Introduction
BRCA1-associated protein-1 (BAP1) tumor predisposition syndrome (BAP1-TPDS) is an inherited cancer syndrome arising from pathogenic germline variations in BAP1. Affected individuals have increased risk for uveal melanoma, cutaneous melanoma, renal cell carcinoma, and malignant mesothelioma as well as characteristic benign cutaneous lesions (BAP1-inactivated nevi), basal cell carcinomas (BCCs), and other malignancies.1, 2, 3 The full phenotypic neoplastic spectrum is still being investigated. We present the case of a 15-year-old girl with uveal melanoma leading to a diagnosis of BAP1-TPDS.
Case report
A 15-year-old girl presented to ophthalmology clinic with sudden onset of a stable grey line in the central vision of her left eye, accompanied by ipsilateral loss of peripheral vision. Clinical examination and ultrasound scan suggested ocular melanocytosis and choroidal melanoma. Enucleation and fine-needle aspiration of the choroidal lesion were performed. Pathologic evaluation found bilobed melanoma of the choroid surrounding the optic nerve with scleral invasion. Fine-needle aspiration of the choroidal lesion was submitted for gene expression profiling with a proprietary 15-gene panel and showed a high-risk molecular signature (class 2) with a higher risk for metastatic recurrence.4
Family history included mesothelioma in her paternal grandmother. One paternal great aunt had stomach cancer, and another had unknown ocular pathology requiring eye enucleation. Next Generation Sequence analysis of BAP1 detected a germline, monoallelic single base substitution c.2188T>C, leading to loss of a stop codon, p.X730Arg. This variation, although not previously reported, was favored to be pathogenic, and BAP1-TPDS was diagnosed. Initial staging included computed tomography (thorax and abdomen) and magnetic resonance imaging (abdomen and brain); all were unremarkable for metastasis.
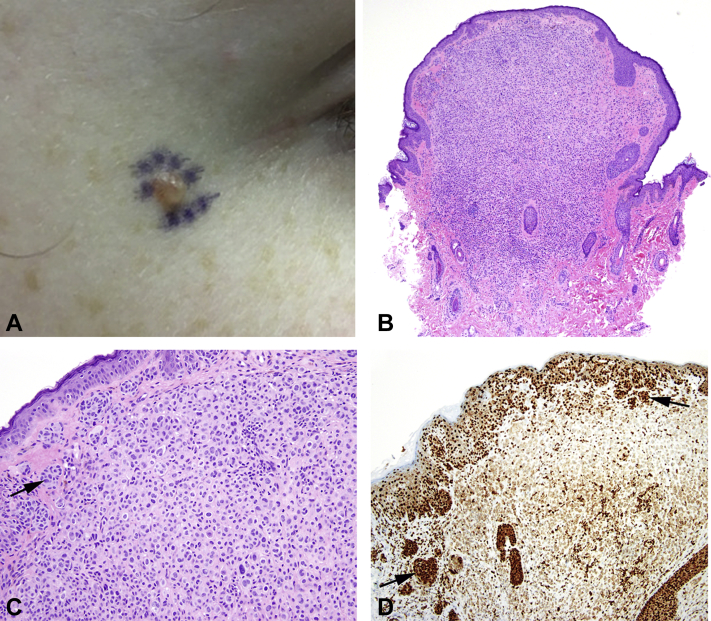
Dermatologic physical examination found several soft pink and pink-tan papules approximately 2-4 mm in size. Pathology of a representative lesion on the lateral canthus (Fig 1, A) showed a dome-shaped biphenotypic melanocytic lesion composed of epithelioid melanocytes with eosinophilic cytoplasmic centrally and ordinary common nevus peripherally (Fig 1, B). The epithelioid component was arranged in a cellular sheet-like growth forming an expansile nodule (Fig 1, C). Both components of the lesion were positive for BRAFV600E. There was loss of nuclear BAP1 expression within the epithelioid component (Fig 1, D). Ki-67/MART1 dual immunostain demonstrated a low proliferation index; p16 expression was retained. The findings were diagnostic for BAP1-inactivated nevus. Removal of several of the patient's other cutaneous lesions also demonstrated histologic features consistent with BAP1-inactivated nevi.
Fig 1.
A, BAP1-TPDS. Clinical presentation of BAP1-inactivated nevus, right lateral canthus. B, Scanning magnification of BAP1-inactivated nevus shows dome-shaped to polypoid silhouette with increased cellularity centrally. C, BAP1-inactivated nevus shows ordinary nevus at periphery (arrow) merging with epithelioid component arranged in a cellular sheet-like configuration. D, BAP1-inactivated nevus shows retention of nuclear BAP1 staining in ordinary nevus component (arrows) and loss of BAP1 staining in epithelioid component. (B and C, Hematoxylin-eosin stain; D, BAP1 immunostain; original magnifications: B, ×40; C and D, ×200.)
