Abstract
Elastin-derived peptides are released from the extracellular matrix remodeling by numerous proteases and seem to regulate many biological processes, notably cancer progression. The canonical elastin peptide is VGVAPG, which harbors the XGXXPG consensus pattern, allowing interaction with the elastin receptor complex located at the surface of cells. Besides these elastokines, another class of peptides has been identified. This group of bioactive elastin peptides presents the XGXPGXGXG consensus sequence, but the reason for their bioactivity remains unexplained. To better understand their nature and structure-function relationships, herein we searched the current databases for this nonapeptide motif and observed that the XGXPGXGXG elastin peptides define a specific group of tandemly repeated patterns. Further, we focused on four tandemly repeated human elastin nonapeptides, i.e., AGIPGLGVG, VGVPGLGVG, AGVPGLGVG, and AGVPGFGAG. These peptides were analyzed by means of optical spectroscopies and molecular dynamics. Ultraviolet-circular dichroism and Raman spectra are consistent with a mixture of β-turn, β-strand, and random-chain secondary elements in aqueous media. Quantitative analysis of their conformations suggested that turns corresponded to half of the total population of structural elements, whereas the remaining half were equally distributed between β-strand and unordered chains. These distributions were confirmed by molecular dynamics simulations. Altogether, our data suggest that these highly dynamic peptides harbor a type II β-turn located in their central part. We hypothesize that this structural element could explain their specific bioactivity.
Significance
Elastin fragmentation products, the so-called elastin peptides, may exhibit a bioactivity toward normal and tumor cells. This phenomenon depends on the sequence motif they harbor. Although XGXXPG sequence bioactivity is explained by the presence of a type VIII β-turn allowing interaction with the elastin receptor complex, the structural reasons for XGXPGXGXG-specific activity remain unexplained. Using data mining, we show that elastin nonapeptides define a specific class of tandemly repeated features. Further, spectroscopic and numerical simulations methods suggest the presence of a type II β-turn in their conformation. This structural element could explain their bioactivity.
Introduction
Elastin is the extracellular matrix protein responsible for the structural integrity and function of tissues undergoing reversible extensibility or deformability (1). This protein is extremely stable and resistant and undergoes virtually no turnover. Nevertheless, during aging, mechanical stress and elastase activities contribute to the fragmentation of this macropolymer into elastin-derived peptides (EDPs) (2).
Elastin is characterized by its elasticity and seems devoid of any other biological activity (1). In contrast, EDPs have been shown to regulate numerous biological processes and are thought to be involved in several aging-related pathologies such as atherosclerosis (3,4) and cancer (5, 6, 7, 8). Most biologically active EDPs, i.e., elastokines, possess a GXXPG consensus sequence adopting a type VIII β-turn structure (9) involved in their binding to the elastin receptor complex (10). The interaction of GXXPG-containing elastokines with the elastin receptor complex and the consequences of their interaction have been considerably documented during the last decades. For a comprehensive review on this topic, the reader is referred to (11).
Besides the classical GXXPG-containing sequences, another class of elastokines has also been reported. In 1988, Long and colleagues reported that nonapeptide sequences from elastin were chemoattractant for fibroblasts (12). In 1989, they further demonstrated that this biological activity could be extended to endothelial cells (13). Strikingly, since then, this peculiar class of EDP has been mostly ignored until 2007, when Maeda and colleagues demonstrated that these peptides could promote macrophages migration via a specific, but unknown, receptor (14). More recently, their biological activity has been further linked to lung carcinoma progression (8) and to promotion of invasion in triple-negative cancer cells via MMP-14 and MMP-2 (15).
The relatively low interest encountered by these nonapeptide EDPs can be explained by the fact that considerable advances have been made on both GXXPG-containing EDPs and their receptor biology. As a consequence, few groups considered investigating on these peculiar peptides.
The nonapeptide AGVPGLGVG and other EDPs share similar structural features: random-coil and β-turn conformations. However, AGVPGLGVG induces angiogenesis, tumor progression, and secretion of proteases higher than other EDPs in carcinomas. Moreover, in vitro, it behaves like amyloid-like peptides harboring the XGGXG motif by forming cross β-sheets at the supramolecular level (16). Very recently, Brassart and colleagues have shown that the receptor mediating AGVPGLGVG biological effects and identified as lactose-insensitive is the ribosomal protein SA (RPSA) (17).
The aim of this work is to analyze the structure and conformational behavior of these peptides. Our structural analysis was conducted using sequence analysis, spectroscopic methods, and molecular dynamics simulations.
Our results show that elastin nonapeptides define a peculiar class among peptides harboring the consensus XGXPGXGXG sequence. In elastin, these peptides are mostly tandemly repeated. Furthermore, our data show that they are engaged in a conformational equilibrium between random-coil and turn conformations at the considered concentrations and temperatures. Molecular dynamics simulations suggest that the dominant conformation of these peptides is a type II β-turn. The functional significance of this conformation is discussed.
Materials and Methods
Sequence analysis
Sequence retrieval
Sequences harboring the XGXPGXGXG consensus motif were searched using Python scripts on UniProtKB (Swiss-Prot and TrEMBL) database Fasta files in which spliced variants were ignored. Only exact matches were retrieved and further cleaned informatically and manually so as to ensure that a sequence belonging to a given species (identified by its unique accession number) was present only once in our results. For each sequence hit, the collected data were its accession number, the originating organism, the length of the protein, and the position(s) of the nonapeptide sequence(s).
Pattern analysis
Nonapeptide exact matches were classified and distributed as a function of the taxonomic hierarchical classification lineage of the source organism of the corresponding parent protein. Hits were either individual hits, i.e., only one occurrence was found in the sequence, or multiple ones. In the latter case, we distinguished three possibilities. Multiple hits could be found in different regions of the protein, and each occurrence was isolated from the others. Another possibility was that the repetitions could overlap, i.e., the end of the first occurrence (-X-G) was the beginning of the following one (X-G-). Finally, we observed that nonapeptide sequences could also be tandemly repeated, one after the other. The tree figure has been made with the Python module ETE3 (18) and the Logo sequences with WebLogo (https://github.com/weblogo/weblogo) (19).
Optical spectroscopy
Molecular compounds
Powder samples of amino acids were purchased from Sigma-Aldrich (Saint Quentin Fallavier, France). Lyophilized samples of nonapeptides were obtained from Genecust (Luxemburg, Luxemburg) as zwitterionic peptides in acetate salts. Their purity was at least 95% as determined by mass spectrometry.
Solution samples
Lyophilized powder of amino acids and peptides was dissolved in pure water from a Millipore filtration system (Guyancourt, France). The ionic strength of all peptide samples was increased by adding 150 mM NaCl to stock solutions. Upon dissolution, the pH was between 4 and 4.5. Raman spectra were recorded between 10 and 80°C. Circular dichroism (CD) spectra were obtained at room temperature between 100 and 200 μM.
CD spectroscopy
Room-temperature ultraviolet-CD spectra were analyzed on a JASCO J-810 spectrophotometer (Lisses, France) within the 190–300 nm spectral region. A pathlength of 1 mm and a spectral resolution of 0.2 nm were selected. Each spectrum corresponding to an average of five scans was recorded with a speed of 100 nm per minute. The measured ellipticity for each sample, referred to as [ϕ]obs, was further normalized to obtain the so-called mean residue ellipticity, [ϕ], by using the expression [ϕ] = [ϕ]obs/ncl, where n, c, and l are the length of the peptide, its molar concentration, and the optical pathlength, respectively. The normalized ellipticity was expressed in deg cm2 dmol−1.
Raman spectroscopy
Room-temperature Stokes Raman spectra were analyzed in bulk samples at right angle on a Jobin-Yvon T64000 spectrometer (Longjumeau, France) at single spectrograph configuration, 1200 grooves/mm holographic grating, and a holographic notch filter. Raman data corresponding to 1200 s acquisition time for each spectrum were collected on a liquid-nitrogen-cooled CCD detection system (Spectrum One, Jobin-Yvon). The effective slit width was set to 5 cm−1. Solution samples were excited by the 488 nm line of an Ar+ laser (Spectra Physics, Evry, France), with 200 mW power at the sample.
Post recording spectroscopic data treatment
Buffer subtraction and smoothing of the observed spectra were performed using the GRAMS/AI Z.00 package (Thermo Galactic, Waltham, MA). Final presentation of Raman spectra was done by means of SigmaPlot package 6.10 (SPSS, Chicago, IL).
Secondary structure analysis
Quantification of protein secondary structures using amide I profile decomposition is a method used commonly, and its intrinsic limits are readily known (20,21). For elastin material, its overall accuracy is ±5% as far as the final structural estimates are concerned (22). The initial step of the reconstruction is to guess how many underlying bands are present in the complex amide I profile to be analyzed. This is achieved using a second derivative analysis of the smoothed spectrum. For our data, four components were suggested. This information was provided to the curve fitting software to reconstruct the experimental spectrum using Gaussian/Lorentzian mixture profiles. The curve fitting procedure was successful when the fit was good (low χ2-value), the number of components was unchanged, and their width at half height was consistent. The secondary structure estimates were obtained by adding the fractional areas of amide I structural components belonging to the same secondary structure group.
