumab and placebo, regardless of whether metronidazole, vancomycin, or fidaxomicin was administered to treat the presenting CDI episode (metronidazole: rate difference [RD], –0.3%; 95% CI, –5.9% to 5.3%; vancomycin: RD, –1.1%; 95% CI, –7.0% to 4.7%; fidaxomicin: RD, 9.7%; 95% CI, –11.0% to 31.3%). Similar ICC rates were also observed in the overall study results, with no difference in the rate between bezlotoxumab-treated (80.0%) and placebo-treated participants (80.3%; RD, –0.3%; 95% CI, –4.3% to 3.7%) (Figure 1).
Figure 1.
Proportion of participants with initial clinical cure (mITT population). aBased on the Miettinen and Nurminen method. Abbreviations: CDI, Clostridioides difficile infection; CI, confidence interval, mITT, modified intent-to-treat.
CDI Recurrence
Differences Between Antibacterial Treatment Subgroups
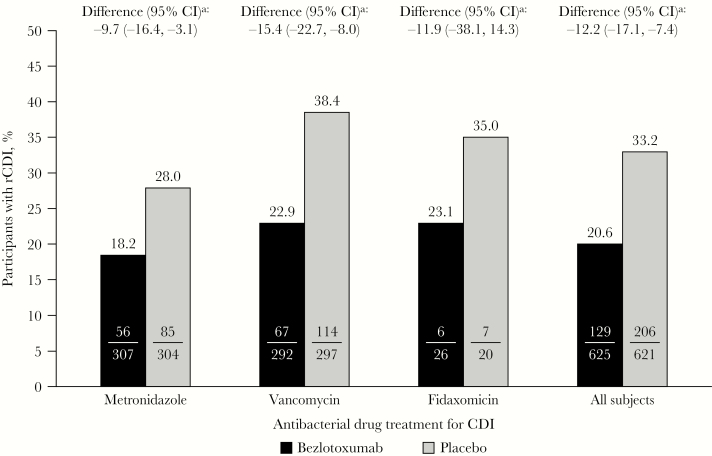
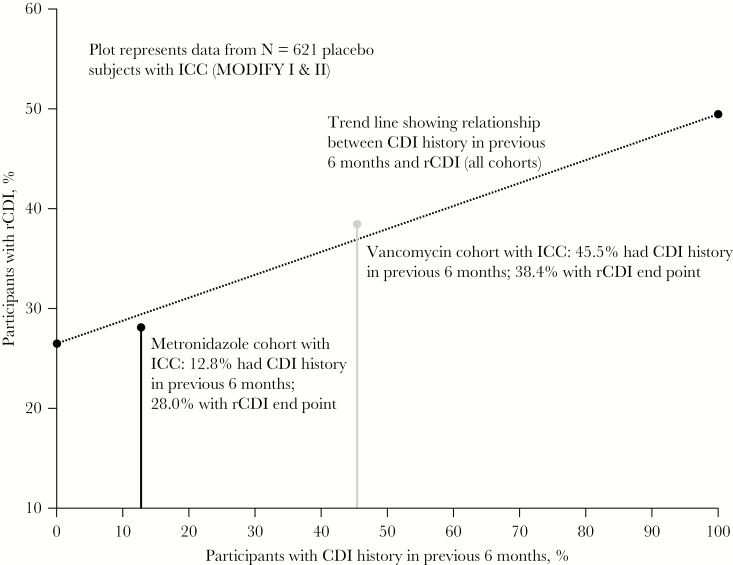
In placebo-treated participants, 28.0% of participants in the metronidazole subgroup experienced rCDI compared with 38.4% of participants in the vancomycin subgroup (RD, 10.4%; 95% CI, 2.9% to 17.9%) (Figure 2). However, the difference in rCDI rate between antibacterial treatment groups decreased when patients were stratified by history of CDI: in participants with primary CDI, 25.5% of metronidazole-treated participants experienced rCDI compared with 27.8% of vancomycin-treated participants (RD, 2.4%); in participants with ≥1 episode of CDI in the past 6 months, 46.2% of metronidazole-treated participants experienced rCDI compared with 50.4% of vancomycin-treated participants (RD, 4.2%). Following adjustment for prior CDI history, the overall difference (vancomycin minus metronidazole) in the proportion of participants who experienced rCDI (among those with ICC) was 2.8% (95% CI, –5.0% to 10.8%). The relationship between CDI history in the previous 6 months and occurrence of rCDI in placebo-treated participants with ICC is presented in Figure 3. As plots for metronidazole and vancomycin fall to the trend line, this suggests that the difference in the observed rCDI rates between these subgroups is largely explained by a baseline difference between the 2 cohorts.
