Dear Editor,
On 02 March 2020, a 36-year-old male came to the Emergency Department of Dr. Masih Daneshvari Hospital in Iran with a 3-day history of fever and dry cough. The patient was a physician with a history of close contact with COVID-19 cases. The patient had no underlying diseases and history of medicine usage.
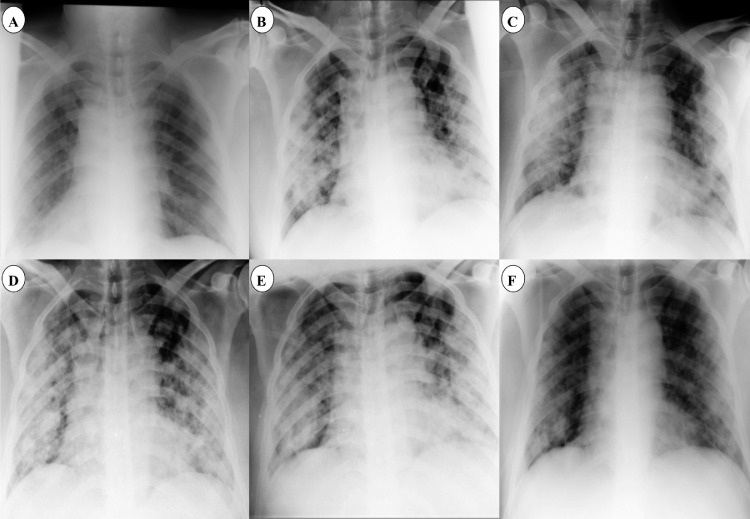
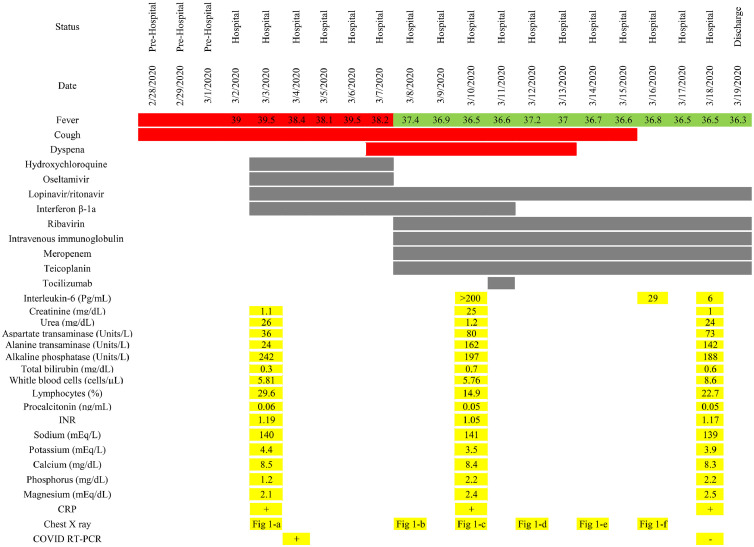
Physical examination revealed a body temperature of 39°C, blood pressure of 120/70 mmHg, heart rate of 90 beats/minute, and peripheral oxygen saturation of 92%; he exhibited no dyspnea. Laboratory results revealed a white blood cell count of 5.81 cells/µL with 29.6% lymphocytes; no other abnormality was seen. The patient's swab specimen tested positive for COVID-19 by reverse transcription polymerase chain reaction (RT-PCR) on 04 March 2020 (cycle threshold value 22.39) [1]. Chest X-ray imaging revealed bilateral lower lobe infiltration (Fig. 1 a). Hence, the patient was diagnosed with COVID-19 pneumonia. The following were commenced: hydroxychloroquine 200 mg p.o. twice a day, oseltamivir 75 mg p.o. twice a day, lopinavir/ritonavir 200/50 mg p.o. in two tablets twice a day, and interferon β-1a 12 million units s.c. every other day.
Fig. 1.
Chest X-ray of the patient during hospitalization. (a, b and c) Progression of lower and upper lobe infiltration. (d, e and f) Recovery of infiltration after tocilizumab administration.