Atomistic molecular dynamics simulations
Ten independent simulations of 500 ns were performed for each of the four human elastin nonapeptides using the amber99SB-ILDN force field (23) and Gromacs 2016.5 software (24). Peptides were built in an extended conformation with PyMOL (25) and solvated with TIP3P water in a 5 nm cubic box. All studied systems were first minimized by steepest descent for 5000 steps. Then, a 1 ns simulation with the peptides under position restraints was run before the production simulations were performed. Different seeds were used for each simulation during the velocity assignments. Periodic boundary conditions were used with a 2 fs time step. The dynamics were carried out under NPT conditions (310 K and 1 bar). The temperature was maintained using the v-rescale method (26), with τT = 0.1 ps, and an isotropic pressure was maintained using the Parrinello-Rahman barostat (27) with a compressibility of 4.5 × 10−5 bar−1 and τP = 2 ps. Short-range nonbonded interactions were treated with a cutoff of 1.0 nm, and long-range interactions were calculated with the particle mesh Ewald method (28) with a grid spacing of 0.16 nm. Bond lengths were maintained with the LINCS algorithm (29) and long-range dispersion corrections for energy and pressure were applied. The 3D structures were analyzed with both PyMOL and VMD (30) softwares.
The secondary structures were computed with DSSP (31), and the different types of β-turns have been assigned based on the ΦΨ angles of two residues following each other as defined by Lewis et al. (32) and Hutchinson et al. (33). Distances between each i and i + 3 Cα have been computed and, if this distance was less than 0.7 nm and the amino acids were not part of a helix conformation, the ΦΨ angles of residues i + 1 and i + 2 were considered. To assign a peculiar type of β-turn, Hutchinson and colleagues (33) considered a range of ±30 or ±40° around the standard angles with a deviation of 50° allowed for one of the angles.
The distribution of ΦΨ angles of residues i + 1 and i + 2 we observed during the simulation permitted us to easily identify type I, I′, II, II′, and VIII β-turn populations as broad plot regions (Fig. S1). Because these regions were larger than those proposed by Hutchinson and colleagues (33), we adapted the considered ΦΨ angle ranges to encompass the ranges we observed. The main effect of this change was the reduction of unassigned turn contribution, i.e., type IV β-turns. The corresponding figure and ΦΨ angle ranges for type I, I′, II, II′, and VIII β-turns are provided as Supporting Material (Fig. S1; Table S1). As in Hutchinson, other β-turn types are considered with a range of ±40° around the standard angles, and type IV β-turns correspond to otherwise unassigned β-turns. Clustering was performed using the gromos method (34) with a cutoff of 0.1 nm on Cα atoms of residues 2–6.
To assess the quality of our simulations, CD spectra have been computed from the peptide trajectories by using the SESCA software (35) with the basis set HBSS-3SC1.
Results
Sequence analysis
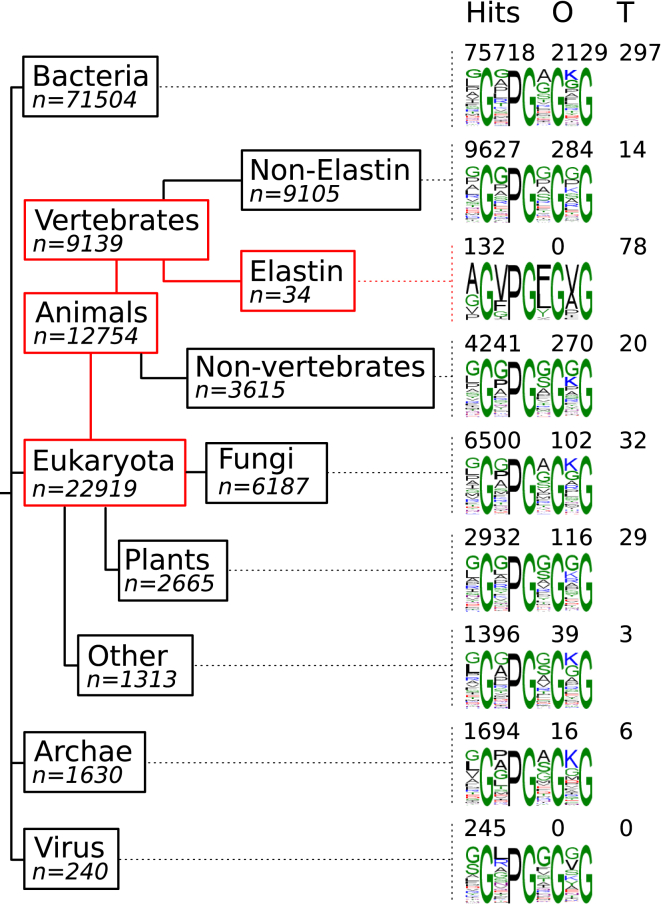
The retrieval of sequences possessing XGXPGXGXG yielded 96,293 unique sequences. These sequences (Table S2) were classed as a function of their taxonomy (Fig. 1). Most sequences were from Bacteria (71,504 sequences for 75,718 occurrences with 2129 overlaps and 297 tandem repeats), then Eukariota (22,919 sequences for 24,828 occurrences with 811 overlaps and 297 tandem repeats), Archae (1630 sequences for 1694 occurrences with 16 overlaps and six tandem repeats), and Virus (240 sequences for 245 occurrences with no overlaps and no tandem repeats). Strikingly, when these results are observed, it becomes clear that elastins define a specific group. They exhibit numerous occurrences per sequences (171 for 38) with no overlap and a very high ratio of tandem repeats. This specificity of elastin nonapeptides is even more evident when the most frequent residues occurring at X positions are considered. Although other groups mostly favor the presence of G, A, K, P, or L at these positions, elastins allow almost none of those, with the AGVPGFGVG being the dominating motif. In fact, out of the 140 million sequences searched, this motif was always found in elastin sequences except for one bacterial sequence.
Figure 1.
Nonapeptide occurrences and distribution. The XGXPGXGXG consensus was found in 96,293 unique sequences, among which 22,919 were from Eukariota, 71,504 from Bacteria, 240 from Virus, and 1630 from Archae. The Eukariota branch distribution is dominated by Animals, in which Vertebrates are predominant. For each branch of the tree, the total number of hits is reported as well as the number of observed overlapping (O) sequences and of tandem (T) repetitions. The consensus motif is also presented with the most frequent residues found at the four X positions.
The nonapeptide sequences found in elastin entries are reported in Table 1, in which they are compared. The sequences are reported as a function of their alphabetical order. In human elastin, nonapeptides can be observed in exon domains 18, 20, or 26. Single occurrences are observed in the two first exons, whereas they appear almost exclusively as tandem repeats in exon 26. Strikingly, the human repeats can also be observed in other species. For instance, the human elastin motif AGVPGFGVG is also present in mouse exon 26. Overall, human exon 26 (or its equivalent in other species) is a sequence domain characterized by tandem repeats of these nonapeptides. As a consequence, those are supposedly important for the biological function of the polymer, and we decided to further analyze the structural behavior of the four human exon 26 nonapeptides, i.e., AGIPGLGVG (hN3), VGVPGLGVG (hN4), AGVPGLGVG (hN5), and AGVPGFGAG (hN6).
Table 1.
Nonapeptide Stack of Elastin Uniprot Entries
| Species | Accession Number | # | Sequence | Position | Exon |
|---|---|---|---|---|---|
| Oreochromis niloticus | I3JQ46 | 8 | AGGPGSGIG | 657–665 | 18 |
| Odobenus rosmarus divergens | A0A2U3ZFC3 | 5 | AGIPGFGAG | 541–549 | – |
| Canis lupus familiaris | J9NW15 | 5 | AGIPGFGVG | 571–579 | 23 |
| Homo sapiens∗ | P15502 | 3 | AGIPGLGVG | 576–584 | 26 |
| Rhinopithecus roxellana | A0A2K6NWE2 | 3 | AGIPGLGVG | 562–570 | 26 |
| Pan troglodytes | A0A2I3SVF0 | 3 | AGIPGLGVG | 580–588 | 27 |
| Macaca mulatta | A0A1D5R663 | 2 | AGIPGVGPG | 312–320 | 15 |
| Papio anubis | A0A2I3MW49 | 3 | AGVPGFGAG | 472–480 | 16 |
| Cercocebus atys | A0A2K5LP54 | 3 | AGVPGFGAG | 512–520 | 18 |
| Felis catus | A0A337S1Z3 | 4 | AGVPGFGAG | 404–412 | 18 |
| Ailuropoda melanoleuca | G1MA94 | 4 | AGVPGFGAG | 499–507 | 23 |
| Canis lupus familiaris | J9NW15 | 6 | AGVPGFGAG | 580–588 | 23 |
| Myotis lucifugus | G1QCG1 | 2 | AGVPGFGAG | 483–491 | 24 |
| Myotis lucifugus | G1QCG1 | 3 | AGVPGFGAG | 492–500 | 24 |
