Highlights
-
•
Employing statistical modeling analyses, we elucidated the effects of a ‘wet market’ on coronavirus disease (COVID-19) transmission dynamics in China, by stratifying cases with contact history with the Huanan Seafood Wholesale Market.
-
•
For market-to-human transmission, we estimated mean R at 0.24 (95% CrI: 0.01–1.38), with the reporting rate identified as being 2–34-fold higher compared with human-to-human transmission.
-
•
The proportion of asymptomatic/mild-symptom patients constitutes a substantial component of the COVID-19 disease burden.
Keywords: Corona virus: market hazard, Transmission, Epidemic
Abstract
Objectives
The novel coronavirus (SARS-CoV-2) originating from Wuhan spread rapidly throughout China. While its origin remains uncertain, accumulating evidence links a wet market with the early spread of SARS-CoV-2 in Wuhan. Similarly, the influence of the marketplace on the early transmission dynamics is yet to be investigated.
Methods
Using the daily series of COVID-19 incidence, stratified according to contact history with the market, we have conducted quantitative modeling analyses to estimate the reproduction numbers (R) for market-to-human and human-to-human transmission, the reporting probability, and the early effects of public health interventions.
Results
We estimated R at 0.24 (95% CrI: 0.01–1.38) for market-to-human transmission and 2.37 (95% CrI: 2.08–2.71) for human-to-human transmission during the early spread in China (2019–2020). Moreover, we estimated that the reporting rate for cases stemming from market-to-human transmission was 2–34 fold higher than that for cases stemming from human-to-human transmission, suggesting that contact history with the wet market played a key role in identifying COVID-19 cases.
Conclusions
Our R estimate tied to market-to-human transmission had substantial uncertainty, but it was significantly lower compared with the reproduction number driving human-to-human transmission. Our results also suggest that asymptomatic and subclinical infections constitute a substantial component of the COVID-19 morbidity burden.
Introduction
A novel coronavirus (SARS-CoV-2) originating from Wuhan spread rapidly around the world to give rise to the most important pandemic event in recent history. As of May 17, 2020, the cumulative number of confirmed cases had reached 3.5 million, including 250,000 deaths (WHO, 2019). Early mean estimates of the reproduction number, based on the epidemic's growth rate, were estimated to be in the range of 1.4–3.5, comparable with estimates for seasonal flu, the 2009 pandemic flu, SARS, and MERS (WHO, 2005, Biggerstaff et al., 2014, Chowell et al., 2004, Chowell et al., 2014, Kucharski et al., 2020, Liu et al., 2020). Considering the explosive spread and significant severity associated with the novel coronavirus, WHO declared this public health emergency a pandemic on March 11, 2020, with the world subsequently coming together to fight the spread of the virus.
Evidence suggests that the novel coronavirus likely jumped from a primary reservoir (e.g. horseshoe bats) to an intermediary reservoir, possibly generating an outbreak among wild animals in at least one wet market in Wuhan, China (By Jon CohenJan, 2020, Li et al., 2020). The virus first infected multiple individuals working at, or visiting, the Huanan Seafood Wholesale Market at an early stage, initiating multiple chains of transmission that ensured sustained transmission in the human population (Yang et al., 2020). While details of the origin of the outbreak remain uncertain, significant evidence strongly links the Huanan Seafood Wholesale Market in Wuhan with the early spread of the novel coronavirus (COVID-19) among humans (Li et al., 2020).
For this study we conducted quantitative modeling analyses to quantify the role of the wet marketplace in the early transmission dynamics of the novel coronavirus in China (Huang et al., 2020). For this purpose, we analyzed and modeled data that stratified the market hazard (market-to-human transmission) and human-to-human transmission dynamics.
