Abstract
Rapidly progressive osteoarthritis (RPO) is a rare condition which is poorly understood. Limited published literature is available. Reported here is a cohort of patients with RPO and acetabular bone loss who underwent primary THA. Risk factors, degree of acetabular bone loss and outcomes are presented. A typical case of RPO is described and investigations discussed. A retrospective audit was undertaken. 49 patients over an 18-year period were included. RPO patients were significantly older (P < 0.01) and had a lower BMI (P = 0.03). The mean acetabular bone loss in this cohort was 10.0 mm. Pathogenesis and risk factors for developing RPO remain unclear and future research is necessary. Repeat imaging in patients with deteriorating symptoms is important and urgent surgery is required maintain bone stock.
Keywords: Rapidly progressive arthritis, Total hip arthroplasty, Outcomes
Introduction
Rapidly progressive osteoarthritis (RPO) of the hip remains a poorly understood clinical entity. Its unpredictable behavior can make it difficult to diagnose early meaning that intervention is often later than ideal, with surgically challenging acetabular bone loss. There are multiple descriptive terms for this condition, such as rapidly progressive coxarthrosis, rapidly destructive osteoarthritis, or rapidly progressive hip disease, thus adding to the confusion. The incidence in the current literature has been reported to be as high as 18% [1,2].
Despite being described as early as 1957 by Forestier [3], the etiology and pathophysiology remain unclear. Numerous mechanisms and triggers for the rapid deterioration in bone stock and symptoms have been proposed. These include the use of nonsteroidal anti-inflammatory drugs (NSAIDs), osteoporotic insufficiency fractures, intra-articular steroid injections, as well as anatomic abnormalities such as inverted labral tissue and posterior pelvic tilt [2,[4], [5], [6]]. Other studies have reported raised levels of proteolytic enzymes and inflammatory markers in the synovial fluid of patients with RPO [7].
RPO has been defined as >5 mm cartilage and bone loss per year. [8] Loss of bone from both the femoral head and acetabulum can occur in these patients. RPO was classified into types 1 to 3 based on the work by Lequesne and Amouroux [9], Postel and Kerboull [10], and Ranieri and Toni [11]. It depends on the amount of bone loss and the time period over which this takes place [[9], [10], [11]].
-
•
Type 1 (rapid): 18 months of chondrolysis followed by 10-15 mm bone loss/year.
-
•
Type 2 (moderate): 18-30 months of chondrolysis followed by bone loss of 5-10 mm/year.
-
•
Type 3 (delayed): normal progression of osteoarthritis for 3-5 years before sudden deterioration into types 1 or 2.
A previous study by Thompson et al [1] identified most acetabular wear patterns to be anterosuperior and superolateral, with superomedial and central patterns being much less common. Complex primary THA includes patients with acetabular bone loss or defects regardless of initial diagnosis. These patients have been shown to be more technically challenging with a higher complication rate [[12], [13], [14]].
The purpose of this audit was to report on a cohort of patients with RPO and acetabular bone loss. We quantified the amount of bone loss using a calibrated preoperative anteroposterior radiograph and report on the outcomes and survivorship for these patients. We have also described the surgical technique that has allowed us to manage these patients with a primary acetabular component and without screw fixation or augmentation.
We have also reported on patient demographics and looked at the recognized risk factors for the development of RPO such as use of NSAIDs and osteoporosis.