On 08 March 2020 the clinical condition of the patient deteriorated, and he exhibited dyspnea with an oxygen saturation of 85%. Fever and cough were persistent, and new chest X-ray imaging revealed progression of bilateral infiltration in the lower and upper lobes (Fig. 1b). It was decided to initiate ribavirin 1,200 mg p.o. b.i.d. and immunoglobulin 20 mg i.v. daily. Meropenem and teicoplanin were also started to cover any probable bacterial sources. After 2 days, on 10 March 2020, his clinical condition worsened. Dyspnea continued with greater severity and he had an oxygen saturation of 83%. The ratio between the partial pressure of oxygen in arterial blood (PiO2) and the fraction of inspired oxygen decreased to 103 mmHg. Chest X-ray imaging did not show significant changes compared with the previous images (Fig. 1c), and the patient was a candidate for intubation and invasive mechanical ventilation. However, this did not happen. Tocilizumab was considered as a last-chance therapy. The patient's IL-6 level was checked and it was >200 pg/mL. QuantiFERON-TB testing was negative for Mycobacterium tuberculosis. Viral markers – including hepatitis B virus, hepatitis C virus, and human immunodeficiency virus – were reported negative. Hence, tocilizumab (Actemra Hoffmann-La Roche Limited) at a single dose of 400 mg was infused over 2 hours. The patient's vital signs were carefully checked during infusion to monitor any adverse effects.
After 2 days, the patient's dyspnea had gradually improved and his oxygen saturation increased to 90%. Chest X-ray imaging also showed less infiltration in comparison with previous imaging (Fig. 1d). Recovery was observed over the next few days, and dyspnea and oxygen saturation significantly improved. The IL-6 levels were checked and found to have decreased from 200 pg/mL to 29 pg/mL and then decreased to 6 pg/mL within a few days. Lung infiltration had remarkably improved in subsequent chest X-ray imaging (Fig. 1e and f). A swab specimen tested negative for COVID-19 by RT-PCR on 18 March 2020. After 18 days of hospitalization, the patient was discharged in an acceptable clinical condition. No bothersome dyspnea was noted, and oxygen saturation was 93% without supplemental oxygen. The timeline of vital signs, therapeutic regimens, and laboratory results are shown in Fig. 2.
Fig. 2.
Timeline of vital signs, therapeutic regimens, and laboratory results during hospitalization.
Cytokine release syndrome may be the underlying pathophysiology in refractory cases of COVID-19. Tocilizumab is a recombinant humanized monoclonal antibody developed against soluble and membrane-bound IL-6 receptors. Tocilizumab prevents the binding of IL-6 to its receptors and reduces the activity of the cytokine by competing with both the soluble and membrane-bound forms of its receptors [2]. The current case was a refractory COVID-19 case who did not respond to conventional therapeutic agents and had tocilizumab administered as salvage therapy. In contrast to hydroxychloroquine, tocilizumab may be a useful agent in severe cases who have not responded to conventional therapy (chloroquine/hydroxychloroquine and antivirals) and those patients with elevated levels of IL-6 [3]. Successful management of tocilizumab was also reported in recent literature. Hammami et al. reported COVID-19 in a liver transplant recipient who responded to tocilizumab therapy [4]. The promising role of tocilizumab has also been reported in pilot studies. Improvements in respiratory and laboratory parameters were also observed in those studies [5,6]. However, while tocilizumab is a promising agent against COVID-19, it is not an appropriate agent in patients with active or latent tuberculosis, bacterial and fungal infections, multi-organ failure, and gastrointestinal perforation [7].
In conclusion, tocilizumab may be considered a salvage therapeutic agent in COVID-19 patients who did not respond to other agents. Clinicians should be aware of the precautions and contraindications of tocilizumab, such as latent infection, and administer the drug with caution.
Declarations
Funding: No funding.
Competing Interests: None.
Ethical Approval: Written informed consent form obtained.
References
- 1.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang C, Wu Z, Li J-W, Zhao H, Wang G-Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Sevestre J, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med Infect Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammami MB, Garibaldi B, Shah P, Liu G, Jain T, Chen PH, et al. Clinical Course of COVID-19 in a Liver Transplant Recipient on Hemodialysis and Response to Tocilizumab Therapy: A Case Report. 2020. [DOI] [PMC free article] [PubMed]
- 5.Sciascia S, Apra F, Baffa A, Baldovino S, Boaro D, Boero R, et al. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin Exp Rheumatol. 2020 [PubMed] [Google Scholar]
- 6.Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID-19 patients with tocilizumab. PNAS. 2020 doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim CH, Chen H-H, Chen Y-H, Chen D-Y, Huang W-N, Tsai J-J, et al. The risk of tuberculosis disease in rheumatoid arthritis patients on biologics and targeted therapy: A 15-year real world experience in Taiwan. PloS One. 2017;12 doi: 10.1371/journal.pone.0178035. e0178035-e. [DOI] [PMC free article] [PubMed] [Google Scholar]