The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pandemic has increased demands for surgical and respirator masks for healthcare workers (HCWs) and other frontline staff. The debate over the importance of airborne transmission of SARS-CoV-2 continues, but air and laboratory studies have shown that SARS-CoV-2 is viable for >12 h in aerosols [[1], [2], [3]]. Low sampling volumes, location of air outlet fan and potential virus damage during sampling may explain the variability in detection of SARS-CoV-2 [1,3].
A limited supply of masks creates a risk for the exposure of HCWs to SARS-CoV-2. Non-traditional materials are widely recommended for public use (source control) and have been considered in place of regulated masks in health care, especially in social care settings. While various materials are effective for filtering large droplets, aerosols generated from sneezing, coughing and aerosol-generating procedures may pass more readily through materials or leakage points [4]. Few data exist on the efficacy of filtration, and no quantitative modelling of efficacies to reduce the risk of infection is currently available.
A probabilistic model was developed to estimate the risk of infection for short (30-s, brief patient check) and long (20-min, duration required for patient intubation) inhalation exposure scenarios. These included situations in a room with a patient with coronavirus disease 2019 (COVID-19) when no mask was worn; when an FFP2 (N95) respirator, FFP3 (N99) respirator or surgical mask was worn; or when a non-traditional material mask (silk, tea towel, vacuum cleaner bag, pillowcase, antimicrobial pillowcase, cotton mix, 100% cotton T-shirt, linen or scarf) was worn.
Inhaled viral dose was estimated using published concentrations (RNA/m3) of SARS-CoV-2 for >4- and 1–4-μm droplets measured in a hospital setting [1]. Ranges from reported concentration data originating from a symptomatic and an asymptomatic patient were used to calculate minimum and maximum values for randomly sampled uniform distributions [1]. Viral exposures for these two size ranges were summed to estimate the total inhaled dose. Doses were estimated for three assumed infectious fractions of total detected viral RNA: 0.1%, 1% and 10%. Inhaled volumes (m3) were estimated using inhalation rates for men and women, where the 5th and 99th percentiles of inhalation rates offered the uniform distribution minimum and maximum, respectively [5].
Filtration efficacies (fraction of total virus filtered out by the material) were used to model the reduction in viral inhalation exposure for each material type. Due to lack of particle-size-specific filtration efficacy data for these materials, it was assumed that filtration efficacy distributions were applicable to both particle size ranges. For each 10,000 combinations investigated, a filtration efficacy was sampled at random from a normal distribution, left- and right-truncated at 0 and 1, respectively. For surgical masks and non-traditional materials, means and standard deviations (SD) of efficacies were informed by MS2 filtration efficacies [6]. Mean efficacies of 95% and 99% were assumed for FFP2 and FFP3 respirators, respectively. SDs were provided by Rengasamy et al. (2009), where larger SDs of two manufacturer versions were chosen as a conservative risk approach [7].
Data from SARS-CoV and human coronavirus 229E (HCoV-229E) dose–response curves were used to estimate a SARS-CoV-2 exact beta-Poisson curve [8]. Based on current epidemiological knowledge, the infectivity of SARS-CoV-2 was assumed to lie between SARS-CoV and HCoV-229E. Pairs of bootstrapped alpha and beta values were used to estimate infection risk per dose.
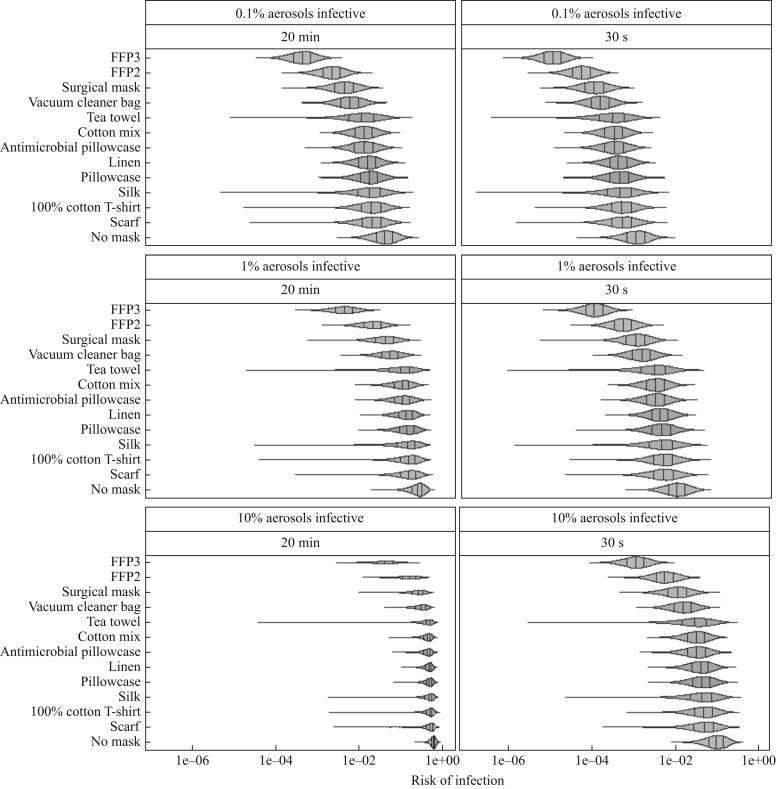
Comparing no protection (baseline) for 20-min and 30-s exposures, it was predicted that the mean risk of infection was reduced by 24–94% and 44–99% depending on the mask. Risk reductions decreased as exposure durations increased. The greatest reduction in estimated mean risk of infection was for FFP3 masks, which reduced baseline mean risks by 94% and 99% for 20-min and 30-s exposures, respectively (Figure 1 ). Of non-traditional materials, the vacuum cleaner bag resulted in the greatest reduction in mean risk of infection (20-min exposure 58%, 30-s exposure 83%), while scarves offered the lowest reduction (20-min exposure 24%, 30-s exposure 44%) (Figure 1). However, large variability in filtration, such as for silk or the tea towel, should be considered when comparing non-traditional mask materials (Figure 1).
