Introduction
Chronic lymphocytic leukemia (CLL) is a mature B-cell neoplasm characterized by a progressive accumulation of monoclonal B-lymphocytes. It is the most common adult leukemia in Western countries, accounting for approximately 30 percent of all leukemias in the United States [1]. Presenting findings are heterogeneous and range from incidental detection on routine laboratory studies to disease-related symptoms, infection, anemia or other non-specific findings.
Clinically apparent neurological involvement by CLL, especially ocular manifestation, is rare [2]. There are few case reports describing orbital, lacrimal, conjunctival, and/or scleral involvement. To date, we have found only two reports of ocular CLL: one report describing retinal and another describing choroidal leukemic infiltration due to CLL [3,4].
Ibrutinib is an oral Bruton tyrosine kinase (BTK) inhibitor, with significant activity in a number of B-cell malignancies, including CLL, mantle cell lymphoma (MCL), those with CNS localization and primary central nervous system lymphoma (PCNSL) [5]. Here we present the first case of ocular relapse of CLL with leukemic retinopathy successfully treated with Ibrutinib.
Case discussion
A 66-year-old male was diagnosed with CLL in 2009 based on the incidental detection of lymphocytosis on routine laboratory studies. Peripheral blood flow cytometry showed a monoclonal B-cell population expressing CD19, CD20, CD5, and CD23. CD20 was dimly expressed. The cells were negative for CD10, ZAP70, and CD38. FISH analysis did not show CCND1-IGH fusion, extra signals or deletions of ATM, trisomy 12, 13q, 17p deletion, or TP53 aberrancies. Bone marrow biopsy showed 45% involvement by CLL. He was maintained on observation, with monthly infusions of intravenous immunoglobulin due to hypogammaglobulinemia accompanied by frequent infections. Six years after diagnosis, systemic treatment was indicated because of progressively worsening lymphadenopathy, fatigue, unintentional weight loss, and splenomegaly. Absolute lymphocyte count was 13,300/µL, hemoglobin 15 g/dl, and platelet count was 243/µL. He was enrolled on a clinical trial comparing Bendamustine-Rituximab (BR) with or without the class I