Case history
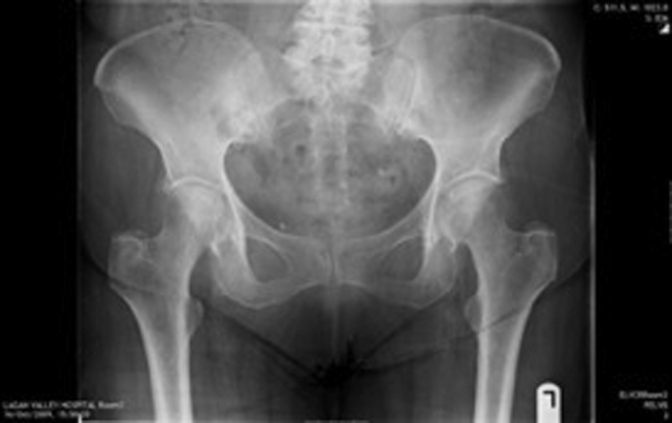
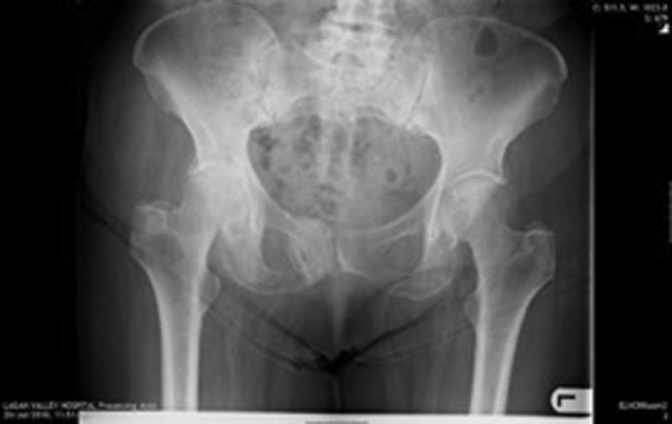
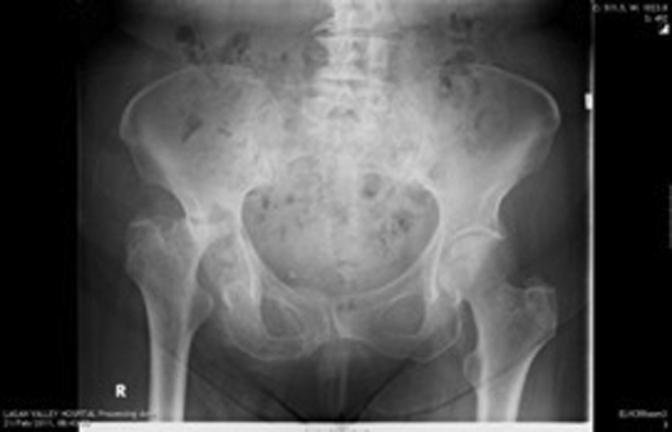
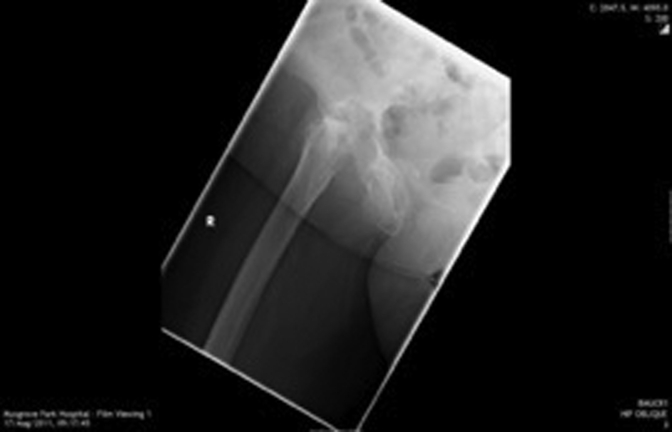
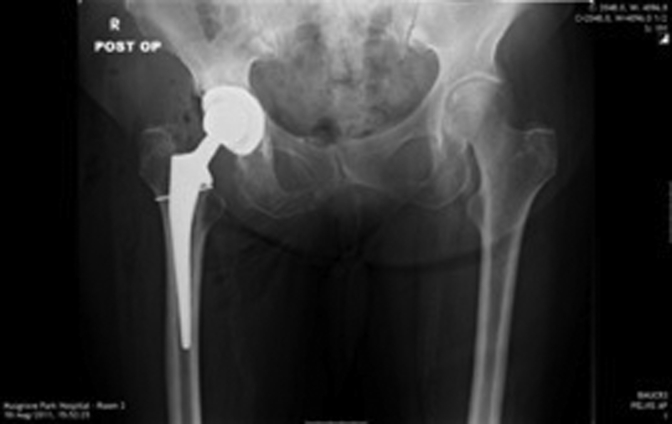
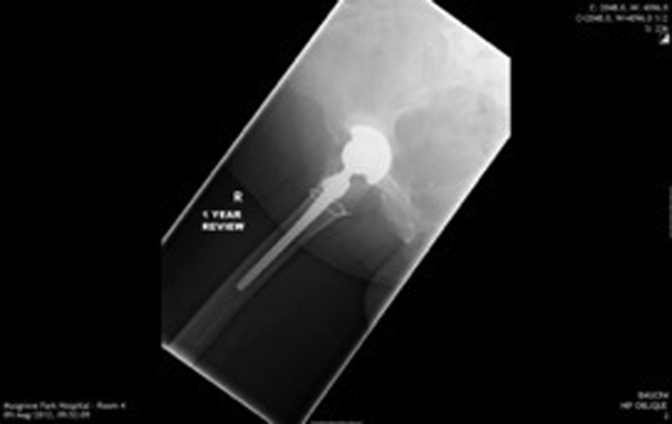
Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 show radiographs of an 81-year-old female patient with type 1 RPO. She initially attended with groin pain (Figure 1, Figure 2), and at presentation to our unit, significant destruction of the acetabulum and femoral head was noted (Figure 3, Figure 4). Inflammatory markers were normal; she had no inflammatory arthropathy or risk factors for avascular necrosis (AVN). She proceeded to have a cementless THA with no acetabular augments. Figure 5 shows her initial postoperative radiograph, with her 1-year lateral film shown in Figure 6. Differential diagnoses for this presentation would have included AVN, subchondral insufficiency fracture, transient osteoporosis, or infection. Inflammatory markers (C reactive protein [CRP] and erythrocyte sedimentation rate [ESR]) were normal, but differentiating between the other possible diagnoses can be difficult. Nelson et al [15] described how to use plain radiographs to determine the likelihood of RPO compared with AVN or subchondral insufficiency fracture particularly using the Tonnis angle [15].
