Abstract
Purpose
Pseudocirrhosis is a radiological term used to describe rapid changes in the contour of liver invaded by metastases and treated with chemotherapy. Our primary objectives were to analyse the clinical and biological characteristics of those patients with breast cancer and to assess the prevalence of complications generally associated with decompensated cirrhosis. We have also assessed associated treatments and response.
Methods
This retrospective study included all women with metastatic breast cancer to the liver who had imaging protocols describing diffuse liver contour abnormalities during systemic treatment between 2003 and 2018 in our centre. The following were identified: neoplastic characteristics, complications presented, treatments administered and response.
Results
48 patients were included. There was a trend towards an increased proportion of luminal cancers (88.2%, n=30, p=0052) when compared with our hospital cancer registry. Most patients (97.9%, n=47) had a widespread liver invasion, 58.3% (n=28) had ascites on physical examination; 90% (n=18) of ascites were classified as transudate. Nearly 23% (n=11) of patients had oesophageal varices and 6.5% (n=3) had an episode of variceal rupture. At the time of the appearance of liver contour abnormalities, the most frequently used molecules were: 5-fluorouracil (22.9%; n=11) and cisplatin (18.8%; n=9). A partial response was observed in 52.1% (n=25) of patients.
Conclusion
This is the largest reported series of patients with pseudocirrhosis. Many patients developed complications related to portal hypertension and liver failure, similar to those observed in decompensated cirrhosis. Luminal subtypes could be over-represented. In our series, pseudocirrhosis appears to develop at the expense of extensive liver disease burden and most often under 5-fluorouracil, or its derivatives, with or without cisplatin, possibly following a response to treatment.
Keywords: complication, liver, breast cancer, pseudocirrhosis
Key questions.
What is already known about this subject?
It has been mainly described in cases reports and little is known about the patients in which pseudocirrhosis occurred and the complication they presented.
What does this study add?
This study, by reporting the largest reported series of patients, provides a clinical picture of pseudocirrhosis, by highlighting in which patients it occurred and their clinical evolution.
How might this impact on clinical practice?
By helping clinicians to make an early diagnostic of pseudocirrhosis this study will allow a better prevention of its complications and hopefully reduce the burden of this disease.
Introduction
After bone, liver is one of the most common sites of breast cancer metastasis.1 A currently unknown fraction of women will present, following one or more treatment lines, a modification of the macroscopic aspect of the liver, which may suggest an image of liver cirrhosis. The liver of these patients has a radiological and macroscopic appearance most often described as irregular, dysmorphic, lobular or nodular and at first sight compatible with a diagnosis of cirrhosis.2 Furthermore, this presentation is accompanied by complications related to portal hypertension (PH) such as ascites and oesophageal varices (EV), but also related to liver failure and may lead to death of patients (figure 1). Currently, 26 articles listed in online supplementary table S-1 have been published, most of the cases described, 120 cases out of 126 were breast cancer patients.
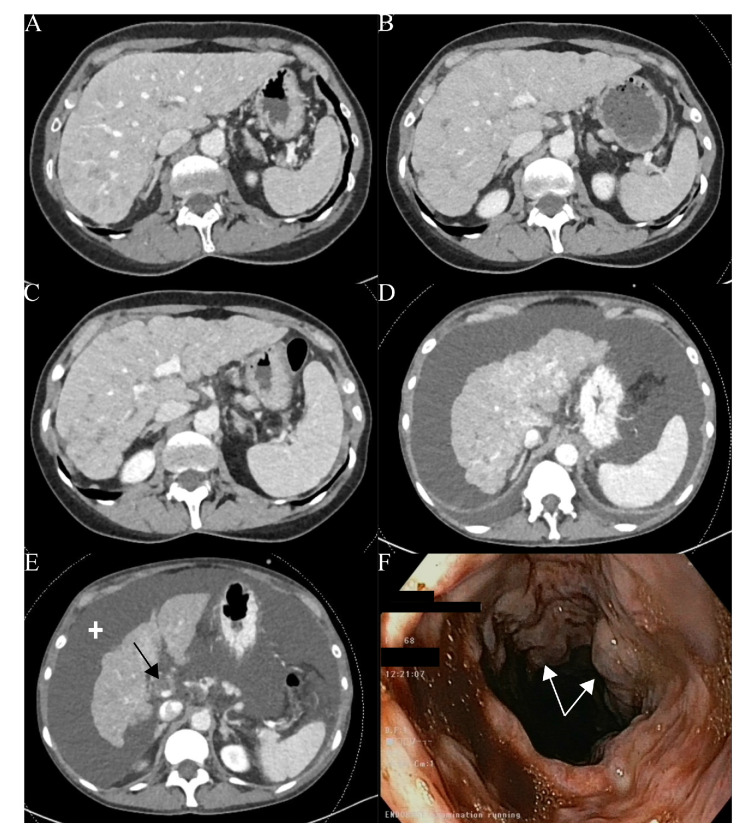
Figure 1.