phosphoinositol-3-kinase delta inhibitor idelalisib. Cytogenetics were repeated as part of trial enrolment and PCR analysis showed unmutated IgHV and absence of TP53 aberrations. A repeat FISH analysis was similar to prior. He was randomized to the idelalisib arm however study drug was discontinued after four weeks due to grade 3 diarrhea and rash. He completed six cycles of BR with resolution of leukocytosis and lymphadenopathy.
Two years after treatment completion, his disease recurred with development of fatigue, worsening lymphocytosis, and lymphadenopathy. Concomitantly he also complained of decreased vision in his right eye. He did not have any pre-existing ocular abnormalities.
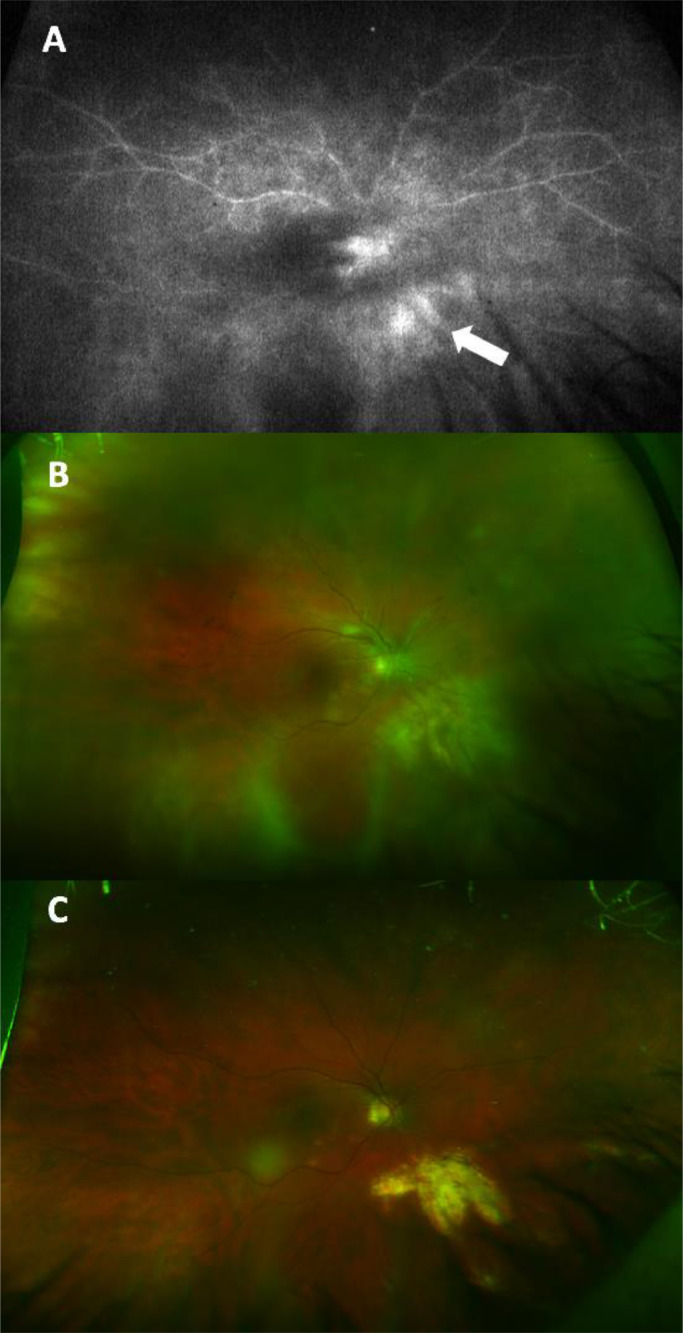
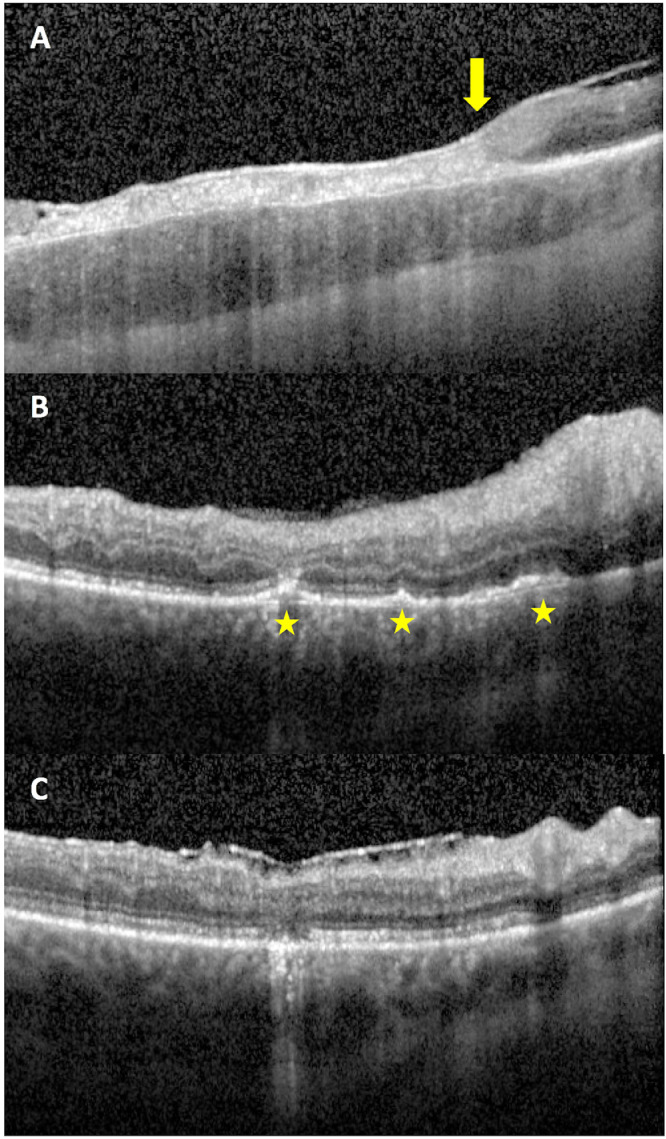
Initial visual acuity examination was counting fingers at one foot for the right eye and 20/25 for the left eye. Intraocular tensions and ocular motility were normal. Confrontational visual fields revealed a right partial outer temporal visual field deficit. Slit lamp bio-microscopy of both eyes showed trace keratic precipitates, moderate anterior chamber inflammation, and sheets of vitreous cells. Funduscopic examination of the right eye revealed vitreous haze, blurred disc margins, diffuse vascular sheathing in the posterior pole, and multiple creamy lesions in the nasal macula and inferonasal to the optic nerve (Figure 1B). Exam of the left eye was notable for a small, subtle, creamy lesion nasal to the optic disc, and otherwise normal appearing optic nerve, macula, and vessels. Optical coherence tomography (OCT) images of the right eye showed full thickness retinal infiltration by a hyperreflective material corresponding to the inferonasal lesion on fundus photographs (Figure 2A) with a sharp demarcation between normal and abnormal retina at the edge of the lesion. OCT images of the macula showed multiple retinal pigment epithelial detachments (PEDs) (Figure 2B). Wide field fluorescein angiography (FA) revealed leakage in nasal macula and inferonasal to the disc on late frames (Figure 1A). FA of the left eye revealed trace leakage corresponding to the lesion nasal to the disc, but without a corresponding PED or retinal infiltrate on OCT.