Figure 1.
Patient presenting with right groin pain.
Figure 2.
Patient referred to orthopaedic team.
Figure 3.
Significant destruction of femoral head and acetabulum on AP radiograph.
Figure 4.
Significant destruction of femoral head and acetabulum on lateral radiograph. Note anterior wear pattern.
Figure 5.
Post operative AP radiograph.
Figure 6.
One year lateral radiograph.
Although not performed in this case, magnetic resonance imaging imaging is now likely to be performed in most cases of suspected RPO. Sugano et al [16] described the early magnetic resonance imaging changes and suggested a small subchondral area of low signal intensity on T1W1 and inhomogeneous high signal intensity on T2W1 in the lateral portion of the femoral head could be an early predictor of disease. They also noted joint effusion, and in cases with more advanced disease, they noted an increased amount of bone edema in the femoral head, neck, and acetabulum. Although it remains difficult to differentiate AVN and RPO in the early stages, this and other studies have also noted that the band-like pattern of low signal on T1W1 and high signal on T2W1 observed in early AVN is not seen in patients with RPO [15,16].
Discussion
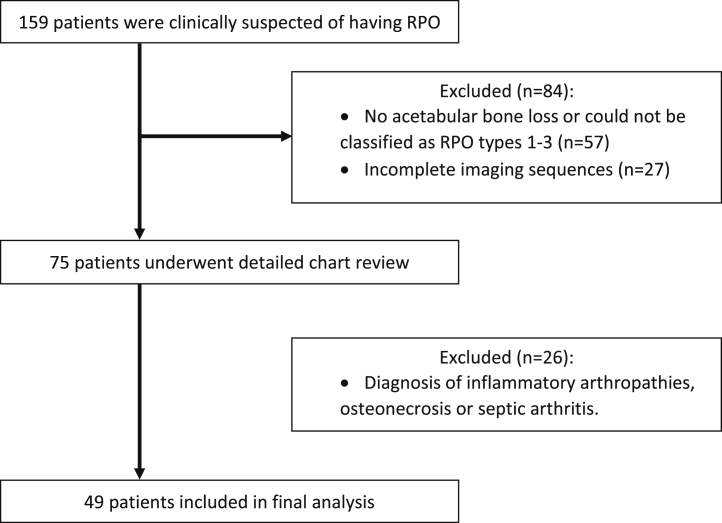
A retrospective audit (Belfast Health and Social Care Trust audit number 5733) was undertaken of all patients with a diagnosis of RPO who had a THA under the care of the senior author, from 1999 to 2017. Patients were included from a prospectively maintained database in whom the senior author at the time of surgery had considered RPO to be a potential diagnosis. A total of 159 patients were identified after interrogation of the database looking for a diagnosis of RPO. Imaging (electronic and hard copies including serial radiographs) results were reviewed by 2 authors (P.K. and A.W.), followed by detailed chart review. Inclusion criteria for the audit are outlined in the consort diagram (Fig. 7).
Figure 7.
Patient selection criteria for RPO audit. RPO, rapidly progressive osteoarthritis.
Baseline demographic details (age, sex, body mass index [BMI], preoperative and postoperative Oxford hip score, 90-day mortality, overall mortality, and waiting times to surgery) were available for all patients.