Materials and methods
Epidemiological incidence cases
Daily series of laboratory-confirmed COVID-19 cases were extracted from a recently published study (Li et al., 2020). From December 8, 2019 to January 21, 2020, we analyzed a total of 425 confirmed cases by date of symptoms onset, including information on whether the case was linked to the Huanan Seafood Wholesale Market. Our unique case series stratified cases with their history of visiting the Huanan Seafood Wholesale Market, which had been suggested as the source of this large epidemic at an early stage (Li et al., 2020), and those arising from human-to-human transmission. Because the corresponding incidence curve was subject to reporting delays, the last 12 epidemic days were excluded from our analysis. Thus, the study period was set from December 8, 2019 to January 9, 2020.
Epidemiological modeling
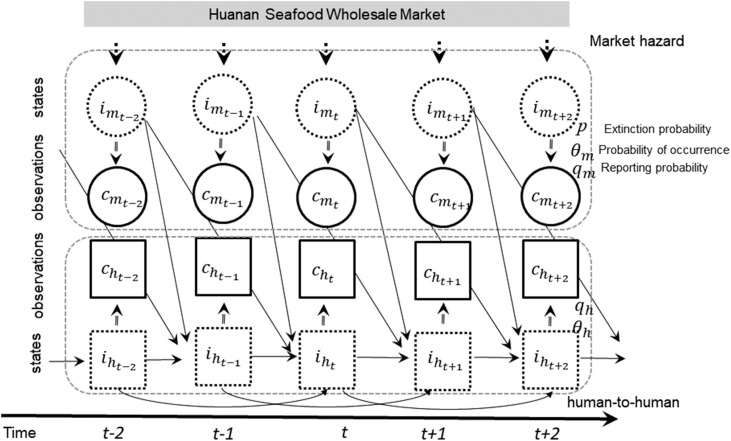
We modeled two types of infection: (a) market hazard (market-to-human transmission, including primary infections arising from zoonotic transmission to some extent); and (b) human-to-human transmission chains. A model schematic of the transmission dynamics is provided in Figure 1 . Primary infection (index case) occurs as a result of a spillover event, and primary infections may generate secondary cases. The number of infected individuals stemming from the market-to-human and the human-to-human routes are denoted by i m and i h, respectively.
Figure 1.
Schematic model diagram of COVID-19 transmission dynamics.
The transmission routes are classified into market-to-human transmission and human-to-human transmission, indicated by im and ih, respectively. Reported primary cases infected through the market-to-human route and secondary or later cases though the human-to-human route are indicated by cm and ch, respectively.
We employed a discrete-time integral equation to capture the daily incidence series with contact history. Let f s denote the probability mass function of the serial interval of length s days, which is given by
for s > 0, where F(s) represents the cumulative distribution function of the lognormal distribution. The expected number of new cases with onset day t after secondary infections, E[c h(t)], is written as follows:
where E[c m(t)] represents the expected number of new cases with onset day t infected through market-to-human transmission, p is the conditional probability of extinction within one generation, given by 1/(R m + 1) (Matsuyama et al., 2017, Nishiura et al., 2013), and R h and R m represent the average number of secondary infections generated by one single infection generated from market-to-human and from human-to-human transmission, respectively.
To account for the type of infection-dependent probability of occurrence, θ j (Li et al., 2011), we assumed that the number of observed cases for infection type j on day t, h j(t), occurred according to a Bernoulli sampling process, with the expected values E(c j; H t−1), where E(c j; H t−1) denotes the conditional expected incidence, on day t, given the history of observed data from day 0 to day (t − 1), denoted by H t−1. Thus, the number of expected newly observed cases is written as follows:
Subsequently, we also accounted for the reporting probability that depended on the type of infection. We assumed that the number of reported cases by infection type j on day t, h j(t), was the product of type-dependent reporting rate, q j, and the actual number of cases, c j(t).