Patient diagnosed with a ductal mammary luminal adenocarcinoma metastatic to the liver and initially treated with a CDK4/6 inhibitor (A). Liver contour abnormalities appear a few months after the initiation of a second line of treatment (B) and continue to develop later (C). The patient receives a third line with persisting changes in hepatic morphology (D) and development of partial portal thrombosis (black arrow) and ascites (cross) requiring supportive treatment (E). Finally, the patient will present an episode of haematemesis and the digestive endoscopy will reveal (F) grade III oesophageal varices (white arrows). The patient died a few days later.
esmoopen-2020-000695supp001.pdf (171.9KB, pdf)
We will use the term pseudocirrhosis, introduced in 1994 by Young et al3 defined as ‘a radiological term used to describe the development of a diffuse nodular aspect of the liver in the context of liver metastases treated with chemotherapy’.4 Other terms have been used, such as ‘Hepar lobatum carcinomatosum’,5–7 although they do not always overlap the same entity. Pseudocirrhosis differs from liver cirrhosis due to the absence of typical histological lesions such as bridging fibrosis between regenerating nodules of hepatocytes4 and a faster evolution. It is likely that pseudocirrhosis constitutes a broad spectrum of pathophysiological mechanisms leading to the same clinical picture. Existing theories include toxicity of systematic therapies (eg, nodular regenerative hyperplasia) and architectural changes in response to the infiltrating tumour.3 5 8 It is considered as resulting from a combined effect of treatment and metastasis. Currently, there are no clear criteria for diagnosis, which is done solely on the basis of imaging.4
Our primary objectives, for conducting this retrospective study, were among patients with metastatic breast cancer to the liver and selected on the basis of the development of diffuse abnormalities of the hepatic contour appearing during systemic treatment and thus compatible with pseudocirrhosis, to:
Describe the clinical and biological characteristics of these patients.
Determine the prevalence of complications generally associated with decompensated liver cirrhosis.
Our secondary objective was to report the treatments administered when liver contour abnormalities appear and the response to them.
Methods
This is a retrospective study based on the medical files of the Jules Bordet Institute. Patients with hepatic imaging suggestive of pseudocirrhosis were first identified by means of an automated textual search of medical records, selected on the basis of a series of keywords (online supplemntary table S-2) and then manually reviewed. This research was conducted on documents belonging to the medical files dated between January 2003 and December 2018.
Participants
We included women with metastatic breast cancer to the liver under systemic therapy and with at least two consecutive imaging protocols (CT scan or MRI) describing a liver contour abnormality generalised to the entire liver. Only the problematic images (positron emission tomography or missing protocols) were reviewed by a radiologist. We also included patients where these abnormalities were described only on the last imaging before their death. Liver contour abnormalities were defined as the presence of at least one of the following terms: contours or liver of nodular or lobular appearance; irregular contours; dysmorphic liver; cirrhotic appearance; cirrhosis; pseudocirrhosis; hepar lobatum carcinomatosum.
Patients were excluded if liver imaging abnormalities were present before the onset of metastases, no reference imaging was available before the onset of liver contour abnormalities, a second neoplasia developed before the onset of liver metastases. In addition, patients with missing follow-up data or treated in another centre were excluded.
Variables
We collected data on age at diagnosis, neoplastic characteristics of the patients, date of diagnosis of the primary tumour, and dates and sites of the various relapses. The neoplastic characteristics were compared with the data from our hospital cancer registry between 2010 and 2017. The liver disease burden was divided in two groups: one with five or fewer hepatic lesions, the other with more than five hepatic lesions or a diffuse invasion. The maximum number of hepatic lesions during the disease was retained. In order to describe the clinical and biological picture, we based ourselves on the natural history of cirrhotic patients.9–11 Data retained for analysis were: the presence of vascular signs of PH on CT scans (enlargement of paraumbilical and presence of collateral veins); endoscopic EV and rupture of EV; ascites on imaging and physical examination, protein and cytological content of ascites, treatment modalities of ascites; spontaneous bacterial peritonitis (SBP) (>250 neutrophils per mm³ of ascites without any intra-abdominal surgically treatable source of infection); splenomegaly on imaging; encephalopathy without any other cause than a liver origin; partial or complete portal vein thrombosis. Coagulation disorders (PT <70%), hyperbilirubinaemia (total bilirubin >2 mg/dL), dilated bile duct and hypoalbuminemia (serum albumin <35 g/L) have also been reported. All these variables were collected only after the first description of the hepatic contour abnormalities. For the biological variables, these required two abnormal results except for SBP. Biological data at the onset of the first liver contour abnormalities were also collected.