| Myotis lucifugus | G1QCG1 | 4 | AGVPGFGAG | 501–509 | 24 |
| Sus scrofa | A0A287AXU0 | 5 | AGVPGFGAG | 599–607 | 25 |
| Homo sapiens∗ | P15502 | 6 | AGVPGFGAG | 603–611 | 26 |
| Rattus norvegicus | Q99372 | 3 | AGVPGFGAG | 642–650 | 26 |
| Rattus norvegicus | Q99372 | 4 | AGVPGFGAG | 658–666 | 26 |
| Mus musculus | P54320 | 2 | AGVPGFGAG | 613–621 | 27 |
| Mus musculus | P54320 | 3 | AGVPGFGAG | 622–630 | 27 |
| Mus musculus | P54320 | 4 | AGVPGFGAG | 631–639 | 27 |
| Mus musculus | P54320 | 5 | AGVPGFGAG | 640–648 | 27 |
| Mus musculus | P54320 | 6 | AGVPGFGAG | 649–657 | 27 |
| Mustela putorius furo | M3YXB8 | 4 | AGVPGFGAG | 608–616 | 27 |
| Pan troglodytes | A0A2I3SVF0 | 6 | AGVPGFGAG | 607–615 | 27 |
| Erinaceus europaeus | A0A1S3W547 | 3 | AGVPGFGAG | 584–592 | – |
| Erinaceus europaeus | A0A1S3W547 | 4 | AGVPGFGAG | 593–601 | – |
| Enhydra lutris kenyoni | A0A2Y9J5B3 | 4 | AGVPGFGAG | 615–623 | – |
| Tursiops truncatus | A0A2U4A8K1 | 1 | AGVPGFGAG | 87–95 | – |
| Ursus maritimus | A0A384CQV9 | 6 | AGVPGFGAG | 583–591 | – |
| Mesocricetus auratus | A0A1U8CCM2 | 2 | AGVPGFGAG | 643–651 | – |
| Mesocricetus auratus | A0A1U8CCM2 | 3 | AGVPGFGAG | 652–660 | – |
| Mesocricetus auratus | A0A1U8CCM2 | 4 | AGVPGFGAG | 661–669 | – |
| Mesocricetus auratus | A0A1U8CCM2 | 5 | AGVPGFGAG | 670–678 | – |
| Dipodomys ordii | A0A1S3GKU4 | 6 | AGVPGFGAG | 587–595 | – |
| Erinaceus europaeus | A0A1S3W547 | 5 | AGVPGFGGG | 602–610 | – |
| Balaenoptera acutorostrata scammoni | A0A384AI97 | 3 | AGVPGFGPG | 584–592 | – |
| Felis catus | A0A337S1Z3 | 2 | AGVPGFGVG | 386–394 | 18 |
| Felis catus | A0A337S1Z3 | 3 | AGVPGFGVG | 395–403 | 18 |
| Canis lupus familiaris | J9NW15 | 3 | AGVPGFGVG | 553–561 | 23 |
| Canis lupus familiaris | J9NW15 | 4 | AGVPGFGVG | 562–570 | 23 |
| Saimiri boliviensis boliviensis | A0A2K6UXV4 | 1 | AGVPGFGVG | 491–499 | 25 |
| Sus scrofa | A0A287AXU0 | 3 | AGVPGFGVG | 581–589 | 25 |
| Sus scrofa | A0A287AXU0 | 4 | AGVPGFGVG | 590–598 | 25 |
| Callithrix jacchus | A0A2R8P8B8 | 3 | AGVPGFGVG | 523–531 | 26 |
| Mustela putorius furo | M3YXB8 | 2 | AGVPGFGVG | 590–598 | 27 |
| Mustela putorius furo | M3YXB8 | 3 | AGVPGFGVG | 599–607 | 27 |
| Odobenus rosmarus divergens | A0A2U3ZFC3 | 4 | AGVPGFGVG | 532–540 | – |
| Enhydra lutris kenyoni | A0A2Y9J5B3 | 2 | AGVPGFGVG | 597–605 | – |
| Enhydra lutris kenyoni | A0A2Y9J5B3 | 3 | AGVPGFGVG | 606–614 | – |
| Ursus maritimus | A0A384CQV9 | 4 | AGVPGFGVG | 565–573 | – |
| Ursus maritimus | A0A384CQV9 | 5 | AGVPGFGVG | 574–582 | – |
| Dipodomys ordii | A0A1S3GKU4 | 4 | AGVPGLGAG | 569–577 | – |
| Dipodomys ordii | A0A1S3GKU4 | 5 | AGVPGLGAG | 578–586 | – |
| Danio rerio | F8W4J7 | 4 | AGVPGLGGG | 568–576 | 18 |
| Ailuropoda melanoleuca | G1MA94 | 3 | AGVPGLGVG | 490–498 | 23 |
| Saimiri boliviensis boliviensis | A0A2K6UXV4 | 2 | AGVPGLGVG | 500–508 | 25 |
| Rhinopithecus bieti | A0A2K6MP70 | 3 | AGVPGLGVG | 522–530 | 25 |
| Bos taurus | P04985 | 1 | AGVPGLGVG | 562–570 | 26 |
| Bos taurus | P04985 | 2 | AGVPGLGVG | 571–579 | 26 |
| Bos taurus | P04985 | 3 | AGVPGLGVG | 580–588 | 26 |
| Homo sapiens∗ | P15502 | 5 | AGVPGLGVG | 594–602 | 26 |
| Rattus norvegicus | Q99372 | 2 | AGVPGLGVG | 633–641 | 26 |
| Callithrix jacchus | A0A2R8P8B8 | 4 | AGVPGLGVG | 532–540 | 26 |
| Callithrix jacchus | A0A2R8P8B8 | 5 | AGVPGLGVG | 541–549 | 26 |
| Callithrix jacchus | A0A2R8P8B8 | 6 | AGVPGLGVG | 550–558 | 26 |
| Colobus angolensis palliatus | A0A2K5JW24 | 3 | AGVPGLGVG | 548–556 | 26 |
| Bos mutus | L8IPH2 | 1 | AGVPGLGVG | 564–572 | 26 |
| Macaca nemestrina | A0A2K6BHK1 | 4 | AGVPGLGVG | 563–571 | 26 |
| Pan troglodytes | A0A2I3SVF0 | 5 | AGVPGLGVG | 598–606 | 27 |
| Ursus maritimus | A0A384CQV9 | 3 | AGVPGLGVG | 556–564 | – |
| Ficedula albicollis | U3KH66 | 1 | AGVPGVGVG | 359–367 | 15 |
| Equus caballus | F7BWV3 | 1 | FGVPGYGVG | 418–426 | 17 |
| Oryzias latipes | A0A3B3HSK9 | 1 | GGAPGFGPG | 383–391 | 14 |
| Tetraodon nigroviridis | H3CXZ1 | 1 | GGAPGSGPG | 373–381 | 8 |
| Sus scrofa | A0A287AXU0 | 2 | GGFPGFGVG | 429–437 | 19 |
| Homo sapiens∗ | P15502 | 2 | GGFPGFGVG | 401–409 | 20 |
| Pan troglodytes | A0A2I3SVF0 | 2 | GGFPGFGVG | 405–413 | 20 |
| Callithrix jacchus | A0A2R8P8B8 | 2 | GGFPGFGVG | 408–416 | 21 |
| Balaenoptera acutorostrata scammoni | A0A384AI97 | 2 | GGFPGFGVG | 440–448 | – |
| Erinaceus europaeus | A0A1S3W547 | 2 | GGFPGYGAG | 426–434 | – |
| Canis lupus familiaris | J9NW15 | 2 | GGFPGYGIG | 399–407 | 17 |
| Ailuropoda melanoleuca | G1MA94 | 2 | GGFPGYGIG | 395–403 | 19 |
| Odobenus rosmarus divergens | A0A2U3ZFC3 | 2 | GGFPGYGIG | 396–404 | |
| Enhydra lutris kenyoni | A0A2Y9J5B3 | 1 | GGFPGYGIG | 456–464 | |
| Ursus maritimus | A0A384CQV9 | 2 | GGFPGYGIG | 426–434 | |
| Felis catus | A0A337S1Z3 | 1 | GGFPGYGVG | 289–297 | 14 |
| Papio anubis | A0A2I3MW49 | 1 | GGFPGYGVG | 338–346 | 14 |
| Cercocebus atys | A0A2K5LP54 | 1 | GGFPGYGVG | 354–362 | 15 |
| Mandrillus leucophaeus | A0A2K5XQM6 | 2 | GGFPGYGVG | 284–292 | 15 |
| Rattus norvegicus | Q99372 | 1 | GGFPGYGVG | 457–465 | 18 |
| Rhinopithecus bieti | A0A2K6MP70 | 2 | GGFPGYGVG | 377–385 | 20 |
| Mus musculus | P54320 | 1 | GGFPGYGVG | 454–462 | 21 |
| Colobus angolensis palliatus | A0A2K5JW24 | 2 | GGFPGYGVG | 405–413 | 21 |
| Mustela putorius furo | M3YXB8 | 1 | GGFPGYGVG | 445–453 | 21 |
| Rhinopithecus roxellana | A0A2K6NWE2 | 2 | GGFPGYGVG | 405–413 | 21 |
| Macaca nemestrina | A0A2K6BHK1 | 2 | GGFPGYGVG | 405–413 | 21 |
| Mesocricetus auratus | A0A1U8CCM2 | 1 | GGFPGYGVG | 466–474 | – |
| Dipodomys ordii | A0A1S3GKU4 | 3 | GGFPGYGVG | 405–413 | – |
| Canis lupus familiaris | J9NW15 | 1 | GGGPGAGLG | 54–62 | 2 |
| Danio rerio | F8W4J7 | 1 | GGGPGAGLG | 33–41 | 2 |
| Odobenus rosmarus divergens | A0A2U3ZFC3 | 1 | GGGPGAGLG | 71–79 | – |
| Dipodomys ordii | A0A1S3GKU4 | 1 | GGGPGAGLG | 81–89 | – |
| Oreochromis niloticus | I3JQ46 | 1 | GGGPGFGGG | 541–549 | 16 |
| Oreochromis niloticus | I3JQ46 | 2 | GGGPGFGGG | 559–567 | 16 |
| Oreochromis niloticus | I3JQ46 | 3 | GGGPGFGGG | 577–585 | 16 |
| Oreochromis niloticus | I3JQ46 | 4 | GGGPGFGGG | 589–597 | 16 |
| Oreochromis niloticus | I3JQ46 | 9 | GGGPGLGLG | 900–908 | 23 |
| Oreochromis niloticus | I3JQ46 | 7 | GGIPGLGYG | 632–640 | 16 |
| Danio rerio | F8W4J7 | 28 | GGIPGVGYG | 1190–1198 | 30 |
| Danio rerio | F8W4J7 | 13 | GGLPGGGAG | 821–829 | 22 |
| Danio rerio | F8W4J7 | 22 | GGLPGGGAG | 1033–1041 | 26 |
| Danio rerio | F8W4J7 | 23 | GGLPGGGAG | 1041–1049 | 26 |
| Danio rerio | F8W4J7 | 26 | GGLPGGGAG | 1117–1125 | 28 |
| Danio rerio | F8W4J7 | 18 | GGLPGGGIG | 956–964 | 24 |
| Danio rerio | F8W4J7 | 16 | GGLPGGGLG | 895–903 | 24 |
| Danio rerio | F8W4J7 | 20 | GGLPGGGLG | 1005–1013 | 26 |
| Danio rerio | F8W4J7 | 21 | GGLPGGGLG | 1025–1033 | 26 |
| Danio rerio | F8W4J7 | 25 | GGLPGGGLG | 1090–1098 | 28 |
| Oreochromis niloticus | I3JQ46 | 11 | GGLPGGGPG | 1844–1852 | 49 |
| Oreochromis niloticus | I3JQ46 | 10 | GGLPGGGTG | 932–940 | 23 |