The patient remains in active melanoma surveillance with annual dilated eye examinations with ophthalmologic imaging. Cancer geneticists guide her surveillance with other physicians. She undergoes physical examinations every 4 months with blood work including liver panel; annual computed tomography of the chest; abdominal imaging every 6 months, alternating ultrasound scan and magnetic resonance imaging; and total body skin examinations every 6 months with dermatology.
Discussion
BAP1-TPDS is inherited in an autosomal dominant manner3 and increases the risk of uveal melanoma, mesothelioma, cutaneous melanoma and clear cell renal cell carcinoma.1,5, 6, 7, 8 Additional dermatologic manifestations include BAP1-inactivated nevi, the most common lesion reported with BAP1-TPDS, and basal cell carcinoma.9, 10, 11 However, the entire associated tumor spectrum is not well defined. Recent reports suggest that meningioma, cholangiocarcinoma, and potentially other cancers may be part of the BAP1-TPDS spectrum.1,12,13
Uveal melanoma is the most common type of malignancy reported with BAP1-TPDS, found in 24%-28% of documented cases.1,2 Patients with germline BAP1 pathogenic variants tend to have more aggressive class 2 tumors with increased risk for metastasis and poorer prognosis.14 Median age of onset of uveal melanoma in BAP1-TPDS is in the 6th decade of life, with the earliest reported at age 16,1,14,15 slightly older than our patient. Current recommendations suggest that screening for uveal melanoma should begin around age 11.3
In patients with BAP1-TPDS who underwent total body skin examinations, BAP1-inactivated nevi (also known as BAP1-inactivated melanocytic tumors, melanocytic BAP1–mutated atypical intradermal tumors, or BAPomas) were identified in 75% of patients and had a median age of detection of 32 years, which represent a significantly higher penetrance and younger age of onset compared with other BAP1-associated tumors.2 These lesions appear as skin-colored to pink, dome-shaped or pedunculated papules 0.2-1 cm in diameter, often clinically indistinguishable from typical nevi; affected patients can have few or many of these lesions.2 Dermoscopy findings can include structureless, pink-to-tan regions with or without irregular dots/globules, peripheral vessels or pigment network.16
Biopsy is required to diagnose BAP1-inactivated nevus. Histopathology usually finds a biphenotypic or combined, predominantly intradermal melanocytic lesion composed of varying components of ordinary nevus and larger epithelioid melanocytes with eosinophilic cytoplasm and distinct cytoplasmic borders. There may be considerable cytologic atypia and nuclear pleomorphism.9,11 When there is high-grade atypia, the term BAP1-inactivated melanocytoma is recommended.17 BAP1 pathogenic variant status correlates well with protein expression on immunohistochemistry. Therefore, cells with loss of both alleles of BAP1 will show negative staining for this protein, whereas those with monoallelic inactivation will still demonstrate nuclear staining with or without cytoplasmic staining.2 Most tumors are also positive for BRAFV600E mutations.9,11 It has been estimated that perhaps 10%-20% of patients with BAP1-inactivated nevi could have pathogenic germline variations in BAP1,18,19 although prospective studies are warranted in this area.
Cutaneous melanomas have been reported in around 18%-19% of patients with known BAP1 germline mutations.1,2 Cutaneous melanomas tend to occur at an earlier age and may have poorer prognosis than sporadic melanomas.14 Loss of BAP1 expression is seen in cutaneous melanomas diagnosed in both sporadic and BAP1-TPDS cases.14,20 Melanoma can arise de novo or within a preexisting BAP1-inactivated nevus/melanocytoma. BCCs, found on chronic ultraviolet light–exposed skin, were recently added to the clinical spectrum of BAP1-TPDS. BCCs in these patients exhibit loss of BAP1 expression, whereas sporadic BCCs do not.10
Dermatologists are critically positioned to identify and care for patients with BAP1-TPDS given the high penetrance and early onset of BAP1-inactivated nevi and the high frequency of cutaneous melanomas in this population. To date, there are no evidence-based screening recommendations for detection of germline BAP1 mutations. However, BAP1-TPDS should be suspected, and referral to cancer genetics suggested, in patients with 2 or more confirmed BAP1-TPDS tumors or one BAP1-TPDS tumor with a suggestive family history.3 Patients with BAP1-TPDS should receive skin cancer education and regular skin examinations and, under the guidance of cancer genetics, should undergo screening for uveal melanoma, renal cell cancer, and other associated cancers.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Walpole S., Pritchard A.L., Cebulla C.M. Comprehensive study of the clinical phenotype of germline BAP1 variant-carrying families worldwide. J Natl Cancer Inst. 2018;110(12):1328–1341. doi: 10.1093/jnci/djy171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haugh A.M., Njauw C.N., Bubley J.A. Genotypic and phenotypic features of BAP1 cancer syndrome: a report of 8 new families and review of cases in the literature. JAMA Dermatol. 2017;153(10):999–1006. doi: 10.1001/jamadermatol.2017.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pilarski R., Rai K., Cebulla C., Abdel-Rahman M. BAP1 Tumor predisposition syndrome. In: Adam M.P., Ardinger H.H., Pagon R.A., editors. GeneReviews((R)). Seattle (WA) 1993. [Google Scholar]