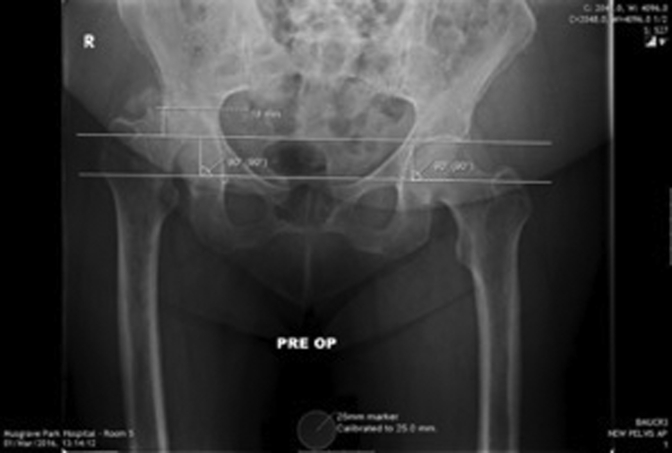
Acetabular bone loss was measured by drawing 2 parallel lines between the sourcil and teardrop on a calibrated preoperative anteroposterior radiograph (as shown in Fig. 8). This was carried out independently by both the authors on 29 patients with electronic imaging to assess interobserver variability, accuracy, and reproducibility. After this, 20 patients with hard-copy images were measured in the same manner. Patients with 1-4 mm of acetabular bone loss were included if they had >5 mm of femoral bone loss.
Figure 8.
Measuring superior acetabular bone loss.
A detailed chart review identified preoperative medications such as NSAIDs, where available inflammatory markers (CRP and ESR) were obtained from chart review to exclude infection. There is good evidence to support their use in ruling out infection preoperatively [17]. Although not required in this cohort, aspiration or rarely intraoperative frozen section and soft-tissue sampling remain the best way to rule out infection [18]. Administration of blood products and histopathology results were also noted from chart review.
Electronic care records and regional imaging systems were also reviewed to ensure no patients underwent revision surgery elsewhere in Northern Ireland (NI).
Basic demographics and outcomes for the RPO cohort were compared with patients without RPO undergoing THA under the care of the senior author. Data for these patients were also obtained from a prospectively maintained database and/or previous published work.
Statistical analysis was carried out using SPSS (version 22, IBM Armonk, NY); all data were assessed for normality using the Shapiro-Wilk test. The chi-squared test was used to compare categorical variables. For nonparametric continuous variables, the Kruskal-Wallis test was used. Statistical significance level was set at P < .05.
Surgical technique
The preparation and surgical technique used for these patients are described in the following paragraphs. Using this technique, standard solid-back acetabular components were used for all patients despite extensive acetabular bone loss in some cases.
Preoperatively, a lateral radiograph will inform the surgeon as to the degree of anterior acetabular destruction. Intraoperatively, during exposure of the acetabulum, it is critical to identify the transverse acetabular ligament (TAL). In RPO, the TAL is invariably present but is often difficult to locate as it is a grade 2 TAL, defined as being covered by soft tissue [19]. Palpating with an index finger can be a helpful aid in confirming its location. Once the TAL is exposed, any abnormal soft tissue is excised from the floor to facilitate acetabular preparation. There may be residual posterior articular cartilage noted, in keeping with the predominant wear patterns mentioned previously. The first acetabular reamer should be a minimum of 3 mm smaller than the premorbid estimated femoral head diameter. This can usually be obtained from the opposite hip or from an earlier calibrated radiograph of the operated hip if the head is also destroyed.
It is important that the first and subsequent reamers are held firmly against the TAL and posterior wall. When this technique is adhered to, the surgeon will observe a large anterosuperior gap which is to be expected in RPO. With successive reaming, the gap becomes smaller, and the surgeon should stop when the appropriately sized reamer starts to touch the anterior wall. The anterior wall is characteristically thin and should not be reamed. In our experience, this prepared acetabular surface will now provide enough bone to obtain satisfactory cup fixation. Historically, a spiked cup has been used (Pinnacle 300 Series, titanium shell with Porocoat (Depuy Synthes, Warsaw, IN) but more recently Gription (titanium, with a different surface finish giving a higher coefficient of friction). We have never used screws with our described technique, although many surgeons would wish to do so. We have been content with 70% cup cover, provided the cup is stable. By stable meaning that when a gentle superior-inferior motion is applied with the index finger and thumb to the cup handle, the cup remains stable. The cup should not be tested in an anterior-posterior direction as that can give a false impression of stability because the cup is squeezed by the anterior and posterior walls. In our experience, this degree of fixation has been adequate and is borne out by the outcomes of this cohort.