| Danio rerio | F8W4J7 | 17 | GGLPGGGVG | 948–956 | 24 |
| Danio rerio | F8W4J7 | 2 | GGLPGIGAG | 144–152 | 5 |
| Danio rerio | F8W4J7 | 19 | GGLPGSGIG | 964–972 | 24 |
| Danio rerio | F8W4J7 | 24 | GGLPGSGIG | 1049–1057 | 26 |
| Danio rerio | F8W4J7 | 9 | GGLPGSGLG | 721–729 | 20 |
| Danio rerio | F8W4J7 | 14 | GGLPGSGTG | 837–845 | 22 |
| Danio rerio | F8W4J7 | 5 | GGLPGSGVG | 661–669 | 20 |
| Danio rerio | F8W4J7 | 6 | GGLPGSGVG | 676–684 | 20 |
| Danio rerio | F8W4J7 | 7 | GGLPGSGVG | 691–699 | 20 |
| Danio rerio | F8W4J7 | 8 | GGLPGSGVG | 706–714 | 20 |
| Danio rerio | F8W4J7 | 10 | GGLPGSGVG | 736–744 | 20 |
| Danio rerio | F8W4J7 | 27 | GGLPGSGVG | 1180–1188 | 30 |
| Oreochromis niloticus | I3JQ46 | 5 | GGVPGFGGG | 613–621 | 16 |
| Myotis lucifugus | G1QCG1 | 1 | GGVPGLGIG | 442–450 | 22 |
| Oreochromis niloticus | I3JQ46 | 6 | GGVPGVGGG | 625–633 | 16 |
| Macaca nemestrina | A0A2K6BHK1 | 3 | GGVPGVGVG | 421–429 | 21 |
| Sus scrofa | A0A287AXU0 | 1 | PGAPGFGPG | 330–338 | 17 |
| Balaenoptera acutorostrata scammoni | A0A384AI97 | 1 | PGAPGFGPG | 315–323 | – |
| Dipodomys ordii | A0A1S3GKU4 | 2 | PGFPGVGAG | 303–311 | – |
| Mandrillus leucophaeus | A0A2K5XQM6 | 1 | PGGPGFGPG | 203–211 | 12 |
| Ailuropoda melanoleuca | G1MA94 | 1 | PGGPGFGPG | 315–323 | 17 |
| Homo sapiens∗ | P15502 | 1 | PGGPGFGPG | 323–331 | 18 |
| Pan troglodytes | A0A2I3SVF0 | 1 | PGGPGFGPG | 327–335 | 18 |
| Rhinopithecus bieti | A0A2K6MP70 | 1 | PGGPGFGPG | 294–302 | 18 |
| Callithrix jacchus | A0A2R8P8B8 | 1 | PGGPGFGPG | 339–347 | 19 |
| Colobus angolensis palliatus | A0A2K5JW24 | 1 | PGGPGFGPG | 322–330 | 19 |
| Rhinopithecus roxellana | A0A2K6NWE2 | 1 | PGGPGFGPG | 322–330 | 19 |
| Macaca nemestrina | A0A2K6BHK1 | 1 | PGGPGFGPG | 322–330 | 19 |
| Ursus maritimus | A0A384CQV9 | 1 | PGGPGFGPG | 333–341 | – |
| Erinaceus europaeus | A0A1S3W547 | 1 | PGVPGFGRG | 343–351 | – |
| Callorhinchus milii | V9KAK8 | 1 | QGEPGLGGG | 243–251 | – |
| Danio rerio | F8W4J7 | 3 | TGLPGIGPG | 463–471 | 12 |
| Danio rerio | F8W4C7 | 1 | TGRPGNGRG | 137–145 | 3 |
| Macaca mulatta | A0A1D5R663 | 1 | VGAPGGGLG | 302–310 | 13 |
| Danio rerio | F8W4J7 | 11 | VGLPGGGLG | 792–800 | 22 |
| Danio rerio | F8W4J7 | 15 | VGLPGGGLG | 875–883 | 24 |
| Balaenoptera acutorostrata scammoni | A0A384AI97 | 5 | VGVPGFGAG | 602–610 | – |
| Odobenus rosmarus divergens | A0A2U3ZFC3 | 3 | VGVPGFGAG | 523–531 | – |
| Trichechus manatus latirostris | A0A2Y9RHH3 | 1 | VGVPGFGAG | 569–577 | – |
| Heterocephalus glaber | G5BG87 | 1 | VGVPGFGAG | 589–597 | – |
| Papio anubis | A0A2I3MW49 | 2 | VGVPGLGVG | 463–471 | 16 |
| Cercocebus atys | A0A2K5LP54 | 2 | VGVPGLGVG | 503–511 | 18 |
| Mandrillus leucophaeus | A0A2K5XQM6 | 3 | VGVPGLGVG | 423–431 | 19 |
| Macaca mulatta | A0A1D5R663 | 3 | VGVPGLGVG | 535–543 | 23 |
| Macaca mulatta | A0A1D5R663 | 4 | VGVPGLGVG | 544–552 | 23 |
| Homo sapiens∗ | P15502 | 4 | VGVPGLGVG | 585–593 | 26 |
| Bos mutus | L8IPH2 | 2 | VGVPGLGVG | 573–581 | 26 |
| Bos mutus | L8IPH2 | 3 | VGVPGLGVG | 582–590 | 26 |
| Macaca nemestrina | A0A2K6BHK1 | 5 | VGVPGLGVG | 572–580 | 26 |
| Pan troglodytes | A0A2I3SVF0 | 4 | VGVPGLGVG | 589–597 | 27 |
| Balaenoptera acutorostrata scammoni | A0A384AI97 | 4 | VGVPGLGVG | 593–601 | – |
| Tursiops truncatus | A0A2U4A8K1 | 2 | VGVPGLGVG | 96–104 | – |
| Danio rerio | F8W4J7 | 12 | YGLPGGGLG | 813–821 | 22 |
Accession number, accession number of the origin sequence. #, number of the hit within the sequence. Position, position of the hit. Exon, exon number (when available).
Human sequences.
CD spectroscopy
To describe the structural behavior of the nonapeptides in solution, their CD spectra were recorded in different (polar versus low dielectric constant) environments. Routinely, methanol is considered as a primary step in studying the peptides conformational behavior in hydrophobic environments such as the membrane interior or hydrophobic pockets of proteins (36).
The analysis of the CD spectra in water (150 mM NaCl), in a water/methanol mixture (50/50, v/v), and in methanol (Fig. 2) reveal a progressive conformational change of the nonapeptides in going from water to methanol solutions.
Figure 2.
Ultraviolet-CD spectra of the four nonapeptides observed in water, water/methanol mixture (50:50), and methanol. The peptides are hN3 (A), hN4 (B), hN5 (C) and hN6 (D).
The CD spectra recorded in water all present a double negative band shape, i.e., composed of a deep minimum at ∼198 nm, followed by a weaker and broader negative band centered at ∼225 nm. It is a matter of fact that random chains are generally identified by a deep negative band at ∼198 nm (37,38). However, a negative double band, like that observed in the case of the nonapeptides (Fig. 2), is an indicator of turns. For instance, certain β-turns are known to give rise to a negative double band. It is to be mentioned that β-turns are recognized as the most frequent ones in proteins (39, 40, 41). Particularly, the CD fingerprints of four categories of β-turns, i.e., type I (37), II, II′ (42, 43, 44), and VIII (45) β-turns, are identified by a double negative band with unequal ellipticities. It is interesting to note that among all the mentioned β-turns, that corresponding to type VIII is formed by a deep minimum at ∼198 nm, followed by a broad shoulder at ∼220 nm (45), i.e., strikingly similar to that observed in the case of the presently analyzed nonapeptides. However, this overwhelming resemblance between CD markers does not permit rejection of possible unordered chains, of which the CD marker can be overlapped with the negative band at ∼198 nm. In the same manner, a probable overlap may occur between the CD fingerprint of β-strands, generally characterized by a unique negative band at ∼215 nm, and the ∼225 nm negative band from β-turns. Nevertheless, any uncertainty about the presence of polyproline-II-type chains can be discarded because of the recent CD studies highlighting the fact that the polyproline II fingerprint is composed of a deep negative band at ∼198 nm, followed by a positive one centered at ∼220 nm. The presence of the latter band cannot be suspected in the CD spectra displayed in Fig. 2.
In methanol (Fig. 2), the CD signals of the nonapeptides are composed of a broad positive band peaking at ∼205 nm, followed by a weak and broad negative band at ∼225 nm. It is worth noting that the CD spectra observed in a water/methanol mixture (1:1, v/v) are somehow an overlap of those observed in water and methanol. To provide a reliable assignment for the CD signal observed in methanol, we can recall that although a type II β-turn gives rise to a positive band located between 190 and 210 nm (37), the observed negative band at ∼225 nm in methanol can be ascribed to β-strands and/or to type I′ β-turn (37). Based on the observed CD spectra, no unordered chain can be expected in the presently studied nonapeptides.