Furthermore, we collected data on treatment in progress when hepatic contour abnormalities appeared (considered as the last line administered if no ongoing treatment), the previous line of treatment and the response of hepatic metastases to these two lines according to CT scan or MRI protocols classified as complete response, partial response, stable or progression. The best response obtained during each line was selected. The molecules administered in combination were dissociated for analysis.
Frequent aetiologies of liver cirrhosis such as alcohol abuse (>20 g alcohol per day) and viral hepatitis serologies have also been collected.
Statistical methodology
Observed distributions of variables were summarised as rate of events (%), mean±SD or median (95% CI) as appropriate. The tumour characteristics were compared by a X2 or Fisher’s exact test when needed. For each variable, patients with missing data were excluded from the analysis. The time to onset of metastases and the different survival distributions were estimated by the Kaplan-Meier non-parametric method. A p<0.05 (bilateral) was considered as statistically significant. Due to the exploratory nature of our study, we did not apply any correction to address the multiple comparison issue. The analyses were performed using the SAS V.9.4 for Windows.
Results
Participants
Out of 469 files identified by the automated search, 65 patients met the inclusion criteria. Seventeen patients were excluded: lost to follow-up (n=7), preexisting liver abnormalities (n=6), no reference imaging (n=2), liver abnormalities before the first line of treatment (n=2). Finally, 48 patients were selected for analysis.
Clinical and biological characteristics
Demographic and neoplastic characteristics of our sample are summarised in table 1.
Table 1.
Description of the population at the time of diagnosis of the primary tumour
| Demographic characteristics (n=48) | |
| Mean age at diagnosis—years±SD | 50.6±11.6 |
| Interval—years | (27–80) |
| Age ≤50 years old—n (%) | 25 (52.1) |
| Median survival since the onset of liver metastases—months (95% CI) | 25.5 (20.1 to 40.4) |
| Median survival since the appearance of liver contour abnormalities—months (95% CI) | 8.5 (6.7 to 10.8) |
| Alcohol abuse | |
| Yes—n (%) (95% CI for %)* | 2 (4.2) (1.2 to 14.0) |
| No—n (%) (95% CI for %) | 40 (83.3) (70.4 to 91.3) |
| N/A—n (%) (95% CI for %) | 6 (12.5) (5.9 to 24.7) |
| n (%) (95% CI for %) | Control—n (%) (95% CI for %)† | |
| Histological subtypes (n=44) | ||
| Ductal | 32 (72.7) (58.1 to 83.7) | 4149 (79.5) (78.4 to 80.6) |
| Lobular | 10 (22.7) (12.8 to 37.0) | 671 (12.9) (12.0 to 13.8) |
| Mixed | 1 (2.3) (0.4 to 11.8) | 215 (4.1) (3.6 to 4.7) |
| Others | 1 (2.3) (0.4 to 11.8) | 184 (3.5) (3.1 to 4.1) |
| P value: Fisher’ exact test | 0.27 | |
| Disease subtypes (n=34)‡ | ||
| Luminal | 30 (88.2) (73.4 to 95.3) | 3517 (69.6) (68.3 to 79.9) |
| Luminal A | 8 | – |
| Luminal B§ | 13 | – |
| Ki67 not available | 9 | – |
| HER2 positive | 3 (8.8) (3.0 to 23.0) | 803 (15.9) (14.9 to 16.9) |
| Triple negative | 1 (2.9) (0.5 to 14.9) | 737 (14.6) (13.7 to 15.6) |
| P value: X2 test | 0.052 | |
| Difference in disease subtypes in our population and the control | |
| Luminal difference | 18.6% (95% CI for the difference: 3.7% to 25.8%) |
| HER2 positive difference | 7.1% (95% CI for the difference: −12.9% to 7.1 %) |
| Triple negative difference | 11.7% (95% CI for the difference: −14.3% to 0.4%) |
*>20 g alcohol/day.