Figure 1.
Wide field fluorescein angiography of the right eye reveals retinal vascular leakage inferonasal to the optic nerve (arrow) corresponding to the infiltrative retinal lesion on fundus photographs (A). Wide field fundus photograph of the right eye shows vitritis and multiple creamy lesions in the nasal macula and inferonasal to the optic nerve on presentation (B), and subsequent resolution of retinal lesions with residual scarring, atrophy, and hypopigmentation at 8 months follow up (C).
Figure 2.
Optical coherence tomography (OCT) images of the right eye at initial presentation show a transition zone (arrow) from a normal retina nasally to a full thickness retinal infiltration by a hyperreflective material temporally (A); multiple retinal pigment epithelium detachments (PEDs) in the inferior macula (star) (B); and resolution of PEDs with resultant retinal atrophy at a two year follow up (C).
A focused uveitis workup was negative for syphilis, tuberculosis, toxoplasmosis, or sarcoidosis. MRI of the brain and orbits with and without contrast revealed diffuse leptomeningeal enhancement. Patient underwent a pars plana vitrectomy with vitreous biopsy, which was inconclusive. A lumbar puncture [LP] was done with the cerebrospinal fluid (CSF) showing an atypical lymphoid infiltrate. CSF flow cytometry was positive for involvement by a small population (3% total cells) of CLL/SLL cells, expressing CD19, CD5, Cd23, CD20 (dim), and SIG-lambda (negative for FMC7), confirming CNS relapse. He received two cycles of intrathecal cytarabine.
Concurrently, the patient also experienced vertigo, muscle spasms, imbalance, and seizures which were treated successfully with levetiracetam.
In September 2017, he was started on Ibrutinib at a dose of 560 mg daily for CNS penetration. Within 4 weeks, the patient reported a significant improvement in vision in both eyes except for some peripheral visual field defect, tunnel vision and scotomatas in the right eye. He did not experience any further seizures. A repeat MRI brain showed resolution of leptomeningeal enhancement, and CSF flow cytometry showed 1% involvement by CLL/SLL cells, thought to be peripheral blood contamination. Best corrected visual acuity at 8 months follow up improved to 20/40 and 20/20 in right and left eyes, respectively. Bio-microscopy was notable for resolution of vitritis, decrease in the size of the retinal lesions with resultant focal retinal atrophy and scarring on the right (Figure 1C and 2C).
He subsequently developed intolerance to ibrutinib, manifested by grade 3 diarrhea with urgency and fecal incontinence, unresponsive to supportive measures, and extensive bruising. He was switched to the second generation BTK inhibitor, acalabrutinib at a dose of 100 mg twice daily. He has tolerated that drug extremely well and, after a year, remains asymptomatic and in a clinical remission. His vision, including peripheral, continues to improve, such that he is now able to drive and ride a bicycle. He no longer requires anti-seizure medications. Physical examination reveals no lymphadenopathy or splenomegaly. Latest CBC shows a WBC of 5100/ul including 1200 lymphocytes, hemoglobin of 13.8 g/dl, and platelets of 222,000/ul.