Raman spectroscopy
Raman spectra of the nonapeptides were recorded in the middle wavenumber region (1800–550 cm−1). They presented a low water contribution enabling an accurate analysis of the observed bands (Fig. 3, A and B). Whatever the nonapeptide, the spectra were identical for the two tested concentrations, i.e., 2.5 and 20 mM (data not shown). Indeed, no substantial change, assignable to either a possible conformational transition or a detectable molecular aggregation, could be observed. Because the spectral features were more pronounced at 20 mM (higher Raman intensities due to higher concentration), we will limit our discussion to the Raman spectra recorded at that concentration.
Figure 3.
Room temperature Stokes Raman spectra in the middle wavenumber region for (A) hN3, hN4, hN5 and (B) hN6, compared to F. Spectra were recorded at 20 mM. See Table 2 for tentative assignments.
The tentative assignments of the Raman spectra of the four studied nonapeptides are reported in Table 2. These assignments have been performed in keeping with the features observed in the Raman spectra of their constitutive amino acids (Fig. S2). The bands observed in the 1500–1320 cm−1 range, mainly arising from the bending modes of CH2 and CH3 side-chain moieties, as well as those located below 1200 cm−1 in amino acid spectra, were taken as references for assigning the observed peaks in the spectra of peptides.
Table 2.
Tentative Assignment of the Raman Bands Observed in the Middle Wavenumber Region
| AGIPGLGVG hN3 | VGVPGLGVG hN4 | AGVPGLGVG hN5 | AGVPGFGAG hN6 | Tentative Assignment |
|---|---|---|---|---|
| 1700-1620 (br) | 1700-1620 (br) | 1700-1620 (br) | 1700-1620 (br) | Amide I |
| – | – | – | 1605 (s) | F1 |
| – | – | – | 1586 (m) | F2 |
| 1465 (s) | 1468 (s) | 1466 (s) | – | P, I, L, V, A |
| 1451 (s) | 1452 (s) | 1452 (s) | 1454 (s) | V, L, I |
| 1421 (s) | 1421 (s) | 1420 (s) | 1421 (s) | G, A, L, I, V, P |
| 1391 (m) | 1394 (m) | 1395 (m) | 1392 (m) | G, A, L, I, V, P |
| 1343 (s) | 1345 (s) | 1343 (s) | 1341 (s) | A, L, I, V, P |
| 1315 (s) | 1313 (s) | 1317 (s) | 1320 (s) | A, L, I |
| 1300-1240 (br) | 1300-1240 (br) | 1300-1240 (br) | 1300-1240 (br) | Amide III |
| – | – | – | 1207 (s) | F3 |
| – | – | – | 1183 (m) | |
| 1161 (m) | 1175 (m) | 1161 (m) | 1159 (m) | V, L, I, P |
| 1128 (s) | 1127 (s) | 1126 (s) | 1126 (m) | V, L, I |
| – | – | – | 1101 (m) | P |
| – | – | – | 1031 (s) | F4 |
| 1031 (m) | 1034 (m) | 1031 (m) | – | G, I, P |
| 1011 (m) | 1014 (m) | 1014 (m) | A, V | |
| – | – | – | 1004 (vs) | F5 |
| 960 (s) | 962 (s) | 960 (s) | – | V, L, I |
| 937 (s) | 938 (s) | 937 (s) | 937 (br) | A, V, L, I |
| 891 (s) | 891 (s) | 891 (s) | – | V |
| 857 (m) | 860 (m) | 856 (sh) | 851 (br) | L, P |
| 845 (m) | 840 (m) | 843 (m) | – | A, V, L, I, P |
| – | – | – | 758 (br) | P |
| 736 (br) | 735 (br) | 737 (br) | – | V, L, I, P |
| – | – | – | 621 (m) | F6 |
(vs) very strong; (s) strong; (m) medium; (br) broad Raman bands. F1 to F6 refer to the six characteristic Raman bands of phenylalanine. Amide I and amide III vibrations give rise to a broad and strong Raman band peaking at ∼1685 and 1255 cm−1, respectively.
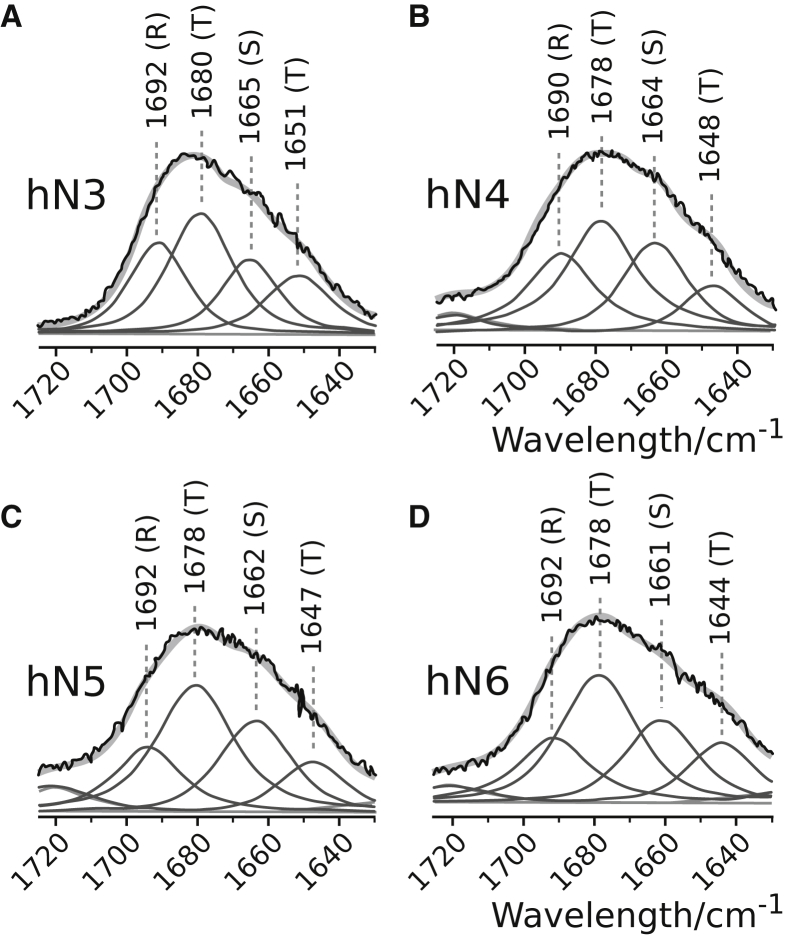
The spectrum of hN6 is characterized by the occurrence of the six Phe characteristic Raman bands, referred to as F1 to F6 (46,47). Being all of the in-plane type and localized in the Phe phenyl ring, the wavenumbers of these six characteristic markers remained very close to those collected from the free amino acid F (Fig. 3 B). The Raman spectra of hN3, hN4, and hN5 (Fig. 3 A) do not present such narrow and resolved aromatic markers, but their striking spectral shape similarity is to be emphasized (Fig. 3). Two regions, corresponding to amide I (1700–1640 cm−1) and amide III (1300–1230 cm−1) vibrations, provide valuable structural information through their decomposition into band components (42, 43, 44). It is noteworthy that whereas amide I vibrations result from the backbone C=O bond stretch motion, more or less coupled to its adjacent N-H angular bending, amide III vibrations mainly arise from the backbone N-H angular bending. The Raman spectra obtained for the free amino acids (Fig. S2) are devoid of amide (I and III) vibrations. However, the presence of low-intensity bands arising from the side chains of Val, Leu, Ile, and Pro falling within the amide III range should be stressed. These bands can be naturally superimposed to those relative to amide III vibrations in peptides. Nevertheless, because of their weakness, one cannot expect a considerable distortion in the structural analysis on the basis of amide III vibrations. In all nonapeptides, amide I and amide III vibrations are characterized by two strong, broad, and incompletely resolved bands peaking at ∼1685 and ∼1265 cm−1, respectively. To go farther in extracting the structural information from amide vibrations, the decomposition of the amide I region for four nonapeptides is displayed in Fig. 4. Through a systematic investigation of the structural analysis of the peptide chains (42, 43, 44,48,49) in an aqueous environment, we have been led to select four band components located at ∼1690 ± 5 (assignable to random chain), ∼1660 ± 5 (assignable to β-strand), and ∼1675 ± 5 and ∼1650 ± 5 (both assignable to turns) to decompose the amide I region in nonapeptides. The populations corresponding to different secondary structural elements, as estimated by the normalized areas of the band components (expressed in percent), are reported in Table 3. As can be seen, between 50 and 60% of the total population is formed by turn structures, and the rest is equally distributed between β-strands and random chains.
Figure 4.
Band decomposition in the amide I region of the nonapeptides observed at 20 mM. The peptides are hN3 (A), hN4 (B), hN5 (C) and hN6 (D). Observed spectra are drawn with black lines and component bands with dark gray lines. The light gray trace corresponds to the sum of component bands (fit). The maximal band wavenumber of each used component is reported. In parentheses: (R) random, (T) turn, (S) β-strand. For the estimated populations of different secondary structural elements, see Table 3.
Table 3.