Further, we modeled the time-dependent variation in transmissibility associated with the implementation of public interventions in Wuhan. This time dependence was modeled by introducing a parameter δ ti, according to transmission route. Time dependence in primary infection and secondary infection is given by
where α 1 and α 2 scale the intensity of public health interventions (where α 1 and α 2 are expected to be less than 1) and period 1 and period 2 define the study periods, according to the timing of the closing of the Huanan Seafood Wholesale Market (December 31, 2019) and the date on which the novel coronavirus was officially declared as the causative pathogen of the outbreak by China CDC (January 8, 2020) (Yang et al., 2020). After the Huanan Seafood Wholesale Market was closed on December 31, we assumed the hazard posed by the market to be negligible.
The number of expected newly observed cases should be updated as
We assumed the incidence, h j, to be the result of the Poisson sampling process, with an expected value of E[h j]. The likelihood function for the time series of observed cases of primary infection through the market-to-human transmission and secondary infection through human-to-human transmission that we employed to estimate the reproduction number and other relevant parameters is given by:
where U indicates parameter sets that are estimated from this likelihood.
The serial interval was assumed to follow a lognormal distribution, with the mean and SD at 4.7 and 2.9 days, respectively, based on ref. (Linton et al., 2020). The maximum value of the serial interval was fixed at 16 days because the cumulative probability distribution of the lognormal distribution up to 16 days reached 0.99.
For sensitivity analyses, we examined the effect of varying the mean of the serial interval on R j by varying the mean serial interval from 2.7 to 6.7 days.
We estimated model parameters and made projections using a Monte Carlo Markov chain (MCMC) method in a Bayesian framework. Point estimates and the corresponding 95% credibility intervals were drawn from the posterior probability distributions. All statistical analyses were conducted using R version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria) and the ‘rstan’ package (No-U-Turn-Sampler (NUTS)).
Results
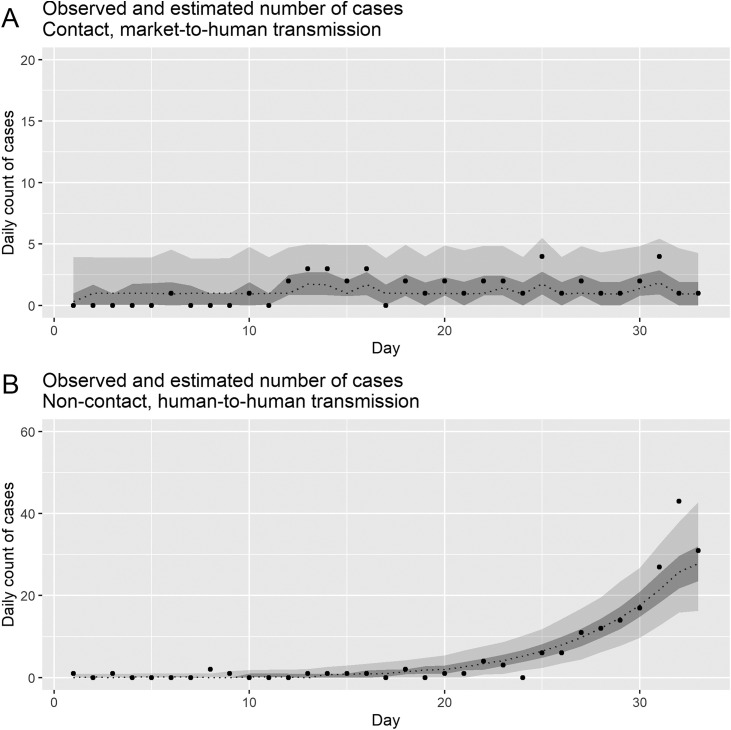
The daily series of COVID-19 incidence in China in 2019–2020 are shown in Figure 2 . Visual inspection indicates that the trend for laboratory-confirmed COVID-19 cases that were not linked to the Huanan Seafood Wholesale Market followed a rapid growth pattern, whereas those cases linked to the Huanan Seafood Wholesale Market followed an overall stable incidence pattern until early January in 2020, likely associated with the closing of the Huanan Seafood Wholesale Market in Wuhan.
