Introduction
Dermatofibrosarcoma protuberans (DFSP) is a low-grade dermal sarcoma that arises from a fibroblast/myofibroblast origin.1 Mohs micrographic surgery (MMS) is the gold standard because of a tendency for local invasion and high rates of recurrence with standard wide excision. We present 2 cases of the rare myxoid variant of DFSP successfully treated with MMS, one of which had the unusual clinical presentation of an atrophic patch.
Case 1
A 22-year-old white man with DFSP diagnosed on punch biopsy (Fig 1) was referred for MMS. He had no other significant medical history. He complained of a mildly painful left upper back lesion that had been present for a few months. He had a soft atrophic patch on the left side of his upper back (Fig 2). Using the Mohs micrographic technique, 2 tissue layers showed a hypocellular, partially spindled proliferation in all sections that was in stark contrast to the expected features of a dense dermal infiltration of spindle cells.
Fig 1.
Myxoid DFSP. Case 1: Hematoxylin-eosin–stained punch biopsy specimen from index case shows deeply infiltrating atypical spindle cell infiltrate extending into the subcutis.
Fig 2.
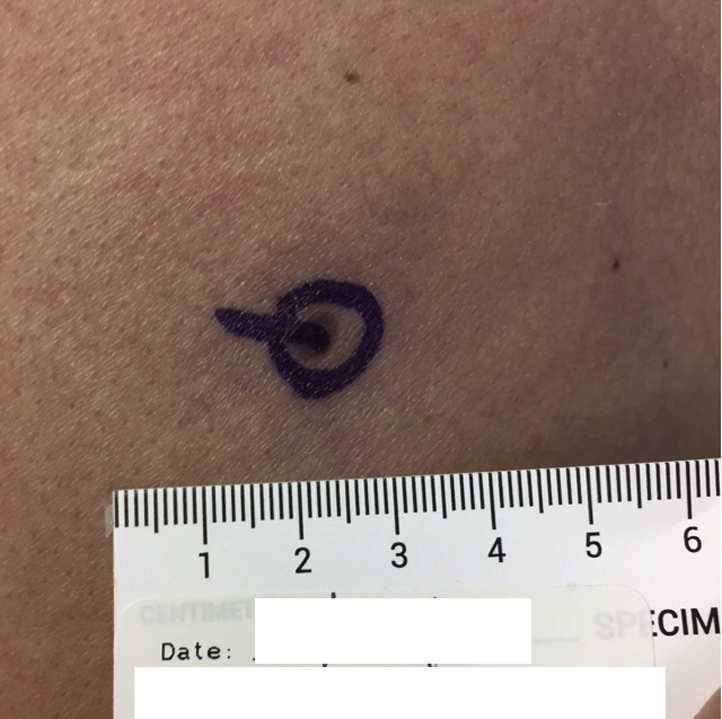
Myxoid DFSP. Case 1: Examination of the left posterior shoulder found a 0.5-cm atrophic patch.
The original pathologist was consulted, and the Mohs sections were sent for confirmatory analysis before proceeding. The pathologist confirmed that the myxoid and spindled presentation that was seen on Mohs frozen sections were in fact DFSP, as shown by a CD34+ deep dermal spindle cell proliferation arranged in loose fascicles with extension into the subcutis. In the deeper aspects of the Mohs excisions, there were interface regions of marked hypercellularity alternating with more evenly spaced hypocellular zones with delicate fibrous tissue and a pale, eosinophilic myxoid stroma (Fig 3). In both the original punch biopsy and the Mohs specimen, the tumor was strongly and diffusely positive for CD34 and negative for CD31, Mart1, smooth muscle actin, and desmin. The final impression from the Mohs specimens confirmed the diagnosis of myxoid DFSP. The patient was cleared by MMS after a total of 4 stages, with MMS being the only modality for margin control. The preoperative size was 1 cm × 1 cm, and the postoperative defect measured 7 cm × 8 cm, which was repaired by the plastic surgery department (Fig 4). The patient is beyond 2 years out from his surgery and has not had any recurrence.
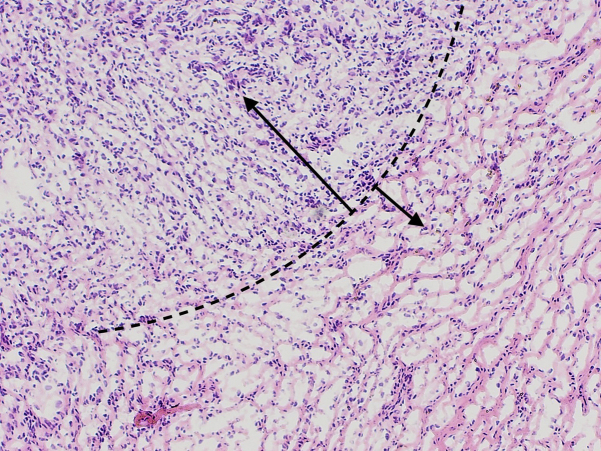
Fig 3.
Myxoid DFSP. Case 1: Hematoxylin-eosin stained Mohs frozen sections. The dashed line represents the interface between the hypercellular zone of spindled cells expected in classic DFSP (larger arrow), compared with the relatively hypocellular zone seen in the myxoid component of this case (smaller arrow). (Original magnification: ×10.)