†Data of primary tumours diagnosed at the Jules Bordet Institute for all patients enrolled in the cancer registry between 2010 and 2017.
‡14 patients did not have labelling for HER2 protein.
§ Ki67 ≥20%.
n, number of patients in the category; NA, not applicable.
The main histological subtypes of the primary tumours, available for 44 patients, were 72.7% (n=32) of ductal and 22.7% (n=10) of lobular adenocarcinomas. The disease subtypes, available for 34 patients, were 88.2% (n=30) of luminal, 8.8% (n=3) of HER2 positive and 2.9% (n=1) of triple negative tumours. There was no statistically significant difference between the proportions of histological subtypes (p=0.27) and disease subtypes (p=0.052) in our sample compared with those observed between 2010 and 2017 in our institution. The local and metastatic management of patients is summarised in the online supplementary table S-3.
In terms of liver disease burden: 97.9% (n=47) of patients had an invasion by more than five lesions or a diffuse invasion. The timing and sites of metastasis are available in the online supplementary table S-3. It should be noted that 20.8% (n=10) of patients also had peritoneal carcinomatosis.
The terms used to describe liver contour abnormalities are available in the online supplementary table S-4. These hepatic contour abnormalities appeared after a median of 13.3 months (8.3–20.9) after the first description of hepatic metastases. The imaging of three patients was reviewed by a radiologist due to a major discrepancy between the protocol and the imaging (n=1) or the need for analysis of positron emission tomography images (n=2).
Estimated median survival was 8.5 months (6.7–10.8) after the onset of liver contour abnormalities and 25.5 months (20.1–40.4) after the description of liver metastases.
Biological variables at the time of description of the liver contour abnormalities are summarised in table 2. We identified six patients (12.5%) with a possible visceral crisis by using total bilirubin ≥2 mg/dL as an indicator.
Table 2.
Biological values at the onset of diffuse liver contour abnormalities
| Analysis | Mean±SD | Units | Baseline values* |
| Carcinoma Antigen 15–3—median (IQR) (n=44) | 283.4 (95.3–494.3) | kUI/L | <26 |
| Total bilirubin (n=48) | 1.0±1.0 | mg/dL | <1.2 |
| Aspartate transaminase (n=48) | 66.3±46.1 | UI/L | <32 |
| Alanine transaminase(n=48) | 47.4±35.4 | UI/L | <33 |
| Gamma-glutamyltransferase (n=48) | 332.0±263.6 | UI/L | 6–42 |
| Alkaline phosphatases (n=48) | 391.4±244.9 | UI/L | 35–104 |
| Serum albumin (n=47) | 35.4±8.3 | g/L | 34–48 |
| International Normalized Ratio (n=40) | 1.2±0.2 | – | 0.95–1.31 |
| Prothrombin time(Quick Time) (n=41) | 83.3±16.0 | % | 70–100 |
| Activated Partial Thromboplastin Time (Time of activated cephalin) (n=39) | 29.9±29.2 | dry | 18.7–32.1 |
| Platelets (n=48) | 182.7±83.7 | 10³/mm³ | 150–410 |
| Urea (n=46) | 32.1±14.7 | mg/dL | 17–48 |
| Plasma creatinine (n=46) | 1.0±0.9 | mg/dL | 0.50–0.90 |
| Haemoglobin (n=48) | 11.0±1.4 | g/dL | 12.0–16.0 |
Bold values indicate value outside reference values.
*Reference values in use at the Jules Bordet Institute in April 2019.
n, number of patients analysed.
In the year of the appearance of liver contour abnormalities, 25 patients (52%) underwent serology testing for viral hepatitis, five patients (20%) had anti-HBc antibodies but all five had negative HBsAg. No positive anti-HCV serology was reported.
Complications
All the complications, usually associated with decompensated cirrhosis, identified in our patients, are presented in table 3.
Table 3.