Figure 2.
Proportion of participants with rCDI (clinical cure population). aBased on the Miettinen and Nurminen method. Abbreviations: CDI, Clostridioides difficile infection; CI, confidence interval; rCDI, recurrent C. difficile infection.
Figure 3.
Relationship between CDI history in the previous 6 months and occurrence of rCDI in placebo-treated participants receiving metronidazole or vancomycin. The relationship between CDI history in the previous 6 months and occurrence of rCDI in placebo-treated participants with ICC (n = 621) is represented by the dotted trend line. The difference in the percentage of participants in the metronidazole and vancomycin subgroups with a history of CDI in the previous 6 months is represented by the distance between the black (metronidazole) and gray (vancomycin) reference lines. Abbreviations: CDI, Clostridioides difficile infection; ICC, initial clinical cure; rCDI, recurrent C. difficile infection.
Differences Between Bezlotoxumab and Placebo Within Antibacterial Treatment Subgroups
In the metronidazole and vancomycin subgroups, participants who received bezlotoxumab experienced a significantly lower rate of rCDI compared with placebo (metronidazole: RD, –9.7%; 95% CI, –16.4% to –3.1%; vancomycin: RD, –15.4%; 95% CI, –22.7% to –8.0%). In the fidaxomicin subgroup, there was a trend for a reduction in rCDI rate in bezlotoxumab-treated participants compared with placebo (RD, –11.9%; 95% CI, –38.1% to 14.3%) (Figure 2).
Other Outcomes
All-cause and CDI-related rehospitalization was similar across antibiotic treatment subgroups for placebo-treated participants, but there was a higher rate of CDI-associated readmissions in vancomycin-treated participants (vancomycin, 14.0%; metronidazole, 8.3%; fidaxomicin, 7.7%) (Table 3). CDI-related rehospitalizations were lower for bezlotoxumab compared with placebo in the metronidazole (4.9% vs 8.3%) and vancomycin subgroups (5.2% vs 14.0%) (Table 3). Mortality rates were similar regardless of antibiotic treatment or bezlotoxumab administration (Table 3).
Table 3.
Proportion of Participants With 30-Day Rehospitalization and All-Cause Mortality by Treatment Arm and Antibacterial Subgroups
| Bezlotoxumab | Placebo | |||||
|---|---|---|---|---|---|---|
| Metronidazole | Vancomycin | Fidaxomicin | Metronidazole | Vancomycin | Fidaxomicin | |
| 30-day rehospitalizations, % (n/N) | ||||||
| All-cause | 22.0 (n = 54/246) | 22.7 (n = 61/269) | 53.3 (n = 8/15) | 25.6 (n = 62/242) | 27.9 (n = 74/265) | 30.8 (n = 4/13) |
| CDI-related | 4.9 (n = 12/246) | 5.2 (n = 14/269) | 6.7 (n = 1/15) | 8.3 (n = 20/242) | 14.0 (n = 37/265) | 7.7 (n = 1/13) |
| Mortality, % (n/N) | ||||||
| 30-day | 4.0 (n = 15/377) | 3.2 (n = 12/380) | 0.0 (n = 0/29) | 4.2 (n = 16/380) | 2.7 (n = 10/374) | 3.7 (n = 1/27) |
| 90-day | 7.4 (n = 28/377) | 6.3 (n = 24/380) | 6.9 (n = 2/29) | 8.4 (n = 32/380) | 6.7 (n = 25/374) | 7.4 (n = 2/27) |
Abbreviation: CDI, Clostridioides difficile infection.
Discussion
rCDI remains a central unmet need in the management of CDI, with no uniformly effective treatment approach. Following significant revisions to treatment guidelines, vancomycin and fidaxomicin are the recommended antibacterial drugs for the treatment of CDI [1]. However, studies have demonstrated that the choice of antibacterial drug treatment may affect the likelihood of further rCDI episodes [8–10]. In MODIFY I/II, bezlotoxumab was shown to substantially reduce the rate of rCDI in participants receiving metronidazole, vancomycin, or fidaxomicin over 12 weeks compared with placebo [14].