Results
The 49 patients included were compared with 8041 THAs carried out by the senior author over the same time period. There was no significant difference in the gender of patients with RPO; the female-to-male ratio was 1.58:1 compared with 1.26:1 (P = .43). Patients with RPO were significantly older, with a mean age of 72.7 years (range 51-88) compared with 68.8 years (range 17-96, P = .006). The BMI was statistically significantly lower in the non-RPO group, with a mean of 28.1 compared with 29.1 (P = .03). A right-to-left ratio of 2.5:1 was also noted in the RPO group. Normal preoperative inflammatory markers (CRP and/or ESR) were available for 45 patients. The remaining 4 patients had a detailed note review (including clinic and theater notes) to ensure no suspicion of infection remained.
Twenty patients were of RPO type 1, 18 type 2, and 11 type 3. There was no difference in outcome or bone loss between the 3 groups. The mean acetabular bone loss as measured was 10.0 mm (range 1-29). Twenty-two patients had 10 mm or greater acetabular bone loss. Thirty-two patients (65.3%) were using NSAIDs for analgesia before their surgery. This was compared with a previous audit of 1000 joint replacements under the care of the senior author, which included 487 THAs; of these, only 30% used NSAIDs before surgery (P < .001). Only 3 patients (6.1%) had a recorded diagnosis of osteoporosis preoperatively. Measurement of acetabular bone loss by both authors produced an interobserver error of 0.97.
The mean time to surgery from boarding was 19 weeks (range 2-79 weeks). Ten patients were boarded as routine (a mean waiting time of 33 weeks), and 31 were boarded as urgent (a mean waiting time of 14 weeks). Information on the boarding status was not recorded for 8 patients. Of the 10 patients boarded as routine, 7 had no evidence of bone loss on booking radiographs. All subsequently developed bone loss while awaiting surgery (mean of 9.1 mm). The remaining 3 patients had no acetabular bone loss but did have femoral head bone loss and by the time of surgery also had acetabular bone loss.
The operative time was significantly longer in the RPO group, with a mean operative time of 61.7 minutes (range 39-87) compared with 57.7 minutes (range 24-225) for all other THAs (P = .01). Preoperative Oxford hip scores were available for 43 patients; the mean score was 7.7 (range 1-15). Postoperative Oxford hip scores at 1 year were available for 42 patients, with a mean score of 36.6 (range 6-48). Overall, 19 patients (38.8%) required blood transfusion postoperatively; 9 patients required 1 unit; 6 patients, 2 units; 2 patients, 3 units; and 2 patients, 4 units.
The mean length of stay was 5.9 days (range 1-34). No patients in this cohort died within 90 days of surgery. To date, 11 (22.4%) patients have died with a mean time to death of 7.7 years (range 1.7-16.2). The mean follow-up time postoperatively was 2.3 years (range 61 days-12.9 years).
All patients in this cohort had a primary cementless acetabular cup without screws or augments, 39 of the stems were cementless and the remainder cemented. Then, the mean cup size was 54 mm, with a range of 48-64 mm.
To date, 3 patients have been revised with no more planned. One patient had early cup loosening and periacetabular fracture after a fall on day 10. This patient had dementia and other comorbidities and was converted to a hip resection arthroplasty. The second had a washout with liner and head exchange at day 21 for infection with no recurrence to date (2 years from washout). The third patient had a stem-only revision at 13.3 years for a periprosthetic fracture around a loose cemented stem.
Histopathology results were only available for 3 patients, and all identified features were consistent with RPO with no evidence of infection or acute inflammation. Areas of severe nonspecific degenerative change were seen with minimal or no osteonecrosis. There was minimal reparative response in any of the specimens.
Current controversies and future considerations
We have reported outcomes for 49 patients with RPO and acetabular bone loss undergoing THA. Our demographic data are in keeping with other studies [1,8,13]. Patients with RPO were significantly older (mean 72.7 years compared with 68.8 years, P = .006), with significantly lower BMI (mean 28.1), and although the proportion of female patients in the RPO group was higher, this was not statistically significant (P = .43).