Figure 2.
Observed numbers and posterior estimates of daily COVID-19 cases in China, 2019–2020.
The total numbers of cases for our study period, with or without contact history with the Huanan Seafood Wholesale Market, were 43 and 187, respectively. The dots indicate the observed, laboratory-confirmed cases. The dashed lines indicate 50th percentiles, with light and dark gray areas representing the 95% and 50% credible intervals (CrI) for posterior estimates, respectively. (A) Cases arising from market-to-human transmission. (B) Observed cases infected through human-to-human transmission. Epidemic day 1 corresponds to December 8, 2020.
Figure 2 shows the observed and model-based posterior estimates of the daily numbers of laboratory-confirmed COVID-19 cases by transmission route in China, from December, 2019 to January, 2020. Overall, our dynamic models were able to capture the temporal dynamics (i.e. incidence) for both market-to-human and human-to-human transmission routes. Importantly, our posterior estimates of the basic reproduction numbers for China in 2019–2020 were estimated at 0.24 (95% CrI: 0.01–1.38) for market-to-human transmission and 2.37 (95% CrI: 2.08–2.71) for human-to-human transmission. Other parameter estimates gauging the probability of occurrence and reporting rate were estimated at 0.93 (95% CrI: 0.73–1.00) and 0.028 (95% CrI: 0.019–0.040) for market-to-human transmission, and 0.90 (95% CrI: 0.72–0.98) and 0.004 (95% CrI: 0.001–0.012) for human-to-human transmission, respectively. A Tukey-Kramer test indicated significant differences in probabilities of occurrence (p < 0.001) and reporting probability (p < 0.001) according to infection route. Moreover, the proportionate reduction in transmissibility brought about by the enhanced public health interventions was estimated to be 0.04 (95% CrI: 0.00–0.17) after January 1 and 0.42 (95% CrI: 0.02–0.95) after January 8, 2020, which was measured using a time-dependent scaling factor, α.
The total numbers of estimated laboratory-confirmed cases (i.e. cumulative incidence) were 43.6 (95% CrI: 27.2–63.8) for market-to-human transmission and 173.4 (95% CrI: 134.0–216.5) for human-to-human transmission. For comparison, the actual numbers of reported laboratory-confirmed cases during our study period were 43 and 187, respectively.
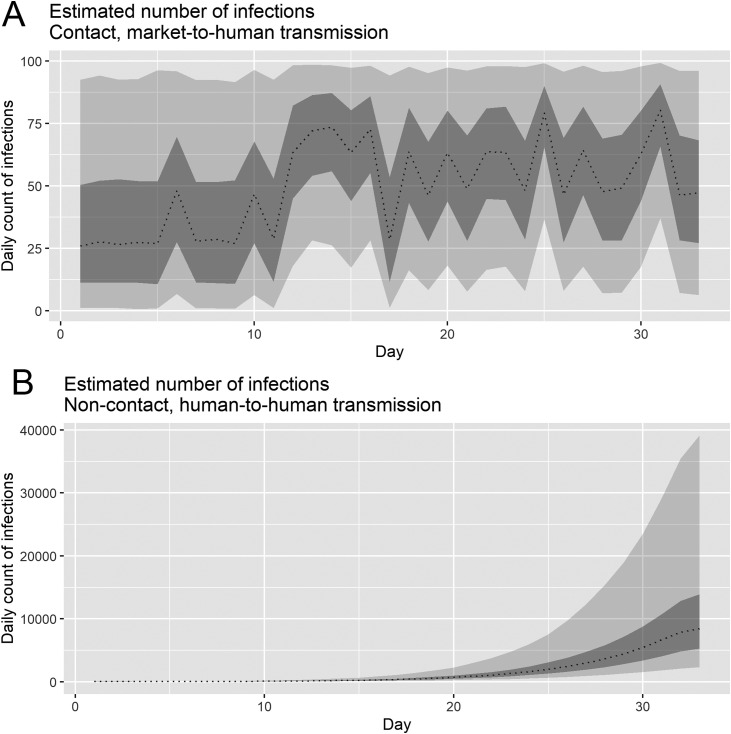
Furthermore, we inferred the total number of COVID-19 infections by transmission route in order to quantify the COVID-19 burden in China during our study period. Our results indicated that the total number of unobserved cases (i.e. cumulative infections) by subgroup were 1687.8 (95% CrI: 1369.2–2020.1) for market-to-human transmission and 52,381.0 (95% CrI: 15,794.0–225,314.9) for human-to-human transmission.