Underlying Secondary Structure Elements in the Amide I Profile of the Nonapeptides and Fractional Areas
| Position (Assignment) | hN3 | hN4 | hN5 | hN6 |
|---|---|---|---|---|
| ∼1690 (random) | 20 | 20 | 24 | 25 |
| ∼1680 (turn) | 41 | 40 | 37 | 38 |
| ∼1660 (β-strand) | 26 | 23 | 21 | 25 |
| ∼1640 (turn) | 13 | 17 | 18 | 12 |
| Fractional Areas | ||||
| Random | 20 | 20 | 24 | 25 |
| Turn | 54 | 57 | 55 | 50 |
| β-strand | 26 | 23 | 21 | 25 |
Component positions are ±5 cm−1. During the computation, their widths were kept between 15 and 25 cm−1. The figures correspond to the normalized areas of the components expressed in percent of the whole amide I profile. Fractional areas estimate the global secondary structures with an accuracy of ±5% (22).
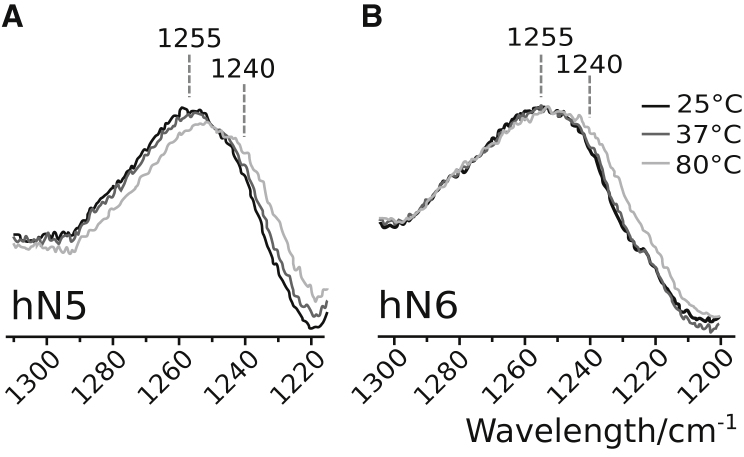
Solution samples containing nonapeptides at 20 mM were heated up to 80°C. Few changes appeared in their Raman spectra, but slight changes could be observed in the amide III region. Fig. 5 exemplifies this behavior for hN5 and hN6. The main effect is a wavenumber downshift of the spectral profile, consistent with a progressive shift from turn to β-strand upon increasing temperature. Albeit the turns remain the major population of secondary structures in the 10–80°C temperature range (data not shown), they show a minor tendency to be transformed into β-strands, presumably because of the weakening or breakdown of the interturn stabilizing hydrogen bonds occurring upon thermal annealing.
Figure 5.
Effect of temperature on the amide III vibrations of nonapeptides. The spectra were recorded for a concentration of 20 mM. Gray scales are used to display the spectra obtained as a function of temperature. The considered peptides are hN5 (A) and hN6 (B).
Atomistic molecular dynamics simulations
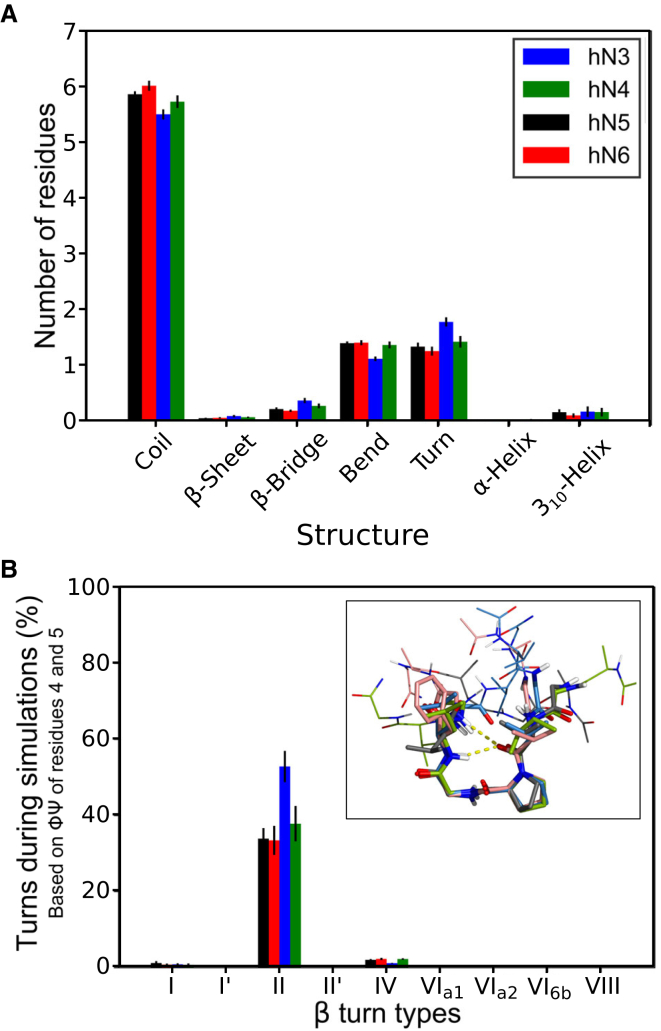
To get a more detailed representation of the structures that can be adopted by the four nonapeptides, 10 molecular dynamic simulations of 500 ns have been performed for each peptide. The adopted conformations, analyzed with DSSP (31), are presented in Fig. 6 A and Fig. S3. The figures show that the peptides mainly adopt random-coil conformations, with some residues favoring turns, but no stable structure can be observed. The formation of β-turns is evidenced by distances between Cα atoms of residues i and i + 3 being less than 0.7 nm with residues i + 1 and i + 2 being nonhelical, which is mainly observed in the middle of the peptides between residues 3 and 6 and notably for the hN3 peptide (Fig. S4 A). The type of β-turns can be identified by analyzing the ΦΨ angles of two amino-acid residues following each other as determined by Lewis et al. (32) and Hutchinson et al. (33). Fig. 6 B shows that type II β-turns are formed during a third to a half of the simulation time (52.9 ± 4.1% for hN3, 37.8 ± 4.7% for hN4, 33.9 ± 2.8% for hN5, and 33.5 ± 3.8% for hN6, mean ± SD) for the PG residues being at positions 4 and 5. PG residues are strongly favored at i + 1 and i + 2 positions in experimental structure of type II β-turns, and they are known to promote this type of β-turn. The population of type II β-turns is greater for the hN3 peptide than for the hN4, hN5, and hN6 peptides. Fig. S4, B–E show that type I, I′, II, II′, and VIII β-turns can also be formed in smaller amounts at other positions of the peptides and that type VIII β-turns are mainly observed at the start of the peptides (up to 3% of the simulation time). A proline at position 4 has been shown to promote the formation of this type of turn (33). Analysis of the simulation underlines that the structures are rapidly changing on the nanosecond timescale from extended to more packed conformations, which mainly corresponds to type II β-turns (Fig. S4 F). The population of β-turns has also been analyzed through clustering methods, and the central structures of the dominant cluster population of each peptide is presented as an inset in Fig. 6 B. Altogether, our analysis of molecular dynamics data strongly suggests that the PG motif of the nonapeptides could mainly adopt a type II β-turn conformation.
Figure 6.
Conformational analysis of the molecular dynamics simulations. (A) Secondary structures adopted during the simulation calculated with DSSP (31) are shown. (B) The occurrence of β-turn types during each frame of the simulation trajectories is shown. β-turns are computed considering the ΦΨ angles of the PG residues at positions 4 and 5. Inset shows an overlay of the conformation representative of the main cluster computed for each of the four nonapeptides. Residues 3–6 are represented with a thicker line. Carbon atoms have the same colors as those defined for each peptide in (B). Error bars are standard deviations between the 10 simulations.
To test the agreement of these results with the measured CD spectra, CD spectra were computed from our simulated trajectories with the SESCA software (35). The results (Fig. S5) show a very good agreement between the measured and computed spectra despite noticeable differences around ∼220 nm. This suggests that the computed profiles might have failed to detect several experimental signals. Nevertheless, they reinforce our proposal that β-turns, and notably type II β-turns, are the prevailing structures adopted by the nonapeptides.
Discussion
Among elastin peptides, elastin nonapeptides have been identified initially as chemoattractants for fibroblasts (12). Now, there are growing evidences that these elastin peptides could define a peculiar class of matrikines.
Brassart and colleagues have recently shown that hN5 (i.e., AGVPGLGVG) plays a key role in tumor progression by promoting tumor cell blebbing and extracellular vesicle shedding after its interaction with RPSA (17). The structure of hN5 has been analyzed previously by CD, FT-IR, and NMR spectroscopies (16). This report concluded that the structure of the peptide was a mixture of random coils and β-turns. Our data fully agree with these findings, but they also expand our knowledge of the nature and structural behavior of these elastin peptides.
The occurrence of the XGXPGXGXG consensus sequence was analyzed in a large sequence data bank. Our results show that among the observed hits, the group of elastin sequences defines a specific group (Fig. 1). Further, our analysis underlines the fact that these peptides mostly occur in tandem repeats in human exon 26 (and corresponding sequences in other species). Finally, as far as human sequences are concerned, we observed that similar sequences were also present in numerous species (Table 1). The most striking observation is that hN6 is present in numerous species and is tandemly repeated five times in the mouse sequence. In contrast, hN3 is only found in three species. These observations suggest that, besides their matrikine activity, these sequences could play a key and yet undescribed role in elastin biology, possibly elastin assembly. Indeed, among elastin sequences, these sequences appear much more conserved than the canonical elastin hexapeptide sequence (say, VGVAPG), the occurrence of which is mostly limited to human exon 24 sequence. Further works are needed to test this hypothesis.
Our data (Fig. 1) show that the XGXPGXGXG consensus sequence can be found in most phyla and/or species. To the best of our knowledge, sequences harboring this consensus have caught attention only in elastin because these elastin peptides are bioactive (8,12, 13, 14, 15,17). Up until now, neither a biological activity nor a dedicated receptor has been described for the nonelastin sequences.