Here, we show that the majority of participants in MODIFY I/II received oral metronidazole or oral vancomycin, which is consistent with the treatment guidelines that were in effect at the time of study enrollment [5]. We also demonstrate that the rate of ICC was ~80% in participants who received placebo, regardless of whether metronidazole, vancomycin, or fidaxomicin was administered. A single infusion of bezlotoxumab did not affect ICC rates with any antibacterial drug.
In participants receiving a placebo infusion, the rate of rCDI was lowest in the metronidazole subgroup compared with the vancomycin or fidaxomicin subgroups. However, this is likely explained by some notable differences in participant characteristics between antibacterial subgroups; compared with participants in the metronidazole subgroup, participants in the vancomycin subgroup were more likely to be older, have a history of CDI, have ≥1 predefined risk factor for rCDI, be an inpatient, or have severe CDI disease at baseline. Participants in the fidaxomicin subgroup were more likely to have ≥1 episode of CDI ever, and a higher proportion of participants had ≥3 prior CDI episodes compared with those who received metronidazole or vancomycin. These findings suggest a selection bias toward metronidazole treatment in lower-risk participants and vancomycin or fidaxomicin treatment in higher-risk participants, thus explaining the lower rate of rCDI in participants treated with metronidazole.
Results from logistic regression models based on placebo data from these trials, which have been published elsewhere, demonstrated that the choice of antibacterial drug treatment for CDI was not predictive of rCDI; however, a history of CDI in the previous 6 months was associated with increased risk [17, 18]. To further explore the apparent difference in rCDI between antibacterial treatment subgroups, an analysis of rCDI that stratified according to history of CDI was conducted and demonstrated similar rCDI rates between the metronidazole and vancomycin subgroups. These results are in agreement with previous studies, which also reported similar rCDI rates for metronidazole and vancomycin, although the clinical success (defined as resolution of diarrhea and absence of severe abdominal discomfort for >2 consecutive days including day 10) of metronidazole was reported as inferior to vancomycin for participants with severe CDI [6].
In this analysis, few participants were rehospitalized due to CDI within 30 days of antibiotic infusion; however, CDI-related readmissions were numerically higher for placebo-treated participants compared with bezlotoxumab. The proportions of participants who died within 30 and 90 days of antibiotic infusion were similar across all subgroups.
Our study demonstrates that in participants who received metronidazole or vancomycin to treat the presenting CDI episode, bezlotoxumab was associated with statistically significant and clinically meaningful reductions in rates of rCDI compared with placebo (35% and 40% relative reductions, respectively). In participants who received fidaxomicin, the bezlotoxumab relative recurrence reduction was 34% but did not meet statistical significance, which was likely due to a small number of participants in the fidaxomicin subgroup. As the administration of metronidazole, vancomycin, or fidaxomicin was determined by the treating physician, this large, pooled data set provides real-world evidence of the efficacy of bezlotoxumab in individuals receiving these antibacterial drugs.
The limitations of the study include the small sample size in the fidaxomicin subgroup. In addition, as the study was not designed to compare the efficacy of different antibacterial drugs, the comparisons are confounded by differences in participant baseline characteristics associated with rCDI and CDI severity. As a result, comparisons of the outcomes in the placebo group by antibacterial therapy should not be considered conclusive.
Conclusions
Participants in the MODIFY I/II trials who received bezlotoxumab with vancomycin or metronidazole demonstrated a significantly lower rate of rCDI compared with antibiotic treatment alone. A similar effect size for reduction in the rate of rCDI was observed for participants who received bezlotoxumab with fidaxomicin.
Acknowledgments
We gratefully acknowledge the MODIFY I/II trial investigators and especially the individuals who consented to participate. Medical writing support, under the direction of the authors, was provided by Hannah Logan, PhD, of CMC AFFINITY, McCann Health Medical Communications, in accordance with Good Publication Practice (GPP3) guidelines. This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Financial support. This work was supported by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Potential conflicts of interest. A.M., R.T., and M.B.D. are current employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD), and may own stock and/or stock options in Merck & Co., Inc., Kenilworth, NJ, USA. E.R.D. has conducted research for MSD, Pfizer, Rebiotix, and Synthetic Biologics and is or has been a consultant to MSD, Pfizer, Rebiotix, Sanofi Pasteur, Summit, and Synthetic Biologics. D.N.G. holds patents for the treatment and prevention of CDI, is an advisory board member for Actelion, Ferring, MSD, Rebiotix, and Summit, is a consultant for Da Volterra, Medpace, MGB Pharma, Pfizer, and Sanofi Pasteur, and holds research grants from the Centers for Disease Control and Prevention and the Department of Veterans Affairs Research Service. C.P.K. has received consulting fees from Artugen, Facile Therapeutics, First Light Diagnostics, Finch, Matrivax, MSD, Seres Health, and Vedanta, and received grant support from the National Institutes of Health, Institut Merieux, and MSD for work related to CDI. K.W.G. has conducted research for MSD for work related to CDI. G.R. has nothing to disclose. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. E.R.D., D.N.G., C.P.K., A.M., R.T., and M.B.D. were involved in the design of this analysis. E.R.D., G.R., A.M., and M.B.D. were involved in conducting the study. E.R.D., D.N.G., C.P.K., K.W.G., G.R., R.T., and M.B.D. were involved in the analysis of data. All authors were involved in drafting and revising this manuscript and provided final approval of the version to be published. All authors vouch for the accuracy of the content included in the final manuscript.