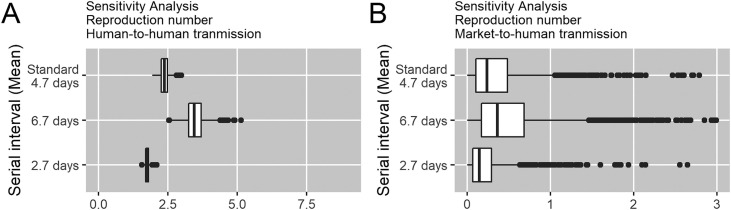
Results of sensitivity analyses on the mean serial interval, ranging from 2.7 to 6.7, are shown in Figure 3 . We found that estimates of R from human-to-human transmission were somewhat sensitive to changes in the mean serial interval, ranging from 1.75 (95% CrI: 1.63–1.89) to 3.45 (95% CrI: 2.87–4.21), while R estimates for primary infections showed a relatively similar range, lying below the epidemic threshold of 1.0.
Figure 3.
Sensitivity analysis of serial interval on reproduction number by infection route.
Discussion
We conducted statistical modeling analyses to assess the effect of a wet market on 2019 novel coronavirus transmission dynamics in China from December, 2019 to January, 2020 by stratifying cases according to transmission route. In particular, we reported estimates of the reproduction numbers for market-to-human and human-to-human transmission, as well as the occurrence reporting probability and estimates of the early effects of public health interventions.
Our posterior estimates of basic reproduction numbers for China in 2019–2020 were estimated to be 0.24 (95%CrI: 0.01–1.38) for market-to-human transmission and 2.37 (95% CrI: 2.08–2.71) for human-to-human transmission. These R estimates, which account for more details of the underlying transmission dynamics, shed light on the impact of the COVID-19 epidemic. Our analysis suggested that the total number of unobserved cases (i.e. cumulative infections) were estimated to be 1687.8 (95% CrI: 1369.2–2020.1) for market-to-human transmission and 52,381.0 (95% CrI: 15,794.0–225,314.9) for human-to-human transmission. Our R estimates accounting for the unobserved cases (infections) were consistent with previous estimates based solely on observed cases (WHO, 2005, Kucharski et al., 2020, Liu et al., 2020). More importantly, our results indicated a substantial contribution of mild and asymptomatic cases to the epidemic. This could influence estimates of R derived from a single epidemic curve of observed reported cases, which does not fully capture the transmission dynamics (Rothe et al., 2020, Kupferschmidt, 2020).
Our method is a powerful tool for estimating the underlying cases, including asymptomatic ones and those with mild symptoms. Our results suggested that these cases constituted a large proportion of the epidemic. This is supported by the results of a study involving careful screening, detailed examination, and follow-up studies of 565 Japanese returnees evacuated from Wuhan city by government-chartered planes from January 29–31, 2020, to confirm that the people did not turn symptomatic at a later stage, which revealed a ratio of four asymptomatic to nine symptomatic cases, with the estimated asymptomatic proportion reported to be 30.8% (95% confidence interval: 7.7–53.8) (MOH, 2020, Nishiura et al., 2020). For the COVID-19 outbreak that unfolded aboard the Diamond Princess cruise ship, the proportion of truly asymptomatic infections was estimated at 17.9% (95% CrI: 15.5–20.25) among a largely senior population (Mizumoto et al., 2020). Another study reported that the majority of seasonal coronavirus infections were asymptomatic by most symptom definitions, with only 4% of individuals experiencing a seasonal coronavirus infection episode seeking medical care for their symptoms (Shaman and Galanti, 2020). Taken together, this evidence further underscores the need to account for a significant fraction of asymptomatic cases. Future serological surveys among the local residents in the epicenter of the epidemic and in other areas will shed light on the true magnitude of the pandemic at different spatial scales.