As evidenced by the consensus profile reported for each of them (Fig. 1), the nonelastin sequences (96,259 sequences) present a sequence pattern that is mostly dominated by the occurrence of G, A, or K at X positions. These residues are not favored in elastin sequences in which AGVPGFGVG is the prevailing motif. This is remarkably pronounced when nonelastin animal sequences are considered. Their consensus is GGGPGGGGG. These important sequence differences suggest that nonapeptides found in nonelastin sequences would most probably be structured and behave differently than elastin nonapeptides. Nevertheless, a comprehensive structural analysis is required to address this point.
In our study, we focused our analysis on the nonapeptide sequences found in human exon 26 aiming at understanding their structure and structural behavior. First, this investigation was performed using optical spectroscopies. The results gathered by both CD and Raman spectra are consistent with previous reports underlining a conformational equilibrium between random, strand, and turn conformations within elastin sequences (50). The Raman data underline that the conformation of these peptides is dominated by β-turns, as deduced from the quantitative analysis of their amide I profiles. These conformations appear very favored because increasing the temperature up to 80°C did not drastically change the spectra and hence their structure, although a small increase of the β-strand content could be observed upon heating. Altogether, our experimental data suggest that the structures of the nonapeptides are dominated by β-turns and that these conformations could be engaged in a conformational equilibrium with extended and random structures.
To have a better understanding of the structural behavior of these peptides, molecular dynamics simulations were undertaken in explicit water. 10 independent 500-ns simulations were performed for each of the four nonapeptides. The results obtained are extremely consistent with our experimental data. Indeed, they show that the most common structure observed during the simulation is a type II β-turn occurring at the X-P-G-X motif. Quantitatively, this structure seems more abundant in hN3 (∼50%) than hN4, hN5, or hN6 (25–35%). This finding is in very good agreement with the conclusions of Lessing and colleagues (51), who observed that the preference for the X-Pro-Gly sequence to form a β-turn increased with the complexity and hydrophobicity of the side chain at position X (Gly < Ala < Val). In contrast to hN4, hN5, and hN6, which harbor a valyl residue before PG, hN3 possesses an isoleucyl residue at that position. Thus, the higher propensity of hN3 to adopt a β-turn when compared to the other three nonapeptides is consistent with the work of Lessing and colleagues.
Since the first report of their bioactivity on bovine fibroblasts (12), nonapeptide sequences have been tested on various cellular systems: bovine endothelial cells (13), rat macrophages (14), and human tumor cell lines (8,9). Nevertheless, among those publications, few used more than one peptide sequence, thereby preventing the comparison of their respective effects. In the work of Maeda and colleagues, hN5 and hN6 were identified as chemoattractants for rat macrophages (14). In this work, the authors also analyzed the effect of the canonical VGVAPG sequence. Their conclusion was that the two nonapeptides could compete for receptor binding on the elastin binding protein (EBP). Indeed, although VGVAPG appeared as an exclusive ligand for EBP, they underlined that hN5 and hN6 could also bind EBP and a lactose-insensitive receptor, later identified as RPSA. Their work therefore suggest that the EBP-binding motif can be harbored by both hexa- and nonapeptides, whereas the RPSA binding motif can be only adopted by nonapeptides. It has been established that VGVAPG bioactivity relies on its ability to adopt mainly a type VIII β-turn conformation (10). Our molecular dynamics simulations show that the major conformation of nonapeptides could be the type II β-turn but also that type VIII turns can be present. Our experimental data also suggest that both type VIII and type II β-turns can be present in the conformation of the nonapeptides. Thus, at the difference of VGVAPG, nonapeptides could be favorably structured to bind EBP and RPSA. However, it has to be emphasized that the sequences of the hexa- and nonapeptides are different. That way, binding affinity could also be supported by favorable residue side-chain interactions in addition to the locally adopted conformation. Sequence differences could also contribute to the differential bioactivity exhibited by these peptides.
Altogether, our data suggest that the structural signature of elastin nonapeptide is a type II β-turn. Consequently, it is reasonable to propose that this conformation could be relevant of the observed bioactivity of these peptides. This proposal is supported by the observations made by Toupance and co-workers, in which they measured a lesser biological effect of hN6 toward human tumor cells than hN5 (8). In our work, hN6 is predicted to present fewer β-turn conformations than hN5. Therefore, it seems plausible that the bioactivity of hN5 is greater than that of hN6, supporting the proposal that the turn motif is the conformation sustaining bioactivity of these peptides. If our prediction is correct, then one can anticipate that hN3 would be a more bioactive peptide than others.
Despite decades of efforts, the structure-function relationship of RPSA is still an open question (52). Notably, the interaction site of elastin nonapeptides on RSPA is unknown. In contrast, RPSA laminin binding site was described as a secondary function acquired during evolution (53). This site corresponds to the 161–180 region of the human sequence. However, the crystal structure of the protein (54) locates this binding site mostly inside the fold. Therefore, it is thought that RPSA could possess multiple sites and/or associate with other compounds to exert its functions. Consequently, our current knowledge of RPSA structure cannot explain where and how it binds elastin nonapeptides. Further structural explorations are required to address this question.
Conclusions
Our data throw new light on the nature, structure, and conformational behavior of elastin nonapeptides. These peptide sequences exhibit a peculiar composition as demonstrated by our bioinformatic analysis of current databases. Furthermore, experimental (CD and Raman spectroscopies) and theoretical data (molecular dynamics) strongly suggest that the dynamic structure of these peptides is dominated by the type II β-turn conformation. We propose that the observed bioactivity of these peptides toward stromal and tumor cells could be driven by the recognition of this structural feature by their cognate receptor, RPSA. Molecular modeling of their interaction is underway.
Author Contributions
B.H. and S.G.K. performed the Raman and CD measurements and analyzed the spectra. S.B. conceived and ran the simulations. J.M.C., J.T., and N.B. performed the sequence analyses. S.B. and M.D. funded the project and edited the manuscript. L.D. supervised the work and wrote the manuscript.
Acknowledgments
The authors thank the MAgICS chair (Université de Reims Champagne Ardenne) and the Centre National de la Recherche Scientifique for financial support. Molecular dynamics calculations were performed at the ROMEO high-performance computing center (https://romeo.univ-reims.fr).
Editor: Margaret Cheung.
Footnotes
Supporting Material can be found online at https://doi.org/10.1016/j.bpj.2020.04.019.
Supporting Material
Sequences Presenting the XGXPGXGXG Motif
References
- 1.Mecham R.P. First Edition. Springer; Berlin, Heidelberg: 2011. The Extracellular Matrix: An Overview. [Google Scholar]
- 2.Duca L., Blaise S., Maurice P. Matrix ageing and vascular impacts: focus on elastin fragmentation. Cardiovasc. Res. 2016;110:298–308. doi: 10.1093/cvr/cvw061. [DOI] [PubMed] [Google Scholar]
- 3.Maurice P., Blaise S., Duca L. Elastin fragmentation and atherosclerosis progression: the elastokine concept. Trends Cardiovasc. Med. 2013;23:211–221. doi: 10.1016/j.tcm.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Gayral S., Garnotel R., Laffargue M. Elastin-derived peptides potentiate atherosclerosis through the immune Neu1-PI3Kγ pathway. Cardiovasc. Res. 2014;102:118–127. doi: 10.1093/cvr/cvt336. [DOI] [PubMed] [Google Scholar]
- 5.Pocza P., Süli-Vargha H., Falus A. Locally generated VGVAPG and VAPG elastin-derived peptides amplify melanoma invasion via the galectin-3 receptor. Int. J. Cancer. 2008;122:1972–1980. doi: 10.1002/ijc.23296. [DOI] [PubMed] [Google Scholar]
- 6.Coquerel B., Poyer F., Vannier J.P. Elastin-derived peptides: matrikines critical for glioblastoma cell aggressiveness in a 3-D system. Glia. 2009;57:1716–1726. doi: 10.1002/glia.20884. [DOI] [PubMed] [Google Scholar]
- 7.Devy J., Duca L., Debelle L. Elastin-derived peptides enhance melanoma growth in vivo by upregulating the activation of Mcol-A (MMP-1) collagenase. Br. J. Cancer. 2010;103:1562–1570. doi: 10.1038/sj.bjc.6605926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toupance S., Brassart B., Birembaut P. Elastin-derived peptides increase invasive capacities of lung cancer cells by post-transcriptional regulation of MMP-2 and uPA. Clin. Exp. Metastasis. 2012;29:511–522. doi: 10.1007/s10585-012-9467-3. [DOI] [PubMed] [Google Scholar]
- 9.Brassart B., Fuchs P., Debelle L. Conformational dependence of collagenase (matrix metalloproteinase-1) up-regulation by elastin peptides in cultured fibroblasts. J. Biol. Chem. 2001;276:5222–5227. doi: 10.1074/jbc.M003642200. [DOI] [PubMed] [Google Scholar]