The pathogenesis and etiology of this condition remain unclear. We did note a significantly higher use of NSAIDs in the RPO cohort (P < .001). Some studies have implicated these drugs in the formation of RPO, suggesting they impair bone turnover [1,20]; however, this has been challenged by other literature [21]. Intra-articular steroid injections have also been linked to RPO [2]. To our knowledge, no patient in this cohort underwent steroid injection preoperatively. The classification system described by Lequense and Amouroux [9] has not previously been shown to be clinically relevant. We also noted no difference in patients of types 1-3.
It has been proposed that osteoporosis or osteopenia with subsequent subchondral fracture leads to the early and dramatic collapse of the femoral head [22]. A study by Okano et al [23], however, showed no difference in bone mineral density between patients with RPO and those with typical osteoarthritis [24]. Our results concur with this as there was no significant difference in the number of female patients, and only 3 patients of 49 had a confirmed diagnosis of osteoporosis.
The importance of urgent surgery has been previously highlighted to minimize loss of acetabular bone stock, which can increase the complexity of the primary surgery [8]. Acetabular defects have been linked to poorer results and decreased survivorship of THA [12]. Within our study, only 60% of patients were boarded urgently for surgery, with a mean waiting time of 19 weeks. Importantly, of the 10 patients boarded as routine, 7 had no bone loss on booking radiographs and all subsequently developed acetabular bone loss. Irwin et al [8] previously described the importance of identifying patients “at risk” of developing RPO. However, no robust features have been borne out in the literature other than the absence of osteophytes giving rise to the name the “atrophic hip” [8,15], as can be seen in Figure 1, Figure 2, Figure 3, Figure 4. Zazgyva et al [24] attempted to describe a new grading system and classification for patients to identify patients with RPO, but this has not been validated outside of their study. As a result, most cannot be predicted, and therefore inevitably, some patients will come to harm on long waiting lists. Consequently, it is important that patients on a long waiting list have repeat radiographs if there is a reported deterioration in symptoms.
Dealing with acetabular bone loss in primary THA remains a challenge with various techniques having been described, such as the use of acetabular screws through to more complex customized acetabular components [13,25]. As we have shown, the amount of acetabular bone loss in these patients can be significant (45% > 10 mm). It has been the practice of our senior author for more than 2 decades to implant a solid-back acetabular shell without screws or supplementary fixation. There has only been 1 loss of cup fixation in the series as a result of a fall on day 10. These results are comparable with those of other published studies of patients undergoing THA under the care of the senior author [26,27]. Preoperative and postoperative Oxford hip scores at 1 year also confirm successful outcomes for most patients. Other studies have concluded that patients with RPO perform similarly after arthroplasty to patients with primary osteoarthritis undergoing THA [28,29].
The rate of blood transfusion within this cohort was much higher than would be expected today with 38% of patients requiring at least 1 unit of packed red cells postoperatively. Comparatively, a recent publication by Magill et al [30] in 2018 revealed a transfusion rate of 12.5% in 393 patients undergoing THA under the care of the senior author. It is important to note that the rate of transfusion after THA was historically much higher over the past decade than that in the present day. The reasons for this are multifactorial and certainly not confined to our unit. Given the study time period, this may in part explain the higher transfusion rate. We have also shown a significant difference in operative time in RPO cases (mean: 61.7 minutes, P = .01) which would also influence blood loss and transfusion rate. This is also reported by other studies [1]. There is insufficient evidence to support the inflammatory component of the disease process as a reason for increased blood loss [14,22].
There are limitations to this study, one being the lack of histopathology results. Although it confirmed the diagnosis in 3 of our patients, ideally it would have been confirmed in all cases. Quite frequently, the senior author did not send specimens to histopathology if the diagnosis of RPO was clear cut. Other significant forms of arthropathy have largely been excluded by the detailed chart review and preoperative blood results where available. We detected a low rate of osteoporosis in this cohort but accept that, especially at the start of the study period, screening was likely to be inconsistent. A further limitation is the potential loss of patients to follow-up with a mean follow-up time of 2.3 years. This was reduced by reviewing electronic care records and imaging systems for all trusts throughout NI, as a means to ensure no revisions took place within NI without our knowledge. A recent publication by Cassidy et al [31] regarding follow-up for patients who underwent THA under the care of the senior author has described how loss of follow-up in our clinical environment is likely to be minimal.
This study has shown the potential for extensive acetabular bone loss in patients with RPO. We have described our technique of acetabular preparation, which allows the use of primary acetabular components despite considerable bone loss. We have experienced no late cup loosenings to date with 1 early loosening after a fall.