We also identified a remarkable decrease in the reproduction number, with this decline coinciding with the timing of the closing of the Huanan Seafood Wholesale Market (identified as the origin of the epidemic) and the official announcement about the epidemic by China CDC. We found that R for human-to-human transmission decreased from 2.37 (95% CrI: 2.08–2.71) to 2.25 (95% CrI: 1.94–2.57), with a relative risk reduction (RRR) of 0.04 (95% CrI: 0.00–0.17), and to 1.38 (95% CrI: 0.12–2.36), with an RRR of 0.42 (95% CrI: 0.02–0.95). However, these estimates need to be interpreted with the epidemic context in mind. Specifically, the first intervention targeting market-to-human transmission is unlikely to have mitigated the risk of secondary infections arising from the unfolding transmission chains. The latter decrease is likely the result of enhanced public interventions. However, in the context of rapid and widespread national transmission, efforts to mitigate further spread were complicated by difficulties in tracking multiple chains of transmission involving cases of the disease with a wide spectrum of illness, including asymptomatic and subclinical cases.
Furthermore, reporting probabilities for cases stemming from market-to-human and human-to-human transmission were estimated to be low, but we estimated that the reporting rate for the market-to-human route was 2–34 fold higher, suggesting that contact history with the Huanan Seafood Wholesale Market played a key role in identifying cases with COVID-19. This was in the context of the influenza season, when many influenza patients with non-specific symptoms, fever, and respiratory symptoms, were visiting medical facilities, which further complicated the accurate diagnosis of COVID-19 based on non-specific symptoms (CNIC, 2020). Indeed, during the initial disease stages, individuals infected with COVID-19 exhibit clinical features that are similar to those of other common respiratory diseases (Zou et al., 2020).
Our results are not free from limitations. We cannot rule out the possibility that some cases with contact history with the Huanan Seafood Wholesale Market got infected by contact with humans. However, our low R estimate for market-to-human transmission indicates that such a proportion is quite low.
In summary, we focused on the effect of a wet market on 2019 novel coronavirus transmission dynamics, and have provided epidemiological and transmission parameters for COVID-19 in China from 2019 to 2020, using an ecological model. Regarding our estimate of around 0.25 for the reproduction number relating to market-to-human transmission, transmission stemming from the wet marketplace appeared to be relatively low compared with human-to-human transmission. The power of our approach lies in the ability to infer epidemiological parameters with quantified uncertainty from limited data collected by surveillance systems.
Conflicts of interest
The authors declare no conflicts of interest.
Ethical approval
No ethical approval was requested.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
KM acknowledges support from the Japan Society for the Promotion of Science (JSPS) KAKENHI (grant numbers 18K17368 and 20H03940) and from the Leading Initiative for Excellent Young Researchers (Ministry of Education, Culture, Sport, Science and Technology of Japan). KK acknowledges support from the JSPS KAKENHI (grant numbers 18K19336 and 19H05330). GC acknowledges support from NSF grant 1414374 as part of the joint NSF-NIH-USDA Ecology and Evolution of Infectious Diseases program.
Footnotes
Supplementary material related to this article can be found, in the online version, at https://doi.org/10.1016/j.ijid.2020.05.091.