Conclusions
In earlier case series, the incidence of neurological complications in CLL was reported to be 4-11.3%; however, direct CNS localization occurred in only 0.4-0.8% of cases [2,6]. Post-mortem studies have reported an incidence of 7-20%, indicating underdiagnosis, difficulty in diagnosis or a high occurrence of sub-clinical disease [7,8].
Ocular involvement in CLL may be either direct via leukemic infiltration or indirect due to immune compromise, hyperviscosity, thrombocytopenia, anemia, or treatment. Whereas ocular involvement is rare [9], [10], [11] it may be the first and sole site of disease relapse. In a retrospective cohort of 30 CLL patients with ocular or CNS involvement, less than half had progressive CLL and 20 had never been treated for CLL [12]. There was no apparent correlation between Rai/Binet stage and occurrence of neurological or ocular disease [12,13].
Varied ophthalmological anomalies have been noted, including subcapsular cataracts, conjunctival anomalies, ophthalmoplegia, extraocular muscle infiltration, ptosis, optic nerve infiltration, optic neuropathy, [11,12,[14], [15], [16], [17]] eyelid and orbital soft tissue masses, [18] bilateral lacrimal gland infiltration, episcleral infiltration, [19] and bilateral posterior scleritis [20]. Anterior segment pathology included one case of anterior uveitis with hypopyon. [18] Vitreous involvement has been documented in a patient with CLL, who developed intraocular Richter transformation. [9] Other secondary ocular complications of CLL include fungal endophthalmitis [10,21] and acute retinal necrosis. [11]. However, there are only two other case reports describing leukemic retinal [4] or choroidal [22] infiltrates due to CLL.
The current case is the first to present OCT findings in this condition. OCT images of the macula in our patient showed several focal PEDs with subsequent resolution of hyperreflective lesions in the inferonasal macula following treatment, with persistent focal photoreceptor disruption. OCT images through the retinal lesion inferonasal to the optic nerve corresponding to the area of retinal vascular leakage on fluorescein angiography, showed hyperreflectivity and disruption of all retinal layers that persisted following treatment and was associated with choroidal thinning. OCT findings of retinal leukemic infiltration have been described in one other case report; however, the patient had chronic myelogenous leukemia. Herein, OCT revealed lesions of uniform reflectivity involving all layers of the retina with subsequent resolution of hyperreflective lesions at 3 months without consecutive atrophy of the inner retinal layers upon resolution. This case too demonstrated persistent focal disruption of the photoreceptor outer segments where there was initial leukemic involvement. [23]
Diagnostic work-up typically involves neuroimaging; however, MRI has a lower sensitivity for detection of meningeal involvement in primary CNS lymphoma [20-37.5%] compared to solid tumors [24,25] and the absence of MRI abnormalities may not rule out CNS CLL. CSF examination, while important, is challenging, because of the low burden of CLL cells in the CSF as well as the risk of peripheral blood contamination during the procedures, increasing false-positive rates. Flow cytometry significantly increases the sensitivity of detection [26]. In anecdotal case reports leptomeningeal biopsy has been used for diagnosis [27], a procedure not usually performed. Ultimately, CSF flow cytometry remains the gold standard, and diagnosis of CNS localization can be made despite a low percentage of malignant cells in the CSF, especially in the presence of symptoms. Given rarity of intraocular CLL involvement, the yield of vitreous biopsy is unknown however, data from primary intraocular lymphoma show high false negative rates of vitreal biopsy with the diagnostic yield dependent on collection technique, prompt cytopathologic analysis, and appropriate fixative agent [28,29]. Ophthalmoscopic exam may also be of low yield as optic neuropathy may not be accompanied by concomitant optic disc edema. Hence, despite the negative vitreal biopsy in our case, the concordance of OCT, CSF, and clinical findings align with diagnosis of ocular CLL and underscore the need for a high clinical index of suspicion.
No specific guideline-based recommendations for treatment of patients with CNS involvement of CLL exist [30] and combinations of systemic and intrathecal chemotherapies, rituximab monotherapy, localized radiotherapy or ibrutinib have been used in case reports [12,13]. Prognosis appears to be determined by the underlying CLL characteristics rather than neurological/ocular involvement [12].