Prevalence of complications generally associated with hepatic cirrhosis following the development of diffuse liver contour abnormalities and management (n=48)
| N (%) (95% CI for %) | |
| Related to portal hypertension | |
| Ascites | |
| Radiological ascites | 39 (81.3) (68.1 to 89.8) |
| Ascites on physical examination | 28 (58.3) (44.8 to 71.2) |
| Characteristics of ascites (n=20) | |
| Protein (mean)—g/L±SD | 14.2±8 |
| Transudate (<30 g/L) | 18 (90.0) (69.9 to 97.2) |
| Exudate (>30 g/L) | 2 (10.0) (2.8 to 30.1) |
| Presence of neoplastic cells | 5 (25.0) (11.2 to 46.9) |
| Absence of neoplastic cells | 15 (75.0) (53.1 to 88.1) |
| Ascites management (n=28) | |
| Use of diuretics | 21 (75.0) (56.6 to 87.3) |
| Large volume paracentesis | 24 (85.7) (68.5 to 94.3) |
| Albumin transfusions | 9 (32.1) (17.9 to 50.7) |
| Transjugular intrahepatic portosystemic shunt | 1 (3.6) (0.6 to 17.7) |
| Radiological vascular signs of portal hypertension* | 13 (27.1) (16.6 to 41.0) |
| Radiological splenomegaly† | 13 (27.1) (16.6 to 41.0) |
| Oesophageal varices (endoscopic)‡ | 11 (22.9) (13.3 to 36.5) |
| Rupture of oesophageal varices | 3 (6.5) (2.2 to 17.5) |
| Portal vein thrombosis§ | 5 (10.4) (4.5 to 22.2) |
| Spontaneous bacterial peritonitis | 3 (6.3) (2.1 to 16.8) |
| Related to liver failure | |
| Hypoalbuminaemia¶ | 36 (75.0) (61.2 to 85.1) |
| Hyperbilirubinaemia** | 31 (64.6) (50.4 to 76.6) |
| Radiological bile duct dilation | 0 (0) (0 to 7.4) |
| Coagulation disorders†† | 27 (56.3) (42.3 to 69.3) |
| Hepatic encephalopathy | 11 (22.9) (13.3 to 36.5) |
*Enlargement of paraumbilical or collateral veins on abdominal imaging.
†Two patients had lesions suspected of splenic metastases on imaging.
‡14 patients underwent a diagnostic upper gastrointestinal endoscopy.
§Of these five patients, four had endoscopic oesophageal varices.
¶Serum albumin <35 g/L.
**Total bilirubin >2 mg/dL.
††PT <70% PT.
n, number of patients analysed; n, number of patients in the category.
Treatments
Liver contour abnormalities occurred after a median of 2 lines of chemotherapy and after a median of 4 lines of all systemic treatments for metastatic disease. Median number of endocrine therapy lines is two for all settings ((neo)adjuvant and metastatic) and 1.5 for metastatic setting.
Analysis of the best radiological response of hepatic metastases to ongoing treatments at the onset of hepatic contour abnormalities showed a partial response in 52.1% (n=25) of patients, stable in 33.3% (n=16) and progression in 10.4% (n=5) (table 4).
Table 4.
Response of liver metastases to ongoing treatments at the description of diffuse hepatic contour abnormalities and to the previous line of treatment according to CT or MRI protocols (n=48)
| Response | Ongoing treatment n (%) (95% CI for %) |
Previous treatment n (%) (95% CI for %) |
| Complete | 0 (0) (0 to 7.4) | 0 (0) (0 to 7.4) |
| Partial | 25 (52.1) (38.3 to 65.5) | 16 (33.3) (21.7 to 47.5) |
| Stable | 16 (33.3) (21.7 to 47.5) | 8 (16.7) (8.7 to 29.6) |
| Progression | 5 (10.4) (4.5 to 22.2) | 11 (22.9) (13.3 to 36.5) |
| Not evaluable or applicable | 2 (4.2) (1.2 to 14.0)* | 13 (27.1) (16.6 to 41.0)† |
| P value: test of Χ²‡ | 0.021 | |
*In two patients, diffuse hepatic contour abnormalities were identified on positron emission tomography, with the absence of contrast injection making it impossible to assess the lesions using tomography images.
†Six patients had diffuse liver contour abnormalities during their first line of treatment.
‡Excluding not evaluable or applicable patients.
N, number of patients analysed; n, number of patients in the category.
The proportion of stable patients or partial responders during this line, compared with those in progression, was statistically significantly higher than during the previous line (p=0.021). Histological (p=1) and disease (p=0.431) subtypes did not show significant statistical association with the response. At the time of the appearance of liver contour abnormalities, the most frequently used treatments were: 5-fluorouracil (5-FU) (n=11; 22.9%), cisplatin (n=9; 18.8%), paclitaxel (n=8; 16.7%) and capecitabine (5-FU prodrug) (n=7; 14.6%) (online supplementary figure 2). Eight patients (16.7%) received 5-FU in combination with cisplatin, one (2.1%) received capecitabine combined to cisplatin and one patient (2.1%) received trastuzumab in addition to cisplatin and 5-FU.