Fig 4.
Myxoid DFSP. Case 1: The extensive and subclinical extensions of this tumor require complete excision, as shown by this 7 cm × 8 cm postoperative defect following 4 stages of MMS. The patient was referred to plastic surgery for repair.
Case 2
A 58-year-old white man with history of melanoma and type 2 diabetes mellitus presented for full body skin examination. He was found to have a painless red papule measuring 1 cm × 1 cm on the left dorsal foot. The pathology report described a circumscribed dermal proliferation of strongly CD34+ spindle cells with wavy nuclei and interspersed small blood vessels, consistent with DFSP. Immunostaining was negative for AE1/AE3, SOX10, and S100. During the subsequent MMS, we again encountered a myxoid, hypocellular stroma with subtle infiltration of spindled cells. He was declared free of tumor after 2 stages of MMS, and the postoperative defect was 1.3 cm × 1.2 cm, which was repaired by primary intention. The patient has not had any recurrence in the 11 months since his surgery.
Discussion
DFSP is a locally aggressive low-grade dermal malignancy of fibroblast/myofibroblast origin that rarely metastasizes.1 The tumor is well known for a tendency to recur with standard wide excision. Complete surgical resection, preferably by MMS, is the gold standard for treatment.
The typical appearance of classic DFSP depends on the time at which a patient is presenting after onset. Early lesions may appear as an asymptomatic indurated plaque, which over time accumulates protuberant nodules, giving rise to a larger multinodular mass that may be violaceus, red, or brown in color and most commonly occurring on the trunk or extremities.2 The clinical presentation of case 1 as a small, soft, atrophic patch is unusual compared with a case series of 23 patients with myxoid DFSP who presented clinically as raised, nodular, and subcutaneous masses.3 We know of only 1 other case describing myxoid DFSP treated with MMS; in that case, the clinical presentation was of a pink scalp nodule in an adolescent male.4
The histopathology of classic DFSP on low-power microscopy is characterized as dense fascicles of spindle cells that invade aggressively into the dermis with a honeycomb pattern of trapped adipocytes in the underlying subcutis. The myxoid variant features tumor cells that may be dispersed among a more pale-staining eosinophilic stroma; by convention, this appearance must constitute 50% of the tumor stroma to be considered a myxoid DFSP.3
One of the most challenging aspects of complete removal of DFSP is the capability of the tumor to spread via contiguous extensions into adjacent tissues such as fascia and muscle.5 The use of MMS optimizes the detection and excision of these extensions of tumor while simultaneously preserving healthy tissue. However, the myxoid variant poses unique challenges to the Mohs surgeon. During MMS in our cases, the tumor components with myxoid stroma lacked the classic appearance of DFSP and instead took on a paler and subtle appearance on hematoxylin-eosin stain with foci of hypocellularity and dispersed tumor cells. The treatment of a myxoid DFSP variant can be challenging because of the unexpected hypocellular pathology. The Mohs surgeon should be aware that these hypocellular zones contain spindled tumor cells and should be removed in their entirety.
Myxoid DFSP is a rare variant of an already rare tumor. If unexpected, the myxoid stroma of a DFSP can be a hurdle that Mohs surgeons encounter when treating DFSP. Furthermore, the unusual clinical appearance of case 1 added another layer of uncertainty. Mohs surgeons should be aware of this variant that presents with a soft, atrophic patch and a myxoid, hypocellular pathology.
Acknowledgments
We acknowledge and thank Nicole Dominiak, MD, (Assistant Professor, Department of Pathology, University of Toledo Medical Center) for her role in obtaining a higher resolution photo of the frozen sections used for Fig 3. She has no conflicts of interest to disclose.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Mentzel T., Scharer L., Kazakov D.V., Michal M. Myxoid dermatofibrosarcoma protuberans: clinicopathologic, immunohistochemical, and molecular analysis of eight cases. Am J Dermatopathol. 2007;29:443–448. doi: 10.1097/DAD.0b013e318145413c. [DOI] [PubMed] [Google Scholar]
- 2.Llombart B., Serra C., Requena C. Guidelines for diagnosis and treatment of cutaneous sarcomas: dermatofibrosarcoma protuberans. Actas dermo-sifiliograficas. 2018;109:868–877. doi: 10.1016/j.ad.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Reimann J.D., Fletcher C.D. Myxoid dermatofibrosarcoma protuberans: a rare variant analyzed in a series of 23 cases. Am J Surg Pathol. 2007;31:1371–1377. doi: 10.1097/PAS.0b013e31802ff7e7. [DOI] [PubMed] [Google Scholar]
- 4.Greywal T., Wang A.S., Jiang S.I., Krakowski A.C. Rare myxoid dermatofibrosarcoma protuberans masquerading as a pilar cyst in a child. JAAD Case Rep. 2015;1:129–131. doi: 10.1016/j.jdcr.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson R.A., Arlette J.P. Mohs micrographic surgery and dermatofibrosarcoma protuberans: a multidisciplinary approach in 44 patients. Ann Plast Surg. 2008;60:667–672. doi: 10.1097/SAP.0b013e31813376a5. [DOI] [PubMed] [Google Scholar]