Ibrutinib has changed the landscape of CLL treatment in recent years with better progression-free survival, overall response rates, and overall survival than standard chemo-immunotherapy. Ibrutinib provides benefit regardless of adverse prognostic factors, such as del(17p)/TP53 mutation and del(11q) [31]. Pre-clinical data with Ibrutinib has shown a high level of brain distribution, and correlates with plasma concentrations [32] providing the justification for the dose used in the current patient of 560 mg rather than the standard 420 mg typically administered in CLL. Ibrutinib has shown promising results in treatment of CNS involvement with histologic subtypes of non-Hodgkin lymphoma including MCL [33,34],Waldenström macroglobulinemia (WM) [35], [36], [37], and, relapsed/refractory primary CNS lymphoma. In relapsed/refractory primary CNS lymphoma, response rates as high as 68% have been reported, albeit relatively short-lived [5]. Unfortunately, one of the more serious complication of its administration is spontaneous bruising or bleeding, which may involve the central nervous system or eye.
This case represents a rare presentation of intraocular CLL with retinal and choroidal infiltration, successfully treated with Ibrutinib monotherapy, highlighting the safety and efficacy of this agent regardless of site or type of relapse. Clinical benefit persists despite switching to acalabrutinib. This BTK inhibitor has demonstrated efficacy similar to that reported with ibrutinib in relapsed and refractory CLL, and has been shown to be of benefit in patients intolerant to ibrutinib [38]. Clinicians should be aware of the possibility of ocular involvement in CLL in patients with prior history of this disease and complaints of neurologic or ocular abnormalities. Administration of ibrutinib should be considered for rapid and sustained clinical benefit.
Contributor Information
Ayushi F Chauhan, Email: ayushi.chauhan@gunet.georgetown.edu.
Narine Viruni, Email: nabgary1@jhmi.edu.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA. Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Strati P, Uhm JH, Kaufmann TJ. Prevalence and characteristics of central nervous system involvement by chronic lymphocytic leukemia. Haematologica. 2016;101(4):458–465. doi: 10.3324/haematol.2015.136556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moazzam AA, Drappatz J, Kim RY, Kesari S. Chronic lymphocytic leukemia with central nervous system involvement: report of two cases with a comprehensive literature review. J Neurooncol. 2012;106(1):185–200. doi: 10.1007/s11060-011-0636-z. [DOI] [PubMed] [Google Scholar]
- 4.Treppendahl MB, Andersen N, Jurlander J, Geisler C. A case of chronic lymphocytic leukemia with deletion 17p and bilateral retinal leukemic infiltrates. Eur J Haematol. 2009;82(1):79–80. doi: 10.1111/j.1600-0609.2008.01160.x. [DOI] [PubMed] [Google Scholar]
- 5.Grommes C, Gavrilovic IT, Kaley TJ. Updated results of single-agent ibrutinib in recurrent/refractory primary (PCNSL) and secondary CNS lymphoma (SCNSL) J Clin Oncol. 2018 [Google Scholar]
- 6.Bower JH, Hammack JE, McDonnell SK, Tefferi A. The neurologic complications of B-cell chronic lymphocytic leukemia. Neurology. 1997;48(2):407–412. doi: 10.1212/wnl.48.2.407. [DOI] [PubMed] [Google Scholar]
- 7.Barcos M, Lane W, Gomez GA. An autopsy study of 1206 acute and chronic leukemias (1958 to 1982) Cancer. 1987;60(4):827–837. doi: 10.1002/1097-0142(19870815)60:4<827::aid-cncr2820600419>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 8.Cramer SC, Glaspy JA, Efird JT, Louis DN. Chronic lymphocytic leukemia and the central nervous system: a clinical and pathological study. Neurology. 1996;46(1):19–25. doi: 10.1212/wnl.46.1.19. [DOI] [PubMed] [Google Scholar]