Discussion
The histological and disease subtypes of our pseudocirrhotic patients are not statistically different from those usually encountered in clinical practice. Even though the recruitment period for the control group was not as long as our recruitment period for patients with pseudocirrhosis, it is unlikely that significant changes have occurred concerning the repartition of breast cancer subtypes. It should be highlighted that the 95% CI for the difference in the incidence of the luminal subtype is wholly positive, favouring an over-representation in the group with pseudocirrhosis compared with the control group. The lack of statistical significance may be partly explained by the lack of power associated to the low number of patients, but a real lack of difference can not be excluded. The under-representation of triple negative tumours could be explained by relapses less sensitive to chemotherapy and short median survival that do not allow time for pseudocirrhosis to develop.12 13 On the other hand, it seems clear that pseudocirrhosis develops in patients with extensive liver invasion, described as diffuse or with more than five metastatic lesions.
When diagnosing pseudocirrhosis by radiological imaging, it is interesting to note that, although biological values already show signs of cholestasis, there is no major anomaly in the average liver function of patients, suggesting that the synthetic function of the liver is not impaired.
Despite their probable fundamental differences at the histological and biological level, cirrhosis and pseudocirrhosis seem to share some of the same complications. First, ascites is the most common complication, being present in 81% of patients. Although a mixed aetiology is not unlikely for patients with peritoneal carcinomatosis, the overall protein content of ascites (90% of transudates) seems to be in favour of an origin related to PH.14 Although the albumin gradient between serum and ascites would be more appropriate to confirm this hypothesis, unfortunately only one patient had available data.15 Treatment with diuretics alone often seems insufficient, one patient has been treated with a transjugular intrahepatic portosystemic shunt (TIPS). Geeroms et al also report that TIPS procedures were performed in the context of pseudocirrhosis. Two of their three patients showed an improvement in their clinical condition with discontinuation of paracentesis, and the third died soon after the technical procedure.8 Second, other signs such as splenomegaly, enlargement of the paraumbilical vein or development of portosystemic collateral veins also illustrate the presence of PH in pseudocirrhosis. Qayyum et al had previously studied the frequency of hepatic contour abnormalities and PH under chemotherapy in metastatic breast cancer to the liver.16 Among their 16 patients with diffusely nodular contours resembling cirrhosis, 37.5% (n=6) had signs of PH on imaging, compared with 39.6% (n=19) of our patients using the same criteria (p=0.882). Unfortunately, only 14 of our patients had an upper gastrointestinal endoscopy after the appearance of liver contour abnormalities, which does not allow to define the prevalence of EV in our sample; however, it should be reminded that 52.2% of cirrhotic patients have EV.17 Third, signs of hepatic failure, such as hypoalbuminaemia, hyperbilirubinaemia and coagulation disorders, are frequently reported, but many confounding factors are present. For example, factors influencing albuminaemia, such as malnutrition, are present in our patients. In a study on cachexia in oncological patients, 28.3% of patients had hypoalbuminaemia (albumin <32 g/L) compared with 75.0% in our study (albumin <35 g/L).18
A recently published article reviewing 37 cases of pseudocirrhosis in breast cancer, similar to our study but with broader inclusions criteria, did also found cirrhosis-like characteristics with 68% of patients with ascites, 11% with PH signs and 8% with splenomegaly but no modification of the synthetic function.19
The first radiological signs of pseudocirrhosis appeared after a median of 2 lines of chemotherapy for metastatic disease. The treatment during which these changes occurred most frequently was 5-FU and particularly in combination with cisplatin. The combination of cisplatin and 5-FU is used in some cases as a so-called salvage therapy for metastatic breast cancer, and more particularly in the extensive liver disease burden with impaired liver function.20 However, it is not excluded that this result may partially reflect the practice of our centre in terms of management of diffuse liver metastases and does not establish causality. The next most administered regimen in our series was paclitaxel, a medication largely used in patients with metastatic breast cancer.21 Among patients identified with possible visceral crisis: two where treated with 5-FU and cisplatin, two with capecitabin and two with paclitaxel when pseudocirrhosis occurred. On the other hand, pseudocirrhosis appears to develop among patients responding to chemotherapy with 52.1% of patients having a partial response of their hepatic metastases and 33.3% stabilising during the treatment line where the liver contour abnormalities appeared. It should be noted, however, that this analysis was solely based on imaging protocols and that the RECIST were, therefore, not applied to all patients. Fenessy et al showed a correlation between capsular retractions and a significant initial size of metastases, as well as a variation in the size of metastases after treatment (increase or decrease) concluding that these anomalies were not due solely to a reduction in post-treatment size.22 In the same article, they did not find a correlation between the presence of capsular retraction and the number of liver metastases. However, the concept of diffuse anomaly was not present in their study, unlike in ours.