- 10.Blanchevoye C., Floquet N., Debelle L. Interaction between the elastin peptide VGVAPG and human elastin binding protein. J. Biol. Chem. 2013;288:1317–1328. doi: 10.1074/jbc.M112.419929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scandolera A., Odoul L., Duca L. The elastin receptor complex: a unique matricellular receptor with high anti-tumoral potential. Front. Pharmacol. 2016;7:32. doi: 10.3389/fphar.2016.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long M.M., King V.J., Urry D.W. Chemotaxis of fibroblasts toward nonapeptide of elastin. Biochim. Biophys. Acta. 1988;968:300–311. doi: 10.1016/0167-4889(88)90021-3. [DOI] [PubMed] [Google Scholar]
- 13.Long M.M., King V.J., Urry D.W. Elastin repeat peptides as chemoattractants for bovine aortic endothelial cells. J. Cell. Physiol. 1989;140:512–518. doi: 10.1002/jcp.1041400316. [DOI] [PubMed] [Google Scholar]
- 14.Maeda I., Mizoiri N., Okamoto K. Induction of macrophage migration through lactose-insensitive receptor by elastin-derived nonapeptides and their analog. J. Pept. Sci. 2007;13:263–268. doi: 10.1002/psc.845. [DOI] [PubMed] [Google Scholar]
- 15.Salesse S., Odoul L., Debelle L. Elastin molecular aging promotes MDA-MB-231 breast cancer cell invasiveness. FEBS Open Bio. 2018;8:1395–1404. doi: 10.1002/2211-5463.12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Da Silva J., Lameiras P., Brassart B. Structural characterization and in vivo pro-tumor properties of a highly conserved matrikine. Oncotarget. 2018;9:17839–17857. doi: 10.18632/oncotarget.24894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brassart B., Da Silva J., Brassart-Pasco S. Tumour cell blebbing and extracellular vesicle shedding: key role of matrikines and ribosomal protein SA. Br. J. Cancer. 2019;120:453–465. doi: 10.1038/s41416-019-0382-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huerta-Cepas J., Serra F., Bork P. ETE 3: reconstruction, analysis, and visualization of phylogenomic data. Mol. Biol. Evol. 2016;33:1635–1638. doi: 10.1093/molbev/msw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crooks G.E., Hon G., Brenner S.E. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lefèvre T., Rousseau M.E., Pézolet M. Protein secondary structure and orientation in silk as revealed by Raman spectromicroscopy. Biophys. J. 2007;92:2885–2895. doi: 10.1529/biophysj.106.100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolui S., Mondal A., Maiti N.C. Order, disorder, and reorder state of lysozyme: aggregation mechanism by Raman spectroscopy. J. Phys. Chem. B. 2020;124:50–60. doi: 10.1021/acs.jpcb.9b09139. [DOI] [PubMed] [Google Scholar]
- 22.Debelle L., Alix A.J., Legrand P. Bovine elastin and kappa-elastin secondary structure determination by optical spectroscopies. J. Biol. Chem. 1995;270:26099–26103. doi: 10.1074/jbc.270.44.26099. [DOI] [PubMed] [Google Scholar]
- 23.Lindorff-Larsen K., Piana S., Shaw D.E. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins. 2010;78:1950–1958. doi: 10.1002/prot.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abraham M.J., Murtola T., Lindahl E. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1–2:19–25. [Google Scholar]
- 25.Schrödinger, LLC . 2010. The PyMOL molecular graphics system. Version. 1: 0.www.pymol.org [Google Scholar]
- 26.Bussi G., Donadio D., Parrinello M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007;126:014101. doi: 10.1063/1.2408420. [DOI] [PubMed] [Google Scholar]
- 27.Parrinello M., Rahman A. Polymorphic transitions in single crystals: a new molecular dynamics method. J. Appl. Phys. 1981;52:7182–7190. [Google Scholar]
- 28.Essmann U., Perera L., Pedersen L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995;103:8577–8593. [Google Scholar]
- 29.Hess B., Bekker H., Fraaije J.G.E.M. LINCS: a linear constraint solver for molecular simulations. J. Comput. Chem. 1997;18:1463–1472. [Google Scholar]
- 30.Humphrey W., Dalke A., Schulten K. VMD: visual molecular dynamics. J. Mol. Graph. 1996;14:33–38, 27–28. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 31.Kabsch W., Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983;22:2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- 32.Lewis P.N., Momany F.A., Scheraga H.A. Chain reversals in proteins. Biochim. Biophys. Acta. 1973;303:211–229. doi: 10.1016/0005-2795(73)90350-4. [DOI] [PubMed] [Google Scholar]
- 33.Hutchinson E.G., Thornton J.M. A revised set of potentials for β-turn formation in proteins. Protein Sci. 1994;3:2207–2216. doi: 10.1002/pro.5560031206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daura X., Gademann K., Mark A.E. Peptide folding: when simulation meets experiment. Angew. Chem. Int. Ed. 1999;38:236–240. [Google Scholar]
- 35.Nagy G., Igaev M., Grubmüller H. SESCA: predicting circular dichroism spectra from protein molecular structures. J. Chem. Theory Comput. 2019;15:5087–5102. doi: 10.1021/acs.jctc.9b00203. [DOI] [PubMed] [Google Scholar]
- 36.Hernández B., Boukhalfa-Heniche F.Z., Ghomi M. Secondary conformation of short lysine- and leucine-rich peptides assessed by optical spectroscopies: effect of chain length, concentration, solvent, and time. Biopolymers. 2006;81:8–19. doi: 10.1002/bip.20366. [DOI] [PubMed] [Google Scholar]
- 37.Perczel A., Fasman G.D. Quantitative analysis of cyclic β-turn models. Protein Sci. 1992;1:378–395. doi: 10.1002/pro.5560010310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopes J.L., Miles A.J., Wallace B.A. Distinct circular dichroism spectroscopic signatures of polyproline II and unordered secondary structures: applications in secondary structure analyses. Protein Sci. 2014;23:1765–1772. doi: 10.1002/pro.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sibanda B.L., Thornton J.M. Beta-hairpin families in globular proteins. Nature. 1985;316:170–174. doi: 10.1038/316170a0. [DOI] [PubMed] [Google Scholar]
- 40.Gunasekaran K., Ramakrishnan C., Balaram P. Beta-hairpins in proteins revisited: lessons for de novo design. Protein Eng. 1997;10:1131–1141. doi: 10.1093/protein/10.10.1131. [DOI] [PubMed] [Google Scholar]
- 41.Kaur H., Raghava G.P. A neural network method for prediction of beta-turn types in proteins using evolutionary information. Bioinformatics. 2004;20:2751–2758. doi: 10.1093/bioinformatics/bth322. [DOI] [PubMed] [Google Scholar]
- 42.Hernández B., Coïc Y.M., Ghomi M. Octreotide used for probing the type-II’ β-turn CD and Raman markers. J. Phys. Chem. B. 2012;116:9337–9345. doi: 10.1021/jp3036428. [DOI] [PubMed] [Google Scholar]
- 43.Hernández B., Coïc Y.M., Ghomi M. Low concentration structural dynamics of lanreotide and somatostatin-14. Biopolymers. 2014;101:1019–1028. doi: 10.1002/bip.22491. [DOI] [PubMed] [Google Scholar]
- 44.Hernández B., López-Tobar E., Ghomi M. From bulk to plasmonic nanoparticle surfaces: the behavior of two potent therapeutic peptides, octreotide and pasireotide. Phys. Chem. Chem. Phys. 2016;18:24437–24450. doi: 10.1039/c6cp04421b. [DOI] [PubMed] [Google Scholar]
- 45.Fuchs P.F., Bonvin A.M., Tamburro A.M. Kinetics and thermodynamics of type VIII beta-turn formation: a CD, NMR, and microsecond explicit molecular dynamics study of the GDNP tetrapeptide. Biophys. J. 2006;90:2745–2759. doi: 10.1529/biophysj.105.074401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hernández B., Pflüger F., Ghomi M. Vibrational analysis of amino acids and short peptides in hydrated media. VIII. Amino acids with aromatic side chains: L-phenylalanine, L-tyrosine, and L-tryptophan. J. Phys. Chem. B. 2010;114:15319–15330. doi: 10.1021/jp106786j. [DOI] [PubMed] [Google Scholar]
- 47.Hernández B., Pflüger F., Ghomi M. Characteristic Raman lines of phenylalanine analyzed by a multiconformational approach. J. Raman Spectrosc. 2013;44:827–833. [Google Scholar]
- 48.Guiffo-Soh G., Hernández B., Ghomi M. Vibrational analysis of amino acids and short peptides in hydrated media. II. Role of KLLL repeats to induce helical conformations in minimalist LK-peptides. J. Phys. Chem. B. 2007;111:12563–12572. doi: 10.1021/jp074264k. [DOI] [PubMed] [Google Scholar]
- 49.Guiffo-Soh G., Hernández B., Ghomi M. Vibrational analysis of amino acids and short peptides in hydrated media. 3. Successive KL repeats induce highly stable β-strands capable of forming non-H-bonded aggregates. J. Phys. Chem. B. 2008;112:1282–1289. doi: 10.1021/jp0767967. [DOI] [PubMed] [Google Scholar]
- 50.Debelle L., Tamburro A.M. Elastin: molecular description and function. Int. J. Biochem. Cell Biol. 1999;31:261–272. doi: 10.1016/s1357-2725(98)00098-3. [DOI] [PubMed] [Google Scholar]
- 51.Lessing J., Roy S., Tokmakoff A. Identifying residual structure in intrinsically disordered systems: a 2D IR spectroscopic study of the GVGXPGVG peptide. J. Am. Chem. Soc. 2012;134:5032–5035. doi: 10.1021/ja2114135. [DOI] [PubMed] [Google Scholar]
- 52.DiGiacomo V., Meruelo D. Looking into laminin receptor: critical discussion regarding the non-integrin 37/67-kDa laminin receptor/RPSA protein. Biol. Rev. Camb. Philos. Soc. 2016;91:288–310. doi: 10.1111/brv.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ardini E., Pesole G., Ménard S. The 67-kDa laminin receptor originated from a ribosomal protein that acquired a dual function during evolution. Mol. Biol. Evol. 1998;15:1017–1025. doi: 10.1093/oxfordjournals.molbev.a026000. [DOI] [PubMed] [Google Scholar]
- 54.Jamieson K.V., Wu J., Meruelo D. Crystal structure of the human laminin receptor precursor. J. Biol. Chem. 2008;283:3002–3005. doi: 10.1074/jbc.C700206200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequences Presenting the XGXPGXGXG Motif