Summary
The pathogenesis and etiology of RPO remain unclear. The current evidence is limited because of the rarity of the condition and the retrospective nature of the studies. It is unlikely that there will be a clear etiologic cause identified soon, and therefore, a key area of further research is the identification of patients at risk of developing RPO. Determining clinical and/or imaging features that would raise the possibility of RPO could be crucial in guiding further management and preventing extensive bone loss.
These patients require urgent surgery, most importantly to maintain bone stock. In areas with long waiting lists, patients with worsening clinical symptoms or imaging suspicious for RPO should have repeat imaging to ensure no further loss of bone stock.
Funding
The authors wish to acknowledge the Belfast Arthroplasty Research Trust (BART), United Kingdom for funding and support of this project.
Conflict of interest
David Baverland reports speakers bureau/paid presentations and paid consultancy for DePuy Synthes and research support from DePuy Synthes and Zimmer Biomet. All other authors declare no potential conflicts of interest.
For full disclosure statements refer to https://doi.org/10.1016/j.artd.2020.04.005.
Supplementary data
References
- 1.Thompson N., Corr A.M., Geddis C.J. Rapidly Progressive osteoarthrosis of the hip. Hip Int. 2004;14(4):217. doi: 10.1177/112070000401400401. [DOI] [PubMed] [Google Scholar]
- 2.Hess S.R., O’Connell R.S., Bednarz C.P. Association of rapidly destructive osteoarthritis of the hip with intra-articular steroid injections. Arthroplasty Today. 2018;4(2):205. doi: 10.1016/j.artd.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forestier F. 1957. Coxite rhumatismales subaigues et chnoniques. [Thesis]. Paris. [Google Scholar]
- 4.Yamamoto T., Bullough P.G. Subchondral insufficiency fracture of the femoral head: a differential diagnosis in acute onset of coxarthrosis in the elderly. Arthritis Rheum. 1999;42(12):2719. doi: 10.1002/1529-0131(199912)42:12<2719::AID-ANR31>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe W., Itoi E., Yamada S. Early MRI findings of rapidly destructive coxarthrosis. Skeletal Radiol. 2002;31(1):35. doi: 10.1007/s00256-001-0445-0. [DOI] [PubMed] [Google Scholar]
- 6.Newman N.M., Ling R.S.M. Acetabular bone destruction related to nonsteroidal anti-inflammatory drugs. Lancet. 1985;326(8445):11. doi: 10.1016/s0140-6736(85)90058-3. [DOI] [PubMed] [Google Scholar]
- 7.Komiya S., Inoue A., Sasaguri Y. Rapidly destructive arthropathy of the hip. Studies on bone resorptive factors in joint fluid with a theory of pathogenesis. Clin Orthop Relat Res. 1992;284:273. [PubMed] [Google Scholar]
- 8.Irwin L.R., Roberts J.A. Rapidly progressive osteoarthrosis of the hip. J Arthroplasty. 1998;13(6):642. doi: 10.1016/s0883-5403(98)80007-7. [DOI] [PubMed] [Google Scholar]
- 9.Lequesne M., Amouroux J. La coxarthrose destructice rapide. Presse Med. 1970;78(32):1435. [PubMed] [Google Scholar]
- 10.Postel M., Kerboull M. Total prosthetic replacement in rapidly destructive arthrosis of the hip joint. Clin Orthop Relat Res. 1970;72:138. [PubMed] [Google Scholar]
- 11.Ranieri L., Toni A. L’artrosi a rapida evoluzione dell’anziano. Un’entita nosologica. Chir Organi Mov. 1979;65(3):261. [PubMed] [Google Scholar]
- 12.Kobayashi S., Eftekhar N.S., Terayama K. Risk factors affecting radiological failure of the socket in primary Charnley low friction arthroplasty. A 10- to 20-year follow up study. Clin Orthop Relat Res. 1994;306:84. [PubMed] [Google Scholar]