- 4.Onken M.D., Worley L.A., Char D.H. Collaborative Ocular Oncology Group report number 1: prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology. 2012;119(8):1596–1603. doi: 10.1016/j.ophtha.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiesner T., Obenauf A.C., Murali R. Germline mutations in BAP1 predispose to melanocytic tumors. Nat Genet. 2011;43(10):1018–1021. doi: 10.1038/ng.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdel-Rahman M.H., Pilarski R., Cebulla C.M. Germline BAP1 mutation predisposes to uveal melanoma, lung adenocarcinoma, meningioma, and other cancers. J Med Genet. 2011;48(12):856–859. doi: 10.1136/jmedgenet-2011-100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Testa J.R., Cheung M., Pei J. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43(10):1022–1025. doi: 10.1038/ng.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Popova T., Hebert L., Jacquemin V. Germline BAP1 mutations predispose to renal cell carcinomas. Am J Hum Genet. 2013;92(6):974–980. doi: 10.1016/j.ajhg.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carbone M., Ferris L.K., Baumann F. BAP1 cancer syndrome: malignant mesothelioma, uveal and cutaneous melanoma, and MBAITs. J Transl Med. 2012;10:179. doi: 10.1186/1479-5876-10-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de la Fouchardiere A., Cabaret O., Savin L. Germline BAP1 mutations predispose also to multiple basal cell carcinomas. Clin Genet. 2015;88(3):273–277. doi: 10.1111/cge.12472. [DOI] [PubMed] [Google Scholar]
- 11.Wiesner T., Murali R., Fried I. A distinct subset of atypical Spitz tumors is characterized by BRAF mutation and loss of BAP1 expression. Am J Surg Pathol. 2012;36(6):818–830. doi: 10.1097/PAS.0b013e3182498be5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shankar G.M., Abedalthagafi M., Vaubel R.A. Germline and somatic BAP1 mutations in high-grade rhabdoid meningiomas. Neuro Oncol. 2017;19(4):535–545. doi: 10.1093/neuonc/now235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pilarski R., Cebulla C.M., Massengill J.B. Expanding the clinical phenotype of hereditary BAP1 cancer predisposition syndrome, reporting three new cases. Genes Chromosomes Cancer. 2014;53(2):177–182. doi: 10.1002/gcc.22129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rai K., Pilarski R., Cebulla C.M., Abdel-Rahman M.H. Comprehensive review of BAP1 tumor predisposition syndrome with report of two new cases. Clin Genet. 2016;89(3):285–294. doi: 10.1111/cge.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cebulla C.M., Binkley E.M., Pilarski R. Analysis of BAP1 germline gene mutation in young uveal melanoma patients. Ophthalmic Genet. 2015;36(2):126–131. doi: 10.3109/13816810.2015.1010734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yelamos O., Navarrete-Dechent C., Marchetti M.A. Clinical and dermoscopic features of cutaneous BAP1-inactivated melanocytic tumors: results of a multicenter case-control study by the International Dermoscopy Society. J Am Acad Dermatol. 2019;80(6):1585–1593. doi: 10.1016/j.jaad.2018.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elder D.E., Massi D., Scolyer R.A., Willemze R., International Agency for Research on Cancer . IARC Publications; Lyon, France: 2018. WHO Classification of Skin Tumours. [Google Scholar]
- 18.Cabaret O., Perron E., Bressac-de Paillerets B., Soufir N., de la Fouchardiere A. Occurrence of BAP1 germline mutations in cutaneous melanocytic tumors with loss of BAP1-expression: a pilot study. Genes Chromosomes Cancer. 2017;56(9):691–694. doi: 10.1002/gcc.22473. [DOI] [PubMed] [Google Scholar]
- 19.Garfield E.M., Walton K.E., Quan V.L. Histomorphologic spectrum of germline-related and sporadic BAP1-inactivated melanocytic tumors. J Am Acad Dermatol. 2018;79(3):525–534. doi: 10.1016/j.jaad.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Piris A., Mihm M.C., Jr., Hoang M.P. BAP1 and BRAFV600E expression in benign and malignant melanocytic proliferations. Hum Pathol. 2015;46(2):239–245. doi: 10.1016/j.humpath.2014.10.015. [DOI] [PubMed] [Google Scholar]