Figure 1.
Distributions of estimated infection risks for FFP3 respirators, FFP2 respirators, surgical masks, masks made of non-traditional materials (vacuum cleaner bag, tea towel, cotton mix, antimicrobial pillowcase, linen, pillowcase, silk, 100% cotton T-shirt or scarf) and no mask for 30 s or 20 min of inhalation exposure. Vertical lines indicate the 25th, 50th and 75th percentiles of risk of infection.
Limitations include not accounting for viral transfer from the hands to the mask during mask adjustments, and assuming that all masks were worn in the same way. Realistically, the fit of homemade masks is likely to be more variable than the fit of regulated masks. While the HCoV-229E data utilized for the dose–response curve were based on human data, the SARS-CoV dose–response data originated from an animal-feeding study [8]. Future work includes updating the dose–response curve as data on SARS-CoV-2 emerge, and addressing the effects of design/fit on the risk of infection.
This study demonstrated that some materials, such as vacuum cleaner bags, may be effective alternatives to reduce the risk of infection. While N95 masks (and similar respirators) are recommended for HCWs and others in close proximity to aerosol-generating procedures, alternative materials may be useful where there are shortages of personal protective equipment (PPE). This may be of particular relevance in low-resource settings where access to PPE is considerably more limited.
Conflict of interest statement
None declared.
Funding sources
A.M. Wilson was supported by the University of Arizona Foundation and the Hispanic Women's Corporation/Zuckerman Family Foundation Student Scholarship Award through the Mel and Enid Zuckerman College of Public Health, University of Arizona. M-F. King and C.J. Noakes were funded by the Engineering and Physical Sciences Research Council, UK: Healthcare Environment Control, Optimisation and Infection Risk Assessment (https://HECOIRA.leeds.ac.uk) (Grant Code: EP/P023312/1). M. López-García was funded by the Medical Research Council, UK (MR/N014855/1). J. Proctor was funded by EPSRC Centre for Doctoral Training in Fluid Dynamics at Leeds (Grant Code EP/L01615X/1). S.E. Abney was funded by a research assistantship from the US-Israel Binational Agricultural Research Development Fund and through a University of Arizona Graduate Access Scholarship.
References
- 1.Chia K., Coleman K., Tan Y., Ong S., Gum M., Lau S. Detection of air and surface contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in hospital rooms of infected patients. medRxiv. 2020 doi: 10.1101/2020.03.29.20046557. [DOI] [Google Scholar]
- 2.Fears A., Klimstra W., Duprex P., Hartman A., Weaver S., Plante K. Comparative dynamic aerosol efficiencies of three emergent coronaviruses and the unusual persistence of SARS-CoV-2 in aerosol suspensions. medRxiv. 2020 doi: 10.1101/2020.04.13.20063784. [DOI] [Google Scholar]
- 3.Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020 doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber A., Willeke K., Marchioni R., Myojo T., Mckay R., Donnelly J. Aerosol penetration and leakage characteristics of masks used in the health care industry. Am J Infect Control. 1993;21:167–173. doi: 10.1016/0196-6553(93)90027-2. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Environmental Protection Agency . US EPA; Washington, DC: 2011. Exposure factors handbook. EPA/600/R-09/052F. [Google Scholar]
- 6.Davies A., Thompson K.A., Giri K., Kafatos G., Walker J., Bennett A. Testing the efficacy of homemade masks: would they protect in an influenza pandemic? Disaster Med Public Health Prep. 2013;7:413–418. doi: 10.1017/dmp.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rengasamy S., Eimer B.C., Shaffer R.E. Comparison of nanoparticle filtration performance of NIOSH-approved and CE-marked particulate filtering facepiece respirators. Ann Occup Hyg. 2009;53:117–128. doi: 10.1093/annhyg/men086. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe T., Bartrand T.A., Weir M.H., Omura T., Haas C.N. Development of a dose–response model for SARS coronavirus. Risk Anal. 2010;30:1129–1138. doi: 10.1111/j.1539-6924.2010.01427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]