- 13.Boisgard S., Descamps S., Bouillet B. Complex primary total hip arthroplasty. Orthop Traumatol Surg Res. 2013;99S:S34. doi: 10.1016/j.otsr.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Batra S., Batra M., McMurtrie A. Rapidly destructive osteoarthritis of the hip joint: a case series. J Orthop Surg Res. 2008;3:3. doi: 10.1186/1749-799X-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson F.R., Bhanarkar V.S., Woods T.A. Using hip measures to avoid misdiagnosing early rapid onset osteoarthritis for osteonecrosis. J Arthroplasty. 2014;29:1243. doi: 10.1016/j.arth.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Sugano N., Ohzono K., Nishii T. Early MRI findings of rapidly destructive coxarthropathy. Magn Reson Imaging. 2001;19:47. doi: 10.1016/s0730-725x(01)00221-1. [DOI] [PubMed] [Google Scholar]
- 17.Huerfano E., Bautista M., Huerfano M. Screening for infection before revision hip arthroplasty: a meta – analysis of likelihood ratios of erythrocyte sedimentation rate and serum C reactive protein levels. J Am Acad Orthop Surg. 2017;25:809. doi: 10.5435/JAAOS-D-16-00642. [DOI] [PubMed] [Google Scholar]
- 18.Lum Z., Shieh A., Meehan J. Native adult hip with bacterial septic arthritis. BJS Rev. 2018;6(10):e2. doi: 10.2106/JBJS.RVW.17.00211. [DOI] [PubMed] [Google Scholar]
- 19.Archbold H.A.P., Mockford B., Molloy D. The transverse acetabular ligament: an aid to orientation of the acetabular component during primary total hip replacement. A preliminary study of 1000 cases investigating post - operative stability. J Bone Joint Surg Br. 2006;88-B(7):883. doi: 10.1302/0301-620X.88B7.17577. [DOI] [PubMed] [Google Scholar]
- 20.Rønningen H., Langeland N. Indomethacin treatment in osteoarthritis of the hip joint. Acta Orthop Scand. 1979;50(2):169. doi: 10.3109/17453677908989752. [DOI] [PubMed] [Google Scholar]
- 21.Flemming D., Gustas-French C. Rapidly progressive osteoarthritis: a review of the clinical and radiological presentation. Curr Rheumatol Rep. 2017;19(7):42. doi: 10.1007/s11926-017-0665-5. [DOI] [PubMed] [Google Scholar]
- 22.Mavrogenis A.F., Flevas D.A., Panagopolous G.N. Rapid destructive arthritis of the hip revisited. Eur J Orthop Surg Traumatol. 2015;25(7):1115. doi: 10.1007/s00590-015-1676-4. [DOI] [PubMed] [Google Scholar]
- 23.Okano K., Aoyagi K., Enomoto H. Bone mineral density in patients with destructive arthrosis of the hip joint. J Bone Miner Metab. 2014;32(3):312. doi: 10.1007/s00774-013-0501-6. [DOI] [PubMed] [Google Scholar]
- 24.Zazgyva A., Gurzu S., Gergely Clinico-radiological diagnosis and grading of rapidly progressive osteoarthritis of the hip. Medicine. 2017;96(12):e6395. doi: 10.1097/MD.0000000000006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuasa T., Maezawa K., Nozawa M. Midterm outcome of total hip arthroplasty for rapidly destructive coxarthrosis. J Orthop Surg (Hong Kong) 2016;24(1):27. doi: 10.1177/230949901602400108. [DOI] [PubMed] [Google Scholar]
- 26.O’Brien S., Wilson R., Hanratty B. The cemented femoral custom stem – a 10-year review. Hip Int. 2007;17(4):194. doi: 10.1177/112070000701700402. [DOI] [PubMed] [Google Scholar]
- 27.Magill P., Blaney J., Hill J. Impact of a learning curve on the survivorship of 4802 cementless total hip arthroplasties. Bone Joint J. 2016;98-B(12):1589. doi: 10.1302/0301-620X.98B12.BJJ-2016-0203.R1. [DOI] [PubMed] [Google Scholar]
- 28.Charrois O., Kahwaji A., Rhami M. Outcome after total hip arthroplasty performed for rapidly progressive hip destruction. Rev Chir Orthop Reparatrice Appar Mot. 2002;88(3):236. [PubMed] [Google Scholar]
- 29.Kuo A., Ezzet K., Patil S. Total hip arthroplasty in rapidly destructive osteoarthritis of the hip: a case series. HSS J. 2009;5(2):117. doi: 10.1007/s11420-009-9112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magill P., Cunningham E., Hill J. Identifying the period of greatest blood loss after lower limb arthroplasty. Arthroplasty Today. 2018;4(4):499. doi: 10.1016/j.artd.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cassidy R., O hEireamhoin S., Beverland D. Guidelines for the follow up of total hip arthroplasty. Do they need to be revised? Bone Joint J. 2019;101-B(5):536. doi: 10.1302/0301-620X.101B5.BJJ-2018-0853.R2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.