- 9.Hattenhauer MG, Pach JM. Ocular lymphoma in a patient with chronic lymphocytic leukemia. Am J Ophthalmol. 1996;122(2):266–268. doi: 10.1016/s0002-9394(14)72022-7. [DOI] [PubMed] [Google Scholar]
- 10.Kalina PH, Campbell RJ. Aspergillus terreus endophthalmitis in a patient with chronic lymphocytic leukemia. Arch Ophthalmol (Chicago, Ill 1960) 1991;109(1):102–103. doi: 10.1001/archopht.1991.01080010104040. [DOI] [PubMed] [Google Scholar]
- 11.Buchan J, McKibbin M, Burton T. The prevalence of ocular disease in chronic lymphocytic leukaemia. Eye. 2003;17(1):27–30. doi: 10.1038/sj.eye.6700277. [DOI] [PubMed] [Google Scholar]
- 12.Wanquet A, Birsen R, Bonnet C. Management of central nervous system involvement in chronic lymphocytic leukaemia: a retrospective cohort of 30 patients. Br J Haematol. 2017;176(1):37–49. doi: 10.1111/bjh.14387. [DOI] [PubMed] [Google Scholar]
- 13.Timmers NKLM, de Maar JS, van Kruijsdijk RCM, Klein SK. Central nervous system localisation of chronic lymphocytic leukaemia, description of two very distinct cases and a review of the literature. Ann Hematol. 2018 doi: 10.1007/s00277-018-3329-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatton MP, Rubin PAD. Chronic lymphocytic leukemia of the orbit. Arch Ophthalmol (Chicago, Ill 1960) 2002;120(7):990–991. [PubMed] [Google Scholar]
- 15.Mowatt L, Matthews T, Anderson I. Sustained visual recovery after treatment with intrathecal methotrexate in a case of optic neuropathy caused by chronic lymphocytic leukemia. J Neuroophthalmol. 2005;25(2):113–115. doi: 10.1097/01.wno.0000165104.01237.3f. [DOI] [PubMed] [Google Scholar]
- 16.Currie JN, Lessell S, Lessell IM, Weiss JS, Albert DM, Benson EM. Optic Neuropathy in Chronic Lymphocytic Leukemia. Arch Ophthalmol. 1988;106(5):654–660. doi: 10.1001/archopht.1988.01060130708030. [DOI] [PubMed] [Google Scholar]
- 17.Khan K, Malik AI, Almarzouqi SJ. Optic Neuropathy Due to Chronic Lymphocytic Leukemia Proven With Optic Nerve Sheath Biopsy. J Neuro-Ophthalmology. 2016;36(1):61–66. doi: 10.1097/WNO.0000000000000300. [DOI] [PubMed] [Google Scholar]
- 18.Ong YL, White S. Intra-vitreal methotrexate leads to resolution of intraocular chronic lymphocytic leukaemia. Br J Haematol. 2010;148(2) doi: 10.1111/j.1365-2141.2009.07751.x. 181–181. [DOI] [PubMed] [Google Scholar]
- 19.Kincaid MC, Green WR. Ocular and orbital involvement in leukemia. Surv Ophthalmol. 1983;27(4):211–232. doi: 10.1016/0039-6257(83)90123-6. [DOI] [PubMed] [Google Scholar]
- 20.Soon AK, Chan TYB. An atypical case of bilateral posterior scleritis in a patient with chronic lymphocytic leukemia. Can J Ophthalmol. 2015;50(4):e60–e63. doi: 10.1016/j.jcjo.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Machado Od O, de O, Gonçalves R, Fernandes EM, Campos WR, Oréfice F, Curi ALL. Bilateral Aspergillus endophthalmitis in a patient with chronic lymphocytic leukaemia. Br J Ophthalmol. 2003;87(11):1429–1430. doi: 10.1136/bjo.87.11.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MURPHY JA, PITTS JF, DUDGEON J, HOGG RB, HARNETT AN. Retinal detachments due to chronic lymphocytic leukaemia. Clin Lab Haematol. 2008;13(2):217–220. doi: 10.1111/j.1365-2257.1991.tb00272.x. [DOI] [PubMed] [Google Scholar]