References
- 1. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66:e1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Desai K, Gupta SB, Dubberke ER, et al. Epidemiological and economic burden of Clostridium difficile in the United States: estimates from a modeling approach. BMC Infect Dis 2016; 16:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. European Centre for Disease Prevention and Control. Surveillance report: point prevalence survey of healthcare-associated infections and antimicrobial use in European acute care hospitals 2011–2012. Available at: http://ecdc.europa.eu/en/publications/publications/healthcare-associated-infections-antimicrobial-use-pps.pdf. Accessed February 2020.
- 4. Gravel D, Miller M, Simor A, et al. ; Canadian Nosocomial Infection Surveillance Program Health care-associated Clostridium difficile infection in adults admitted to acute care hospitals in Canada: a Canadian Nosocomial Infection Surveillance Program Study. Clin Infect Dis 2009; 48:568–76. [DOI] [PubMed] [Google Scholar]
- 5. Cohen SH, Gerding DN, Johnson S, et al. ; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–55. [DOI] [PubMed] [Google Scholar]
- 6. Johnson S, Louie TJ, Gerding DN, et al. ; Polymer Alternative for CDI Treatment (PACT) investigators Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis 2014; 59:345–54. [DOI] [PubMed] [Google Scholar]
- 7. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45:302–7. [DOI] [PubMed] [Google Scholar]
- 8. Cornely OA, Crook DW, Esposito R, et al. ; OPT-80-004 Clinical Study Group Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis 2012; 12:281–9. [DOI] [PubMed] [Google Scholar]
- 9. Cornely OA, Miller MA, Louie TJ, et al. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis 2012; 55(Suppl 2):S154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Louie TJ, Miller MA, Mullane KM, et al. ; OPT-80-003 Clinical Study Group Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364:422–31. [DOI] [PubMed] [Google Scholar]
- 11. Sheitoyan-Pesant C, Abou Chakra CN, Pépin J, et al. Clinical and healthcare burden of multiple recurrences of Clostridium difficile infection. Clin Infect Dis 2016; 62:574–80. [DOI] [PubMed] [Google Scholar]
- 12. European Medicines Agency (EMA). Zinplava assessment report. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/004136/WC500222643.pdf. Accessed 21 March 2019.
- 13. Merck Sharp & Dohme Corp. Zinplava (Bezlotoxumab) Prescribing Information. Whitehouse Station, NJ: Merck Sharp & Dohme Corp. Revised October 2016. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761046s000lbl.pdf. Accessed 7 March 2018. [Google Scholar]
- 14. Wilcox MH, Gerding DN, Poxton IR, et al. ; MODIFY I and MODIFY II Investigators Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med 2017; 376:305–17. [DOI] [PubMed] [Google Scholar]
- 15. Merck Sharp & Dohme Corp. Prescribing Information, DIFICID® (Fidaxomicin), 12/2015 Revision. Whitehouse Station, NJ: Merck Sharp & Dohme Corp. Revised October 2016. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761046s000lbl.pdf. Accessed 11 December 2018. [Google Scholar]
- 16. Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med 1985; 4:213–26. [DOI] [PubMed] [Google Scholar]
- 17. Yee KL, Kleijn HJ, Zajic S, et al. A time-to-event analysis of the exposure-response relationship for bezlotoxumab concentrations and CDI recurrence. J Pharmacokinet Pharmacodyn. In press. [DOI] [PubMed] [Google Scholar]
- 18. Yee KL, Kleijn HJ, Kerbusch T, et al. Population pharmacokinetics and pharmacodynamics of bezlotoxumab in adults with primary and recurrent Clostridium difficile infection. Antimicrob Agents Chemother. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]