Although this study was not intended to define the impact of pseudocirrhosis on survival, the median survival from liver metastases in our sample (25.5 months) is similar to that reported in the literature (15.5–24.9 months depending on the presence or absence of other metastatic sites).1 Nevertheless, the median survival after the appearance of liver contour abnormalities was only 8.5 months.
Our work has some major limitations. First, the absence of histological analysis at the pseudocirrhotic stage does not rule out the possibility that our patients may have different pathologies leading to the same changes in imaging. Second, the absence of a new radiological analysis and the inclusion solely on the basis of imaging protocols may have constituted a selection bias by excess (anomalies overestimated by the protocol) or by default (absence of a description of the anomalies). The extent of this bias cannot be assessed. However, it should be noted that in Qayyum et al 16 (17.6%) of their 91 patients with metastatic breast cancer in the liver, had a hepatic contour qualified as diffusely nodular.16 Even if our study is not comparable and did not aim to establish the prevalence of pseudocirrhosis, it is possible that with 48 patients over a 15-year period, some cases could have been missed by our selection method. Nevertheless, we checked that some known cases were indeed retrieved by the selection method. In addition, by choosing a definition based on imaging criteria, our study grouped different levels of hepatic contour alterations, with not all patients having a comparable impairment to the patient in figure 1. Third, the absence of controls with hepatic metastases without diffuse hepatic contour abnormalities limits our ability to interpret our results. However, matching patients would not help to achieve our primary objective which was to describe the clinical and biological characteristics of pseudocirrhosis. Fourth, we could not formally exclude other causes of liver cirrhosis. Markers of autoimmune hepatitis or cholangitis and copper/ceruloplasmin levels were too rarely available to be analysed. As the iron homeostasis was severely disrupted by multiple transfusions for some patients, it was excluded from analysis. Pre-existing non-alcoholic fatty liver disease or steatosis linked to breast cancer hormonal therapy were also not investigated.
Conclusion
This is to our knowledge the largest series of patients with pseudocirrhosis in metastatic breast cancer. It would appear that luminal subtypes were over-represented in comparison with our control cohort, but this will need to be confirmed by larger studies. We also showed that some of these patients, in addition to the macroscopic similarities with cirrhosis, developed similar complications related to both PH and hepatic insufficiency. Pseudocirrhosis appears to develop among our patients at the expense of extensive liver disease and most often under 5-FU with or without cisplatin and following a response to treatment. It is, therefore, necessary to draw clinicians’ attention to these patients in order to identify these complications as soon as possible and provide appropriate management.
Footnotes
Contributors: DE, MM, MP and AA designed the study, analysed the results and participated to the manuscript redaction. DE collected the data. YZ reviewed radiological images. MM performed the statistical analysis. AL and YZ participated to the manuscript redaction.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This work received approval from the Ethics Committee of the Jules Bordet Institute, Brussels, Belgium under number CE2912.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information. Individual data are available from DE (dan.engelman@ulb.ac.be).