- 23.Le JQ, Braich PS, Brar VS. Optical coherence tomography findings of bilateral foveal leukemic infiltration. Int Med Case Rep J. 2016;9:237–240. doi: 10.2147/IMCRJ.S103723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pauls S, Fischer A-C, Brambs H-J, Fetscher S, Höche W, Bommer M. Use of magnetic resonance imaging to detect neoplastic meningitis: limited use in leukemia and lymphoma but convincing results in solid tumors. Eur J Radiol. 2012;81(5):974–978. doi: 10.1016/j.ejrad.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 25.Taylor JW, Flanagan EP, O'Neill BP. Primary leptomeningeal lymphoma: International Primary CNS Lymphoma Collaborative Group report. Neurology. 2013;81(19):1690–1696. doi: 10.1212/01.wnl.0000435302.02895.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.French CA, Dorfman DM, Shaheen G, Cibas ES. Diagnosing lymphoproliferative disorders involving the cerebrospinal fluid: increased sensitivity using flow cytometric analysis. Diagn Cytopathol. 2000;23(6):369–374. doi: 10.1002/1097-0339(200012)23:6<369::aid-dc1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Cervilla Muñoz E, Demelo Rodríguez P, García García A, Menarguez Palanca J, Del Toro Cervera J. Brain biopsy in the diagnosis of leptomeningeal involvement in stage I chronic lymphocytic leukemia. Clin case reports. 2017;5(12):1919–1922. doi: 10.1002/ccr3.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karma A, von Willebrand EO, Tommila PV. Paetau AE, Oskala PS, Immonen IJ. Primary Intraocular Lymphoma. Improving the Diagnostic Procedure. Ophthalmology. 2007;114(7):1372–1377. doi: 10.1016/j.ophtha.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 29.E.M. D, M. A, M.R. R, F. S, C. C Vitreous analysis in the management of uveitis. Mediators Inflamm. 2012;2012 doi: 10.1155/2012/863418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robak T, Caligaris-Cappio F, Stilgenbauer S. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018 doi: 10.1182/blood-2017-09-806398. [DOI] [PubMed] [Google Scholar]
- 31.Ibrutinib Deeks ED. A Review in Chronic Lymphocytic Leukaemia. Drugs. 2017;77(2):225–236. doi: 10.1007/s40265-017-0695-3. [DOI] [PubMed] [Google Scholar]
- 32.Goldwirt L, Beccaria K, Ple A, Sauvageon H, Mourah S. Ibrutinib brain distribution: a preclinical study. Cancer Chemother Pharmacol. 2018;81(4):783–789. doi: 10.1007/s00280-018-3546-3. [DOI] [PubMed] [Google Scholar]
- 33.Bernard S, Goldwirt L, Amorim S. Activity of ibrutinib in mantle cell lymphoma patients with central nervous system relapse. Blood. 2015;126(14):1695–1698. doi: 10.1182/blood-2015-05-647834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tucker DL, Naylor G, Kruger A, Hamilton MS, Follows G, Rule SA. Ibrutinib is a safe and effective therapy for systemic mantle cell lymphoma with central nervous system involvement – a multi-centre case series from the United Kingdom. Br. J. Haematol. 2017 doi: 10.1111/bjh.14122. [DOI] [PubMed] [Google Scholar]
- 35.Castillo JJ, Itchaki G, Paludo J. Ibrutinib for the treatment of Bing-Neel syndrome: a multicenter study. Blood. 2019;133(4):299–305. doi: 10.1182/blood-2018-10-879593. [DOI] [PubMed] [Google Scholar]
- 36.Cabannes-Hamy A, Lemal R, Goldwirt L. Efficacy of ibrutinib in the treatment of Bing-Neel syndrome. Am J Hematol. 2016;91(3):E17–E19. doi: 10.1002/ajh.24279. [DOI] [PubMed] [Google Scholar]
- 37.Mason C, Savona S, Rini JN. Ibrutinib penetrates the blood brain barrier and shows efficacy in the therapy of Bing Neel syndrome. Br J Haematol. 2017;179(2):339–341. doi: 10.1111/bjh.14218. [DOI] [PubMed] [Google Scholar]
- 38.Awan FT, Schuh A, Brown JR. Acalabrutinib monotherapy in patients with chronic lymphocytic leukemia who are intolerant to ibrutinib. Blood Adv. 2019;3(9):1553–1562. doi: 10.1182/bloodadvances.2018030007. [DOI] [PMC free article] [PubMed] [Google Scholar]