Appendix A. Supplementary data
The following are supplementary data to this article:
References
- Biggerstaff M., Cauchemez S., Reed C., Gambhir M., Finelli L. Estimates of the reproduction number for seasonal, pandemic, and zoonotic influenza: a systematic review of the literature. BMC Infect Dis. 2014;14:480. doi: 10.1186/1471-2334-14-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- By Jon CohenJan Mining coronavirus genomes for clues to the outbreak's origins. Science. 2020;(January) Available from https://www.sciencemag.org/news/2020/01/mining-coronavirus-genomes-clues-outbreak-s-origins [accessed 03.02.20] [Google Scholar]
- Chowell G., Castillo-Chavez C., Fenimore P.W. Model parameters and outbreak control for SARS. Emerg Infect Dis. 2004;10(7):1258–1263. doi: 10.3201/eid1007.030647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowell G., Blumberg S., Simonsen L. Synthesizing data and models for the spread of MERS-CoV. 2013: key role of index cases and hospital transmission. Epidemics. 2014;9:40–51. doi: 10.1016/j.epidem.2014.09.011. Peng Zhou P, Yang XL, W XG, et al. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. bioRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese National Influenza Center, China. Available from http://www.chinaivdc.cn/cnic/.
- Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 doi: 10.1016/S0140-6736(20)30183-5. pii: S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharski A.J., Russell T.W., Diamond C. Early dynamics of transmission and control of COVID-19: a mathematical modelling study. Lancet Infect Dis. 2020;20(May (5)):553–558. doi: 10.1016/S1473-3099(20)30144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferschmidt K. 2020. Study claiming new coronavirus can be transmitted by people without symptoms was flawed. Available from: https://www.sciencemag.org/news/2020/02/paper-non-symptomatic-patient-transmitting-coronavirus-wrong [accessed 04.02.20] [Google Scholar]
- Li R., Weiskittel A.R., Kershaw J.A., Jr. Modeling annualized occurrence, frequency, and composition of ingrowth using mixed-effects zero-inflated models and permanent plots in the Acadian Forest Region of North America. Can J For Res. 2011;41:2077–2089. [Google Scholar]
- Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;(January) doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton N.M., Kobayashi T., Yang Y. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: a statistical analysis of publicly available case data. J Clin Med. 2020;9(2) doi: 10.3390/jcm9020538. pii: E538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Gayle A.A., Wilder-Smith A. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27(2) doi: 10.1093/jtm/taaa021. pii: taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama R., Miura F., Nishiura H. The transmissibility of noroviruses: statistical modeling of outbreak events with known route of transmission in Japan. PLOS ONE. 2017;12:e0173996. doi: 10.1371/journal.pone.0173996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25(10) doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health, Labour and Welfare, Japan. Available from https://www.mhlw.go.jp/index.html [in Japanese].
- Nishiura H., Mizumoto K., Ejima K. How to interpret the transmissibility of novel influenza A(H7N9): an analysis of initial epidemiological data of human cases from China. Theor Biol Med Model. 2013;10:30. doi: 10.1186/1742-4682-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura H., Kobayashi T., Miyama T. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) Int J Infect Dis. 2020;94:154–155. doi: 10.1016/j.ijid.2020.03.020. Epub 2020 Mar 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe C., Schunk M., Sothmann P. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020 doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaman J., Galanti M. Direct measurement of rates of asymptomatic infection and clinical careseeking for seasonal coronavirus. medRxiv. 2020 doi: 10.1101/2020.01.30.20019612. [DOI] [Google Scholar]
- World Health Organization (WHO) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV); 2005. Statement on the meeting of the International Health Regulations. Available from: https://www.who.int/news-room/detail/23-01-2020-statement-on-the-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov) [Google Scholar]
- World Health Organization (WHO) 2019. Novel Coronavirus (2019-nCoV) situation reports. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. [Google Scholar]
- Yang P. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Chin J Epidemiol. 2020;(February) doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020 doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]