References
- 1.Diamond JR, Finlayson CA, Borges VF. Hepatic complications of breast cancer. Lancet Oncol 2009;10:615–21. 10.1016/S1470-2045(09)70029-4 [DOI] [PubMed] [Google Scholar]
- 2.Jha P, Poder L, Wang ZJ, et al. Radiologic mimics of cirrhosis. AJR Am J Roentgenol 2010;194:993–9. 10.2214/AJR.09.3409 [DOI] [PubMed] [Google Scholar]
- 3.Young ST, Paulson EK, Washington K, et al. CT of the liver in patients with metastatic breast carcinoma treated by chemotherapy: findings simulating cirrhosis. AJR Am J Roentgenol 1994;163:1385–8. 10.2214/ajr.163.6.7992734 [DOI] [PubMed] [Google Scholar]
- 4.Adike A, Karlin N, Menias C, et al. Pseudocirrhosis: a case series and literature review. Case Rep Gastroenterol 2016;10:381–91. 10.1159/000448066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alberti N, Bechade D, Dupuis F, et al. Hepar lobatum carcinomatosum associated with liver metastases from breast cancer: report of five cases. Diagn Interv Imaging 2015;96:73–8. 10.1016/j.diii.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 6.Vilgrain V, Lagadec M, Ronot M. Pitfalls in liver imaging. Radiology 2016;278:34–51. 10.1148/radiol.2015142576 [DOI] [PubMed] [Google Scholar]
- 7.Mamone G, Cortis K, Sarah A, et al. Hepatic morphology abnormalities: beyond cirrhosis. Abdom Radiol 2018;43:1612–26. 10.1007/s00261-017-1351-9 [DOI] [PubMed] [Google Scholar]
- 8.Geeroms B, De Hertogh G, Vanslembrouck R, et al. Transjugular intrahepatic portosystemic shunt for the treatment of portal hypertension-induced refractory ascites due to metastatic carcinomatous liver disease. J Vasc Interv Radiol 2018;29:1713–6. 10.1016/j.jvir.2018.08.007 [DOI] [PubMed] [Google Scholar]
- 9.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver . EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406–60. 10.1016/j.jhep.2018.03.024 [DOI] [PubMed] [Google Scholar]
- 10.Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet 2014;383:1749–61. 10.1016/S0140-6736(14)60121-5 [DOI] [PubMed] [Google Scholar]
- 11.Sanyal AJ, Bosch J, Blei A, et al. Portal hypertension and its complications. Gastroenterology 2008;134:1715–28. 10.1053/j.gastro.2008.03.007 [DOI] [PubMed] [Google Scholar]
- 12.Kassam F, Enright K, Dent R, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer 2009;9:29–33. 10.3816/CBC.2009.n.005 [DOI] [PubMed] [Google Scholar]
- 13.Press DJ, Miller ME, Liederbach E, et al. De novo metastasis in breast cancer: occurrence and overall survival stratified by molecular subtype. Clin Exp Metastasis 2017;34:457–65. 10.1007/s10585-017-9871-9 [DOI] [PubMed] [Google Scholar]
- 14.Runyon BA, Hoefs JC, Morgan TR. Ascitic fluid analysis in malignancy-related ascites. Hepatology 1988;8:1104–9. 10.1002/hep.1840080521 [DOI] [PubMed] [Google Scholar]
- 15.Runyon BA, AASLD Practice Guidelines Committee . Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009;49:2087–107. 10.1002/hep.22853 [DOI] [PubMed] [Google Scholar]
- 16.Qayyum A, Lee GK, Yeh BM, et al. Frequency of hepatic contour abnormalities and signs of portal hypertension at CT in patients receiving chemotherapy for breast cancer metastatic to the liver. Clin Imaging 2007;31:6–10. 10.1016/j.clinimag.2006.09.028 [DOI] [PubMed] [Google Scholar]
- 17.Kovalak M, Lake J, Mattek N, et al. Endoscopic screening for varices in cirrhotic patients: data from a national endoscopic database. Gastrointest Endosc 2007;65:82–8. 10.1016/j.gie.2006.08.023 [DOI] [PubMed] [Google Scholar]
- 18.Vigano AAL, Morais JA, Ciutto L, et al. Use of routinely available clinical, nutritional, and functional criteria to classify cachexia in advanced cancer patients. Clin Nutr 2017;36:1378–90. 10.1016/j.clnu.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 19.Oliai C, Douek ML, Rhoane C, et al. Clinical features of pseudocirrhosis in metastatic breast cancer. Breast Cancer Res Treat 2019;177:409–17. 10.1007/s10549-019-05311-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lekakis L, Tryfonopoulos D, Pistamatzian N, et al. Salvage chemotherapy with cisplatin and 5-fluorouracil in metastatic breast cancer. particular activity against liver metastases. Anticancer Res 2012;32:1833–7. [PubMed] [Google Scholar]
- 21.Cardoso F, Senkus E, Costa A, et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4)†. Ann Oncol 2018;29:1634–57. 10.1093/annonc/mdy192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fennessy FM, Mortele KJ, Kluckert T, et al. Hepatic capsular retraction in metastatic carcinoma of the breast occurring with increase or decrease in size of subjacent metastasis. AJR Am J Roentgenol 2004;182:651–5. 10.2214/ajr.182.3.1820651 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2020-000695supp001.pdf (171.9KB, pdf)